Abstract
Peripheral T-cell lymphomas (PTCLs) are a heterogeneous group of clinically aggressive diseases associated with poor outcome. Studies that focus specifically on PTCL are emerging, with the ultimate goal of improved understanding of disease biology and the development of more effective therapies. However, one of the difficulties in classifying and studying treatment options in clinical trials is the rarity of these subtypes. Various groups have developed lymphoma classifications over the years, including the World Health Organization, which updated its classification in 2008. This article briefly reviews the major lymphoma classification schema, highlights contributions made by the collaborative International PTCL Project, discusses prognostic issues and gene expression profiling, and outlines therapeutic approaches to PTCL. These include the standard chemotherapeutic regimens and other modalities incorporating antifolates, conjugates, histone deacetylase inhibitors, monoclonal antibodies, nucleoside analogs, proteasome inhibitors, and signaling inhibitors. As this review emphasizes, the problem has now evolved into an abundance of drugs and too few patients available to test them. Collaborative groups will aid in future efforts to find the best treatment strategies to improve the outcome for patients with PTCL.
Introduction
Peripheral T-cell lymphomas (PTCL), a subdivision of T-cell non-Hodgkin lymphomas (NHLs) and distinct from the more common cutaneous T-cell lymphomas, are a diverse group of disorders that, for the most part, carry a poor prognosis. Classification of PTCL is complex, has resulted in many classification schemes, and has been further hampered by a paucity of molecular markers. Older lymphoma classification systems include the Rappaport system, which was used until the mid 1970s,1 the Kiel system, introduced in 1974,2 and the National Cancer Institute's Working Formulation, introduced in the 1980s.3 The work of Lukes, Collins, and Lennert in the 1970s first suggested that the T-cell lymphomas should be identified as distinct from B-cell lymphoma, but this was initially met with skepticism. In 1994, the International Lymphoma Study Group proposed the REAL classification (revised European-American Classification of Lymphoid Neoplasms), which featured the major histologic, immunologic, and genetic characteristics of B- and T-cell neoplasms and Hodgkin lymphoma.4 A clinical evaluation of the International Lymphoma Study Group classification of NHL published in 1997 concluded that the classification could be readily applied and identified clinically distinctive types of NHL.5
The International Lymphoma Epidemiology Consortium (InterLymph) proposed in 2007 a nested classification of lymphoid neoplasm subtypes.6 They based their classification on the World Health Organization (WHO) classification of lymphoid neoplasms7 and the International Classification of Diseases for Oncology (3rd ed).8 The fourth edition of the WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues was published in 2008.9 The new WHO classification includes many additional subtypes of PTCL (Table 1); nevertheless, it does not necessarily help clinicians determine the best therapeutic strategy for each specific subtype. The International PTCL Project is a collaborative effort that was designed to gain better understanding of the distribution and outcomes of aggressive T-cell lymphomas. A total of 22 institutions and more than 1300 eligible patients in North America, Europe, and Asia participated in and submitted clinical and pathologic information on PTCLs diagnosed and treated at their respective centers.10
Old and new WHO classifications of PTCLs
| Old WHO classification7 . | New WHO classification9 . |
|---|---|
| Precursor T-cell lymphoma | |
| T-lymphoblastic lymphoma/leukemia | |
| Mature T-cell lymphomas | |
| T-cell prolymphocytic leukemia | T-cell prolymphocytic leukemia |
| T-cell granular lymphocytic leukemia | T-cell large granular lymphocytic leukemia |
| Aggressive NK-cell leukemia | Aggressive NK-cell leukemia |
| Indolent large granular NK-cell lymphoproliferative disorder (provisional) | |
| ATL/adult T-cell leukemia (HTLV1+) | ATL/adult T-cell leukemia |
| Extranodal NK-/T-cell lymphoma, nasal type | Extranodal NK-/T-cell lymphoma, nasal type |
| Enteropathy-type T-cell lymphoma | EATL |
| Hepatosplenanic T-cell lymphoma | Hepatosplenic T-cell lymphoma |
| Subcutaneous panniculitis-like T-cell lymphoma | Subcutaneous panniculitis-like T-cell lymphoma (αβ only) |
| Primary cutaneous γδ T-cell lymphoma | |
| Mycosis fungoides/Sézary syndrome | Mycosis fungoides/Sézary syndrome |
| Anaplastic large-cell lymphoma, systemic or cutaneous | ALCL: ALK+ |
| ALCL: ALK− (provisional) | |
| PTCL, unspecified | PTCL, NOS |
| AITL | AITL |
| Primary cutaneous CD30+ T-cell LPD | |
| LYP and primary cutaneous ALCL | |
| Primary cutaneous CD4+ small/medium T-cell lymphoma (provisional) | |
| Primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma (provisional) | |
| Systemic EBV+ T-cell LPD of childhood | |
| Hydroa vacciniforme-like lymphoma |
| Old WHO classification7 . | New WHO classification9 . |
|---|---|
| Precursor T-cell lymphoma | |
| T-lymphoblastic lymphoma/leukemia | |
| Mature T-cell lymphomas | |
| T-cell prolymphocytic leukemia | T-cell prolymphocytic leukemia |
| T-cell granular lymphocytic leukemia | T-cell large granular lymphocytic leukemia |
| Aggressive NK-cell leukemia | Aggressive NK-cell leukemia |
| Indolent large granular NK-cell lymphoproliferative disorder (provisional) | |
| ATL/adult T-cell leukemia (HTLV1+) | ATL/adult T-cell leukemia |
| Extranodal NK-/T-cell lymphoma, nasal type | Extranodal NK-/T-cell lymphoma, nasal type |
| Enteropathy-type T-cell lymphoma | EATL |
| Hepatosplenanic T-cell lymphoma | Hepatosplenic T-cell lymphoma |
| Subcutaneous panniculitis-like T-cell lymphoma | Subcutaneous panniculitis-like T-cell lymphoma (αβ only) |
| Primary cutaneous γδ T-cell lymphoma | |
| Mycosis fungoides/Sézary syndrome | Mycosis fungoides/Sézary syndrome |
| Anaplastic large-cell lymphoma, systemic or cutaneous | ALCL: ALK+ |
| ALCL: ALK− (provisional) | |
| PTCL, unspecified | PTCL, NOS |
| AITL | AITL |
| Primary cutaneous CD30+ T-cell LPD | |
| LYP and primary cutaneous ALCL | |
| Primary cutaneous CD4+ small/medium T-cell lymphoma (provisional) | |
| Primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma (provisional) | |
| Systemic EBV+ T-cell LPD of childhood | |
| Hydroa vacciniforme-like lymphoma |
The WHO classification for PTCLs was updated in 2008. The new classification expanded some existing disease types and added several new provisional diseases.
WHO indicates World Health Organization; PTCL, peripheral T-cell lymphoma; NK, natural killer; HTLV1, human T-lymphotropic virus type 1; EATL, enteropathy-associated T-cell lymphoma; and ALCL, anaplastic large cell lymphoma; AITL, angioimmunoblastic T-cell lymphoma; and NOS, not otherwise specified.
Treatment advances in PTCLs have been slow compared with other lymphomas. Very often, PTCL patients are treated with the same therapy used in B-cell lymphomas, but this approach generally has a very poor outcome. Relapse is common after the administration of most currently available agents, and there are few effective options for salvage therapy. In addition, only small numbers of patients have each type of PTCL, which further complicates studying new treatments for these diseases in clinical trials.
Classification
The WHO classification system classifies the mature T-cell lymphomas into 4 groups based on their clinical features. Most of the aggressive T-cell lymphomas are included within the nodal, extranodal, and leukemic groups.
The nodal lymphoma group includes PTCL, not otherwise specified (NOS), accounting for 25.9% of cases,10 anaplastic large cell lymphoma (ALCL), and angioimmunoblastic T-cell lymphoma (AITL). ALCL is further separated into the ALK+ and ALK− entities. The ALK− ALCLs are morphologically and immunophenotypically similar to ALK+ ALCL but lack ALK expression and have distinctive molecular features.11 According to the International PTCL study, ALK+ ALCL accounts for 6.6% and ALK− ALCL for 5.5% of PTCL cases.10 The cutaneous ALCLs are included as a separate disease entity because their treatment and prognosis are distinct from the systemic ALCLs. AITL is the second largest category, accounting for 18.5% of cases in the International PTCL Project.10 The lymphoepithelioid cell variant appears to be distinct from the others and is usually characterized by CD8+ T cells and a predominance of epithelioid cells in the background.12
The extranodal group includes a number of less common entities described primarily by their tissue tropism. Hepatosplenic γδ T-cell lymphoma is a disease of children and young adults,13,14 accounting for 1.4% of PTCL cases.10 This tumor is derived from immature or nonactivated γδ T cells, which infiltrate the liver, spleen, and bone marrow sinusoids. A characteristic chromosomal abnormality is isochromosome 7q, and patients with hepatosplenic T-cell lymphoma often have a poor outcome with a median survival of less than 2 years. The disease is more common in young males who often present with B-symptoms and cytopenias. The cells are typically CD2+, CD3+, CD4−, CD5−, CD7+, CD8+, or CD8− and may express natural killer (NK) markers as well.
Enteropathy-associated T-cell lymphoma (EATL) accounts for 4.7% of cases of T-cell lymphoma.10 EATL is more common in geographic areas with a higher incidence of celiac disease, as evidenced by the fact that it represents 5.8% and 9.1% of the PTCLs in North America and Europe, respectively, but only 1.9% of those in Asia. The disease often presents with pain, weight loss, and bowel perforation. There are 2 morphologic variants that are the pleomorphic type, associated with celiac disease and usually CD3+, CD7+, and CD56−, and the monomorphic type, which is CD56+ and often not associated with celiac disease.15 Chromosomal abnormalities found in EATL include gains at chromosome 9q33-q34 in up to 70% of cases.16 The intestinal T- and NK-cell lymphomas diagnosed in Asian countries are associated with Epstein-Barr virus (EBV) infection and are part of the spectrum of nasal-type NK-/T-cell lymphoma.17 The mucocutaneous γδ T-cell lymphomas,18 the nasal type NK-/T-cell lymphomas, and even ALCLs can present as an intestinal lymphoma.19,20
Panniculitis-like T-cell lymphomas constitute only 0.9% of PTCLs, and in the International PTCL Project were more common in men (75% of cases) than in women. Patients usually present with subcutaneous nodules that may become necrotic. The neoplastic cells are typically CD3+, CD4−, and CD8+, with either T-cell receptor (TCR)–α/β+ or TCR-γ/δ+ and infiltrate in a rim-like fashion around the fat cells. The γδ panniculitis-like T-cell lymphomas have now been reclassified as cutaneous γδ T-cell lymphoma because their outcome is significantly inferior to that of patients with the αβ type.21
The leukemia group consists of adult T-cell lymphoma (ATL) associated with human T-lymphotropic virus type I (HTLV-1), T-cell chronic large granular lymphocytic (LGL) leukemia, aggressive NK-cell leukemia, and T-cell prolymphocytic leukemia. Most patients with LGL leukemia have an indolent disorder that is often associated with neutropenia and can be treated with immunosuppressive agents, whereas those with aggressive NK-cell leukemia and ATL often have a poor outcome.
Several other types are delineated in the WHO classification: hydroa vacciniforme-like lymphoma, which is usually of a T-cell origin, NK-cell lymphoma caused by mosquito bite allergy, and systemic EBV-positive T-cell lymphoproliferative disease of childhood, which is part of the spectrum of chronic, active EBV infection.
Incidence of T-cell lymphomas
Based on the United States Surveillance, Epidemiology, and End Results registry, the incidence of PTCL is < 1 case per 100 000 people in the United States.22 Results from the International PTCL Project showed that, around the world, the most common subtypes are the nodal T-cell lymphomas, with PTCL-NOS (25.9%) being the most frequent, along with AITLs (18.5%) and the ALCLs (12%). NK-/T-cell lymphomas composed 10.4%, whereas enteropathy-associated T-cell lymphomas (4.7%), hepatosplenic T-cell lymphomas (1.4%), and panniculitis-like T-cell lymphomas (0.9%) were rare, even in this large study. Among the common subtypes, there are regional differences in frequency. PTCL-NOS is more common in North America and less common in the European and Asian countries; AITL is more common in Europe than in Asia or North America; ALK+ ALCL is more common in North America; ALK− ALCL is slightly more common in Europe; and the NK-/T-cell lymphomas and ATL are more common in Asia.23 The epidemiology of EATL is closely associated with the human leukocyte antigen DQ, which results in either overt or silent celiac disease, a European disease.24 The EBV-associated lymphoproliferative, T-cell, and NK-cell neoplasms are seen mainly in Japan, Korea, and Northern China, but also in Native American populations from Central and South America. NK-cell nasal and nasal-type lymphomas, occurring more frequently in Asia and Latin America, have a greater incidence in males than in females.
Viral associations with T-cell lymphomas
The increased incidence of T-NK-cell lymphomas in East Asia related to the frequency of endemic HTLV-1 and EBV infections. The HTLV-1 virus was first identified by Gallo's group at the National Cancer Institute from a cell line established from a patient with cutaneous T-cell lymphoma.25 At the same time, Yoshida et al in Japan identified a retrovirus that was immunoreactive to sera from patients with T-cell leukemia.26 The unique genome structure of HTLV-1 identified it as distinct from any other animal retroviruses, thus founding a new retroviral group. Other members of this viral group include HTLV-2, STLV, and BLV. Viral transmission is thought to require the transfer of live infected T lymphocytes, that is, T cells in breast milk, T cells in semen, and fresh T cells in blood carriers of HTLV-1 proviruses.
HTLV-1-associated lymphoma
HTLV-1-associated lymphomas include ATL, smoldering ATL, which is characterized by small numbers of circulating leukemia cells without nodal involvement, lymphomatous ATL, which presents with lymphadenopathy without leukemic involvement, and chronic ATL, which is characterized by skin lesions, leukemic, nodal, and visceral disease without hypercalcemia, gastrointestinal involvement, bone, or central nervous system disease. The geographic distribution is reflective of the areas where HTLV-1 infections are prevalent, specifically Japan and the Caribbean basin. ATL is characterized by a clonal expansion of CD4+ T lymphocytes frequently associated with skin rash, lymph node and visceral involvement, and hypercalcemia. It is suggested that the transformation of T cells in the early stages of ATL is mediated by HTLV-1 Tax protein and persistent nuclear factor-κB activation.
Angioimmunoblastic T-cell lymphoma
AITL shows distinctive features, with a polymorphous infiltrate containing medium-sized neoplastic cells, prominent arborizing blood vessels, proliferation of follicular dendritic cells, and scattered EBV+ B-cell blasts.27 Recent studies indicate that AITL is derived from the unique T-cell subset located in the germinal center, which are called the follicular helper T cells (TFH).28-31 Viruses have been implicated in the transformation of these TFH cells. Besides EBV, which is found in B-cell blasts and is likely to play a role in the development of an EBV-associated B-cell lymphoma in some AITL patients, HHV6B, another human herpes virus, has been reported in approximately half of AITL cases. Although viral infection most probably reflects the underlying immune dysfunction, it could also suggest that EBV and/or HHV6B may modulate cytokines, chemokines, and membrane receptors.
NK-/T-cell lymphoma
NK-cell lymphomas include extranodal NK-/T-cell lymphoma, nasal type, blastic NK-cell lymphoma, and aggressive NK-cell leukemia account for 10.4% of PTCL cases. Most NK-cell lymphomas have been recognized and are included in the classification of lymphoid neoplasms, but some other NK-cell lineage neoplasms are not yet categorized by the WHO. These include myeloid/NK-cell precursor acute leukemia, precursor NK-cell acute lymphoblastic leukemia, and chronic reactive NK-cell lymphocytosis.32 EBV has been implicated and found in the tumor cells of nasal/NK-cell lymphomas and aggressive NK-cell leukemia.33 However, there may be other causative factors. In one recent study from China, the occurrence of epithelial carcinomas in 10 of 23 cases of NK-/T-cell lymphoma of the oral cavity suggests that other oncogenic factors may be implicated as well as EBV.34
Prognostic features of T-cell lymphomas
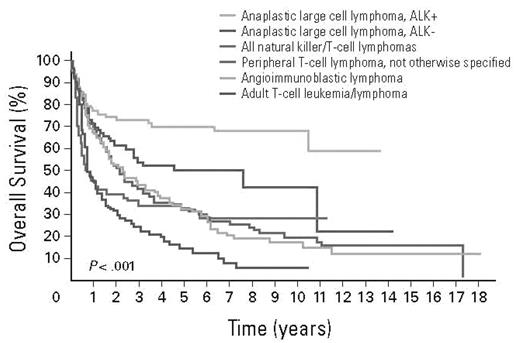
Overall, patients with PTCL had a very poor outcome compared with patients with aggressive B-cell lymphomas.10 The International T-cell Lymphoma Study demonstrated that the overall survival (OS) and failure-free survival (FFS) with PTCL-NOS at 10 to 15 years was 10% (Figure 1). The International Prognostic Index (IPI), when applied to patients with aggressive T-cell lymphomas, identified a group with high IPI score who had an adverse outcome compared with patients with low IPI, similar to diffuse large B-cell lymphoma.35 However, even patients in the best prognostic category did not have a favorable outcome, and patients in the highest risk category had very short survival. When examined by histopathologic subset, the 5-year OS for patients with PTCL-NOS and AITL with IPI of 0 to 1 were 56% and 50%, respectively, whereas those with IPI of 4 or 5 were 11% and 25%, respectively. For patients with ALCL, the 5-year survivals for IPI 0 to 1 patients were excellent at 90% and 74% for ALK+ and ALK− patients, respectively; however, patients with IPI of 4 to 5 had a poor outcome with 5-year survivals of 33% and 13%, suggesting that IPI is an important predictor, even in ALK+ patients.23 The IPI has not been predictive of outcome in patients with ATLL, enteropathy-associated and hepatosplenic T-cell lymphoma, or extranasal NK-/T-cell lymphoma.
OS of patients with the common subtypes of PTCL. Data from Vose et al10 with permission.
OS of patients with the common subtypes of PTCL. Data from Vose et al10 with permission.
A new prognostic index specifically designed for PTCL,36 the prognostic index for PTCL (PIT), is similar to the IPI, including age, lactate dehydrogenase, performance status, and then bone marrow involvement. When applied to patients with PTCL-NOS, the index separated patients into more specific prognostic groups than the IPI. Of 322 patients studied, 20% had no adverse features, 34% had one feature, 26% had 2 features, and 20% had 3 or more. The 5-year OS for the most favorable group with no adverse prognostic features was 62% compared with 18% for patients with 3 or 4 adverse prognostic factors.
Aside from conventional histopathology, chemokine expression and proliferative signature have been shown to have prognostic significance, including p53 and Ki-67 (Table 2). In one study, p53 was the most important prognostic factor and was correlated with expression of P-glycoprotein, which confers resistance to chemotherapy.37 In addition, 40% to 60% of patients with PTCL express BCL2- and BCLXL-associated proteins.38 ALK+ ALCL is usually BCL2 negative, whereas other subtypes with worse outcomes may be BCL2+.
Biologic factors and outcome in PTCL
| Variable . | Prognostic significance . |
|---|---|
| p53 | Worse |
| Ki-67 | Worse |
| BCL-2, BCL-XL | Worse |
| CD26 | Worse |
| EBV | Worse |
| MDR | Worse |
| CCND2 | Worse |
| CCR4 | Worse |
| NF-κB | Favorable |
| CCR3 | Favorable |
| CXCR3 | variable |
| PRDM1 | Worse |
| ALK-1 | Favorable |
| TCR BF1 | Favorable |
| TCR gamma1 | Worse |
| Variable . | Prognostic significance . |
|---|---|
| p53 | Worse |
| Ki-67 | Worse |
| BCL-2, BCL-XL | Worse |
| CD26 | Worse |
| EBV | Worse |
| MDR | Worse |
| CCND2 | Worse |
| CCR4 | Worse |
| NF-κB | Favorable |
| CCR3 | Favorable |
| CXCR3 | variable |
| PRDM1 | Worse |
| ALK-1 | Favorable |
| TCR BF1 | Favorable |
| TCR gamma1 | Worse |
TCR indicates T-cell receptor.
Two chemokine receptors, CXCR3 and CCR4, were found to be expressed in 63% and 34% of PTCL-NOS cancers, respectively.8 The dominant chemokine expression found in this study was CXCR3-positive/CCR4-negative; this phenotype was shown by multivariate analysis to be an independent prognostic factor and significantly prognostic of a poor prognosis in both PTCL-NOS and ALK-negative ALCL.
In a study reported by Rudiger et al of PTCL-NOS category, which excludes AITL, the presence of > 70% transformed blasts, > 25% Ki67 proliferation, CD56 and CD30 expression, EBV infection, and a background of > 10% CD8+ cells were all adverse prognostic factors.39 The results indicated that immunophenotyping is absolutely necessary to determine prognosis. In another study, multivariate analysis of all prognostic factors indicated that, on controlling for IPI, if the number of transformed cells is > 70%, the hazard ratio for OS is 2.2 and 1.6 for FFS.40 In patients with ALCL, the presence of the small cell variant can be associated with a worse prognosis. Finally, the expression of cytotoxic molecules also has an adverse impact on survival.41
Gene expression, cytogenetic and comparative genomic hybridization data
Several studies of gene expression profiling of PTCLs have been published. Importantly, expression profiling of purified normal T-cell subpopulations has provided the framework for understanding cell-of-origin and functional properties of T-cell malignancies.42-47 In aggregate, these studies have helped define the normal counterpart of several specific PTCLs, identified pathways frequently altered in PTCL, including nuclear factor-κB signaling and cell cycle deregulation, and highlighted the importance of proliferation as a potential biomarker for prognosis.48-57 However, most of these studies are largely underpowered to allow definitive statements regarding survival prediction. Large sample sizes will be needed to build molecular outcome predictors in PTCL and will probably require international collaboration.
More recently, gene expression profiling studies of larger numbers of patients have been undertaken and do provide novel insights into several PTCL entities.58,59 Iqbal et al studied 144 cases and were able to confirm several previous observations.58 In addition, they were able to show a major role for the microenvironment in AITL and developed a 15-gene outcome predictor highly correlated with survival and independent of the IPI. Similarly, they discovered a subgroup of PTCL-NOS with cytotoxic characteristics and inferior survival. Huang et al studied a much smaller number of extranodal NK-/T-cell lymphomas, nasal type by combining microarray analysis with array comparative genomic hybridization.59 These authors established marked differences in gene expression in these tumors compared with normal NK cells, particularly the significant overexpression of granzyme H; provided evidence for overexpression of PDGFRA similar to PTCL-NOS; and suggested a possible role for the novel tumor suppressor gene, HACE1, mapping to the 6q21 region, in NK-/T-cell lymphomas.
Classic cytogenetic studies have also provided insight into copy number alterations in PTCL (Table 3).60 A recent study of PTCL-NOS, AITL, and ALK− ALCL revealed recurrent alterations associated with specific entities, including gains of 5q, 21, and 3q in AITL, commonly associated with trisomies of chromosome 5 and 21. Loss of genetic material involving chromosome 6q was also frequent. ALK− ALCL was characterized by gains of 1q and 3p and losses of 16pter, 6q13-21, 15, 16qter, and 17p13. PTCL-NOS showed frequent gains of 7q22-31, 1q, 3p, 5p, and 8q24qter and losses of 6q22-24 and 10p13pter. Cases with a complex karyotype had shorter OS.
Cytogenetics of PTCL subtypes
| . | TCR . | Chromosomal/histopathologic features . | Distinguishing features . | 5-year survival, % . |
|---|---|---|---|---|
| PTCL-NOS | αβ | Loss 13q22.3 adverse prognosis; gains 8q,9p,19q,loss 3q,9p | Heterogeneous, variable morphology | 20-30 |
| AITL | αβ | Follicular dendritic cell signature CXCL13+, PD-1+; gains 2,5,13q22.3 adverse prognosis | Immunodeficiency and immune dysregulation Helper B cells may respond to cyclosporine | 32 |
| ALCL ALK+ | αβ | (2,5) translocation; gain 1q, loss6q, 13q | Bone marrow involvement in only 29%, median age 34 y | 70 |
| ALK ALK− | αβ | Pax 5−, CD15− | Median age 58 y | 49 |
| NK-/T-cell | CD56+, often EBV+ | Stage I/II patients respond to radiotherapy or radiochemotherapy Serum EBV copy number predictive of outcome | Nasal type 64 Extranasal type < 20 | |
| SPTCL- αβ SPTCL- γδ | Αβ γδ | CD3+CD8+CD56−5q,13q gains CD3+CD8− CD56+/− | Nodules and plaques nodules, often ulceration Hemophagocytic syndrome | 82; 11 |
| Hepatosplenic T-cell lymphoma | γδ | Isochromosme 7q, CD3+, CD4−CD8−, may be CD56+ | Infiltration of sinusoids in liver, spleen, bone marrow Erythrophagocytosis | 2-year OS 20 |
| EATL | αβ | Gains at chromosome 9q33-q34 CD3+ CD7+, may be CD8+ CD56+ with monomorphic type | Associated with celiac sprue, malabsorption | 4 |
| . | TCR . | Chromosomal/histopathologic features . | Distinguishing features . | 5-year survival, % . |
|---|---|---|---|---|
| PTCL-NOS | αβ | Loss 13q22.3 adverse prognosis; gains 8q,9p,19q,loss 3q,9p | Heterogeneous, variable morphology | 20-30 |
| AITL | αβ | Follicular dendritic cell signature CXCL13+, PD-1+; gains 2,5,13q22.3 adverse prognosis | Immunodeficiency and immune dysregulation Helper B cells may respond to cyclosporine | 32 |
| ALCL ALK+ | αβ | (2,5) translocation; gain 1q, loss6q, 13q | Bone marrow involvement in only 29%, median age 34 y | 70 |
| ALK ALK− | αβ | Pax 5−, CD15− | Median age 58 y | 49 |
| NK-/T-cell | CD56+, often EBV+ | Stage I/II patients respond to radiotherapy or radiochemotherapy Serum EBV copy number predictive of outcome | Nasal type 64 Extranasal type < 20 | |
| SPTCL- αβ SPTCL- γδ | Αβ γδ | CD3+CD8+CD56−5q,13q gains CD3+CD8− CD56+/− | Nodules and plaques nodules, often ulceration Hemophagocytic syndrome | 82; 11 |
| Hepatosplenic T-cell lymphoma | γδ | Isochromosme 7q, CD3+, CD4−CD8−, may be CD56+ | Infiltration of sinusoids in liver, spleen, bone marrow Erythrophagocytosis | 2-year OS 20 |
| EATL | αβ | Gains at chromosome 9q33-q34 CD3+ CD7+, may be CD8+ CD56+ with monomorphic type | Associated with celiac sprue, malabsorption | 4 |
PTCL indicates peripheral T-cell lymphoma; TCR, T-cell receptor; NOS, not otherwise specified; AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; NK, natural killer; SPTCL, subcutaneous panniculitis-like T-cell lymphoma; and EATL, enteropathy-associated T-cell lymphoma.
Although many subtypes of PTCL are characterized by clonal expansion of TCR rearranged lymphocytes, this may not be a characteristic of all cases of NK neoplasms. Two studies recently examined the lack of TCR rearrangements in these patients and reported rearrangements at the TCR locus in 0.8% to 1.2% of cases.61,62
Comparative genomic hybridization was used to examine genomic alterations in patients with ALK+ and ALK− ALCL. A study of 74 patients showed that ALK+ ALCL samples had gains of 17p and losses of 4q13-q21 and 11q14, whereas gains of 1q and 6p21 were more frequent in ALK− samples.11 Another study, which used genomic profiling in patients with PTCL-NOS (n = 42) and ALK− ALCL (n = 37),63 showed that genetics of PTCL-NOS and ALK− ALCL differ substantially from other T-cell lymphomas, including EATL, T-cell prolymphocytic leukemia, and ATL. Those with PTCL-NOS had recurrent chromosomal gains, high-level amplifications, and recurrent chromosomal losses on 13q, 6q, 9p, 10q, 12q, and 5q. Cutaneous ALCL and ALK+ ALCL did not show imbalances, whereas ALK− ALCL showed chromosomal gains of 1q and losses of 6q and 13q.
Lastly, it is probable that significant progress in our understanding of the genetic complexity of PTCL will require an international effort to study a number of well-annotated cases using next-generation sequencing strategies.64 It can safely be hypothesized that this approach will yield important information about recurrent mutations and novel fusions that underlie the critical events in the biology of these tumors. Matched constitutional DNA will be required to properly annotate all of the somatic changes.
Therapeutic approaches
Standard first-line therapy
T-cell lymphomas have traditionally been treated much like the B-cell lymphomas, with a combination chemotherapy regimen. CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) was the most widely used, although there were no randomized studies that proved that it was the best therapy. A retrospective meta-analysis of 2912 patients treated with CHOP or CHOP-like regimens reported a 5-year OS of 37%.65 Several groups have tried more intensive chemotherapeutic treatments for PTCL patients. The French Groupe d'Etude des Lymphomes de l'Adulte (GELA) used more intensive chemotherapy: ACVBP (dose-intensified doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone) plus consolidation with autologous stem cell transplantation (ASCT) for younger, fitter patients.66 The German High Grade Non-Hodgkin Lymphoma Study Group used several variations of CHOP,67,68 and an Italian group used high-dose sequential chemotherapy with ASCT.69 The M. D. Anderson Cancer Center used an alternating triple therapy regimen and hyper-CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone).70 The GELA demonstrated that ACVBP had higher toxicity but superior event-free survival and OS compared with CHOP.71 CHOP-14 proved superior to CHOP-21 or CHOP plus etoposide in elderly patients,68 and CHOP plus etoposide should be used in younger patients.67
Anthracycline-containing regimens for PTCL have been associated with median 5-year OS rates < 40%.65 ALCL patients have better outcomes than non-ALCL patients, with an overall response rate (ORR) > 75% and 5-year OS rates > 60%.23 ALK+ ALCL patients have slightly higher OS and FFS rates compared with ALK− ALCL patients, but this may be the result of a difference in age, as ALK+ patients tend to be younger than ALK− patients. However, even in ALK+ ALCL patients, 5-year FFS rates were only 49%, suggesting a definite need for new therapies or different subtype-specific treatments. It is unclear why patients with aggressive T-cell lymphomas are less responsive to conventional B-cell lymphoma regimens, but data are emerging regarding the expression of P-glycoprotein and other drug resistance pathways in subsets of patients, particularly patients with NK-/T-cell lymphomas.
Novel approaches
New therapeutic approaches and the incorporation of novel agents into these therapeutic regimens are necessary to improve the outcome for PTCL patients. Table 4 lists some of the new agents that are currently being used or are in clinical trials.
Novel agents in use or trials in PTCL
| Type of agent . | Name . | Description . | Disease(s) . | Status . |
|---|---|---|---|---|
| Antifolates | Pralatrexate | 10-Deazaminopoterin | PTCL, CTCL | Approved for PTCL |
| Conjugates | LMB-2 | Anti-Tac) anti-CD25 fused to Pseudomonas toxin | CTCL, PTCL (especially ATLL) | Phase 2 |
| Denileukin diftitox | IL-2 targeting domain fused with diphtheria toxin | CTCL, PTCL | Approved for CTCL | |
| Brentuximab vedotin | CD30 antibody conjugated to monomethylauristan-E | CD30+ T-cell lymphoma | Phase 2 | |
| HDAC inhibitors | Belinostat | PXD101 | CTCL, PTCL | Phase 2 |
| Panobinostat | LBH589 | CTCL, ATL | Phase 2 | |
| Romidepsin | Depsipeptide | CTCL, PTCL | Approved for CTCL | |
| Vorinostat | Suberoylanilide hydroxamic acid | CTCL | Approved for CTCL | |
| Immunomodulatory agents | Lenalidomide | Derivative of thalidomide | PTCL, CTCL | Phase 2 |
| Immunosuppressive agents | Cyclosporine | Inhibitor of the NF-AT transcription complex | AITL | Phase 2 |
| Monoclonal antibodies | Alemtuzumab | Anti-CD52 | PTCL | Phase 3 |
| Bevacizumab | Anti-VEGF | PTCL (especially AITL), NK-cell | Phase 2 | |
| Iratumumab | Anti-CD30 | CD30+ ALCL | Phase 1/2 | |
| KW-0761 | Anti-CCR4 | ATLL, PTCL | Phase 2 | |
| SGN-30 | Anti-CD30 | CD30+ ALCL | Phase 2 | |
| Siplizumab | Anti-CD2 | PTCL, NK-cell, ATLL | Phase 1 | |
| Zanolimumab | Anti-CD4 | CTCL, PTCL | Phase 2 | |
| Nucleoside analogs | Cladribine | Purine nucleoside analog | PTCL | Phase 4 |
| Clofarabine | Purine nucleoside analog | PTCL, NK-cell | Phase 1/2 | |
| Fludarabine | Purine nucleoside analog | PTCL, CTCL | Phase 2 | |
| Forodesine | Metabolic enzyme inhibitor | PTCL, CTCL | Phase 2 | |
| Gemcitabine | Pyrimidine nucleoside analog | PTCL | Phase 2 | |
| Nelarabine | Purine nucleoside analog | T-ALL, T-NHL | Phase 2 | |
| Pentostatin | Metabolic enzyme inhibitor | PTCL | Phase 2 | |
| Proteasome inhibitors | Bortezomib | Proteasome inhibitor | CTCL | Phase 2 |
| Signaling inhibitors | Enzastaurin | Selective inhibitor of protein kinase C | PTCL, CTCL | Phase 2 |
| R788 | Syk inhibitor | PTCL | Phase 2 |
| Type of agent . | Name . | Description . | Disease(s) . | Status . |
|---|---|---|---|---|
| Antifolates | Pralatrexate | 10-Deazaminopoterin | PTCL, CTCL | Approved for PTCL |
| Conjugates | LMB-2 | Anti-Tac) anti-CD25 fused to Pseudomonas toxin | CTCL, PTCL (especially ATLL) | Phase 2 |
| Denileukin diftitox | IL-2 targeting domain fused with diphtheria toxin | CTCL, PTCL | Approved for CTCL | |
| Brentuximab vedotin | CD30 antibody conjugated to monomethylauristan-E | CD30+ T-cell lymphoma | Phase 2 | |
| HDAC inhibitors | Belinostat | PXD101 | CTCL, PTCL | Phase 2 |
| Panobinostat | LBH589 | CTCL, ATL | Phase 2 | |
| Romidepsin | Depsipeptide | CTCL, PTCL | Approved for CTCL | |
| Vorinostat | Suberoylanilide hydroxamic acid | CTCL | Approved for CTCL | |
| Immunomodulatory agents | Lenalidomide | Derivative of thalidomide | PTCL, CTCL | Phase 2 |
| Immunosuppressive agents | Cyclosporine | Inhibitor of the NF-AT transcription complex | AITL | Phase 2 |
| Monoclonal antibodies | Alemtuzumab | Anti-CD52 | PTCL | Phase 3 |
| Bevacizumab | Anti-VEGF | PTCL (especially AITL), NK-cell | Phase 2 | |
| Iratumumab | Anti-CD30 | CD30+ ALCL | Phase 1/2 | |
| KW-0761 | Anti-CCR4 | ATLL, PTCL | Phase 2 | |
| SGN-30 | Anti-CD30 | CD30+ ALCL | Phase 2 | |
| Siplizumab | Anti-CD2 | PTCL, NK-cell, ATLL | Phase 1 | |
| Zanolimumab | Anti-CD4 | CTCL, PTCL | Phase 2 | |
| Nucleoside analogs | Cladribine | Purine nucleoside analog | PTCL | Phase 4 |
| Clofarabine | Purine nucleoside analog | PTCL, NK-cell | Phase 1/2 | |
| Fludarabine | Purine nucleoside analog | PTCL, CTCL | Phase 2 | |
| Forodesine | Metabolic enzyme inhibitor | PTCL, CTCL | Phase 2 | |
| Gemcitabine | Pyrimidine nucleoside analog | PTCL | Phase 2 | |
| Nelarabine | Purine nucleoside analog | T-ALL, T-NHL | Phase 2 | |
| Pentostatin | Metabolic enzyme inhibitor | PTCL | Phase 2 | |
| Proteasome inhibitors | Bortezomib | Proteasome inhibitor | CTCL | Phase 2 |
| Signaling inhibitors | Enzastaurin | Selective inhibitor of protein kinase C | PTCL, CTCL | Phase 2 |
| R788 | Syk inhibitor | PTCL | Phase 2 |
There are several other experimental agents in various stage clinical trials for T-cell lymphoma.
PTCL indicates peripheral T-cell lymphoma; CTCL, cutaneous T-cell lymphoma; ATLL, adult T-cell lymphoma; HDAC, histone deacetylase; NF-AT, nuclear factor of activated T cell; AITL, angioimmunoblastic T-cell lymphoma; NK, natural killer; ALCL, anaplastic large cell lymphoma; T-ALL, T-cell acute lymphoblastic leukemia; and T-NHL, T-cell non-Hodgkin lymphoma.
Monoclonal antibodies and immunoconjugates
The addition of the anti-CD20 monoclonal antibody rituximab to chemotherapy regimens, such as CHOP, has significantly improved treatment outcomes in B-cell lymphoma. As such, several monoclonal antibodies and targeted immunoconjugates and fusion proteins are currently being tested in PTCL, including alemtuzumab, iratumumab, siplizumab, zanolimumab, denileukin diftitox, and brentuximab vedotin.
Alemtuzumab, an anti-CD52 monoclonal antibody, has been shown to have activity in heavily treated patients with PTCL with an ORR of 36% in one early study.72 Alemtuzumab has been used in combination with CHOP or EPOCH (etoposide, prednisone, vincristine, cyclophophamide, and doxorubicin) with some success in PTCL, with half of patients achieving a complete response (CR).73,74 Another study compared combinations of alemtuzumab with CHOP, EPOCH, and IMVP16-PL (ifosfamide plus mesna, methotrexate, etoposide, and prednisone),75 and a third study used alemtuzmab with CHOP and ESHAP (etoposide, methylprednisolone, high-dose cytarabine, and cisplatin) in newly diagnosed PTCL patients.76 Although overall response rates have been high, there has been an increased risk of opportunistic infections associated with the immunosuppressive effects of alemtuzumab. Patients on these trials have required routine surveillance for reactivation of cytomegalovirus. A randomized trial comparing CHOP-14 to alemtuzumab-CHOP 14 is currently underway as a European intergroup study.
Two anti-CD30 monoclonal antibodies, iratumumab and SGN-30, have shown efficacy in CD30+ ALCL.77,78 In vitro studies have shown that both drugs are synergistic or additive with conventional chemotherapy.79-81 An immunoconjugate of SGN-30 and monomethyl auristatin, brentuximab vetodin (SGN-35), has demonstrated an ORR of 41% with a median response duration of 7.3 months in patients with relapsed and refractory CD30+ lymphomas.82 A study is currently underway to evaluate the combination of CHOP with brentiximab vetodin in newly diagnosed patients with ALCL.
Siplizumab is an anti-CD2 monoclonal antibody. CD2 is an adhesion molecule highly expressed on activated T cells and NK cells and on the majority of cells from patients with T-cell lymphoma and leukemia. Siplizumab eliminated both CD4+ and CD8+ T cells and NK cells without affecting B cells. In a phase 1 trial in patients with CD2+ lymphoproliferative disease, siplizumab showed clinical activity, inducing CRs in 2 patients with LGL leukemia, 3 partial responses (PRs) in patients with ATL, and 1 PR in a patient with cutaneous T-cell lymphoma (CTCL).83 A subsequent dose escalation study produced a PR in a patient with NK-cell LGL and a CR in a PTCL patient.84 However, siplizumab also predisposes patients to the development of lymphoproliferative syndrome,85 although it may be possible to prevent that with prophylactic rituximab.
CD4 is expressed in half of all T cells and by most CTCL and nodal PTCL cells. Zanolimumab, an anti-CD4 monoclonal antibody, is being used in both disease types, although clinical development for CTCL is farther along. Zanolimumab was shown to be active and well tolerated in a study of 21 PTCL patients, with an ORR in 24% of patients.86 Clinical studies of zanolimumab in combination with CHOP are ongoing and include a phase 1/2 dose escalation trial in patients with noncutaneous CD4+ PTCL.
Bevacizumab, an antivascular endothelial growth factor (VEGF) monoclonal antibody, will most probably have the largest impact in AITL, as AITL is characterized by the overexpression of angiogenic factors, such as VEGF. At least one relapsed AITL patient has achieved a CR after treatment with bevacizumab87 and was being studied along with CHOP in a clinical trial for patients with PTCL or NK-cell neoplasms by the Eastern Cooperative Oncology Group. However, preliminary results of this trial reported a high incidence of cardiac events related to the therapy.88
Denileukin diftitox, a fusion protein that combines IL-2 receptor-binding domain with diphtheria toxin, has demonstrated activity in both cutaneous and aggressive T-cell lymphomas. In a single-center phase 2 study at M. D. Anderson Cancer Center, denileukin diftitox at a dose of 18 μg/kg per day for 5 days on a 21-day cycle demonstrated a response rate of 48% in heavily pretreated patients with relapsed PTCL.89 Responses were seen in 4 of 10 patients with PTCL-NOS, 2 of 3 with AITL, and 2 of 2 with ALCL. In this trial, the expression of CD25 by immunohistochemistry was not predictive of response to denileukin diftitox.
Based on these data, a combination of denileukin diftitox and CHOP was studied in untreated patients with PTCL. This study enrolled 49 patients. Denileukin diftitox was administered at 18 μg/kg per day on days 1 and 2 of each cycle, followed by CHOP chemotherapy on day 3, and granulocyte colony-stimulating factor support starting day 4 of each 21-day cycle.90 In 37 efficacy-evaluable patients (> 2 cycles), the ORR was 86% (CR 75%). Median progression-free survival (PFS) was 15 months, and 2-year estimated OS was 60%. A large randomized study comparing CHOP to denileukin diftitox with CHOP is being initiated.90
LMB-2 is an anti-Tac (anti-CD25) single-chain monoclonal antibody conjugated to Pseudomonas toxin. LMB-2 has shown clinical activity in phase 2 trials in CLL, CTCL, and hairy cell leukemia. ATL is the PTCL subtype that is most sensitive to LMB-2, but clinical responses have been limited to do rapid disease progression after > 95% tumor reduction and immunogenic reactions.91 A phase 2 clinical trial for this population is being planned in which LMB-2 will be given after chemotherapy with fludarabine and cyclophosphamide.
HDAC inhibitors
Histone deacetylase (HDAC) inhibitors are potent inducers of histone acetylation, which results in the expression of tumor suppressor genes that had been previously silenced by deacetylation. This gene expression leads to cell cycle arrest and apoptosis. There are a number of HDAC inhibitors being used or studied in T-cell lymphoma, including vorinostat, romidepsin (also known as depsipeptide), panobinostat, and belinostat. Vorinostat and romidepsin have shown single-agent activity in CTCL,92,93 and vorinostat was approved by the Food and Drug Administration (FDA) in 2006 for the treatment of advanced and refractory CTCL. Romidepsin was also recently FDA-approved in 2009 for advanced and refractory CTCL based on a demonstrated ORR in 2 clinical trials of 34%.94 The median response duration was 15 months (range 1-20+), and median time to progression was 8.3 months in early and 6.4 months in more advanced disease. A phase 2 study of romidepsin was completed in patients with relapsed and refractory PTCL.95 This phase 2, open-label, multiarm, multicenter study enrolled 43 PTCL patients from the National Cancer Institute and 9 extramural sites. Of 43 patients, 31 received ≥ 2 cycles of therapy. Mean number of prior therapies was 3.9 (range, 1-12). Objective response rate was 39% overall or 55% for patients who received at least 2 cycles of therapy. The overall median duration of response was 8.3 months (range, 1.6 months to 4.8+ years) for all patients. A multicenter, multinational phase 2B registration study of romidepsin at the same dose and schedule in relapsed and refractory PTCL has completed accrual and results have been recently reported. Of 130 patients with a median of 2 prior therapies, the ORR was 26% with 15% CR by radiographic documentation. The median response duration was 12 months, and toxicities included gastrointestinal and constitutional events and thrombocytopenia.
Belinostat, a hydroxamic acid-derived HDAC inhibitor, has been studied in both intravenous and oral formulations. Belinostat was administered intravenously at 1000 mg/m2 daily for 5 days every 3 weeks in 53 patients, including 19 with refractory PTCL and 29 with refractory CTCL.96 The objective response rate in PTCL was 32% with 2 CR and a median response duration of 8.9+ months, and 14% in CTCL, with a response duration of 9.1 months. A multicenter phase 2 registration trial of belinostat in relapsed PTCL patients is underway, and a cohort dose escalation study of oral belinostat is ongoing in patients with relapsed lymphoma.
Additive and synergistic activity has been demonstrated in vitro for combinations of HDAC inhibitor with a number of agents, including topoisomerase inhibitors, bortezomib, and cytotoxic chemotherapy drugs, and clinical trials are underway to explore the activity of these combinations in T-cell lymphomas.
Antifolates
Pralatrexate is a novel folate antagonist whose activity is associated with binding to the reduced folate carrier.97 In a phase 1/2 dose escalation trial of pralatrexate in refractory lymphoma patients, the ORR was 31%, with response durations ranging from 3 to 26 months.98 The response rate in that trial was 54% for patients with T-cell lymphomas. Based on these encouraging data, the PROPEL trial was initiated. In this trial, 111 patients with relapsed or refractory PTCL were treated with pralatrexate weekly for 6 weeks on a 7-week cycle. The median prior therapies was 3, and 63% of patients had no response to their last line of therapy. The ORR was 29% and the median response duration was 10.1 months. Five patients with relapsed/refractory PTCL who responded to single-agent pralatrexate went onto stem cell transplantation.99 Toxicities included mucositis in70% of patients and thrombocytopenia in 40%. Pralatrexate was approved in September 2009 by the United States FDA as a single agent to treat relapsed or refractory patients with PTCL. A number of recent studies have explored the potential synergy between pralatrexate and other active agents in T-cell lymphoma. A phase 1 study combining pralatrexate with gemcitabine is underway.
Immunomodulators and immunosuppressants
Cyclosporine is an immunosuppressive agent that inhibits the nuclear factor of activated T-cell transcription complex, which activates the genes encoding cytokines and cell surface molecules involved in cell-to-cell communication and death.100 Because AITL is characterized by immune dysregulation, cyclosporine was administered to 12 patients in a phase 2 trial.101 Two-thirds (3 CRs, 5 PRs) of the patients responded, but there were 4 deaths. A phase 2 trial of cyclosporine in AITL was conducted by Eastern Cooperative Oncology Group but closed early because of slow accrual.
Other immune-modulating and antiangiogenic agents, including bevacizumab,87 rituximab,102 lenalidomide, and thalidomide, are also being explored as single agents and in combination with chemotherapy. A phase 2 study of lenalidomide at a dose of 25 mg/m2 daily for 21 days of a 28-day cycle was conducted in 24 relapsed PTCL patients.103 The overall response rate was 30% with a PFS of 95 days. Toxicities included neutropenia and thrombocytopenia in 20% and 33% of patients, respectively.
Nucleoside analogs
Nucleoside analogs are chemotherapeutic agents that primarily inhibit DNA replication and repair. Gemcitabine is the most effective pyrimidine nucleoside analog in PTCL. It has been active both as a single agent104,105 and in combination with alemtuzumab106 and bortezomib.107,108 The purine nucleoside analogs include cladribine, fludarabine, clofarabine, and nelarabine. Both cladribine and fludarabine have shown efficacy in PTCL, and clofarabine and nelarabine are currently in several clinical trials in T-cell lymphoma.
The metabolic enzyme inhibitors, which include deoxycoformycin (pentostatin) and forodesine, do not incorporate into DNA, unlike the other nucleoside analogs. Pentostatin inhibits adenosine deaminase, increasing the deoxyadenosine triphosphate pool, and forodesine inhibits phosphorylase, increasing the deoxyguanosine triphosphate pool. Both agents have shown some efficacy in CTCL. A phase 1/2 study of oral forodesine in relapsed and refractory CTCL patients reported a 53% overall response rate, and a phase 2 trial has been completed.109
Proteasome inhibitors
Bortezomib, a proteasome inhibitor, has been well tolerated and active as a single agent in relapsed or refractory CTCL patients.110 In a phase 2 study of bortezomib in relapsed CTCL or PTCL patients, the ORR was 67% with 2 CR and no grade 4 toxicity.110 The GELA has conducted a phase 2 study of bortezomib with ACVBP chemotherapy in 57 untreated PTCL patients and has reported that 29 patients were withdrawn prematurely because of toxicity.111 The ORRs were similar to ACVBP alone. Bortezomib has been used in combination with gemcitabine plus doxorubicin,107,108 and recent evidence shows that bortezomib may synergize with pralatrexate in T-cell lymphoma (see antifolates section above).112
Signaling inhibitors
Enzastaurin is a selective inhibitor of protein kinase C, which acts in part through the AKT pathway. By targeting the PI3K/AKT pathways, enzastaurin inhibits cell proliferation, induces tumor cell apoptosis, and suppresses tumor-induced angiogenesis in CTCL cell lines.113 Enzastaurin is currently in 2 phase 2 trials: one for patients with several types of NHL, including PTCL and CTCL, and another for relapsed CTCL patients.
Treatment approaches for HTLV-1-associated ATL and NK-/T-cell lymphomas
Optimal treatment approaches for patients with NK-/T-cell lymphoma have not yet been established. A phase 1/2 study of concurrent chemoradiotherapy for untreated localized NK-/T-cell lymphoma was conducted in Japan.114 Extranodal NK-/T-cell lymphoma, nasal type, is generally refractory to CHOP associated with a high expression of the multidrug resistance gene, P-glycoprotein. Patients in this trial were concurrently treated with radiotherapy and chemotherapy consisting of carboplatin, etoposide, ifosfamide, and dexamethasone. With a median follow-up of 32 months, the 2-year OS was 78% (95% confidence interval [CI], 57%-89%). This compared favorably with the historical control of radiotherapy alone (45%). Of the 26 patients assessable for a response, 20 (77%) achieved a CR, with one PR. The ORR was 81%. The most common grade 3 nonhematologic toxicity was mucositis related to radiation (30%). The investigators concluded that concurrent chemoradiotherapy using multidrug resistance-nonrelated agents and etoposide is a safe and effective treatment for localized nasal NK-/T-cell lymphoma.
A novel approach for patients with advanced NK-/T-cell lymphomas incorporates L-asparaginase along with ifosfamide, etoposide, dexamethasone, and methotrexate (SMILE). Overexpression of P-glycoprotein in NK-/T-cell lymphomas has contributed to relative chemoresistance and poor outcomes after CHOP-based therapies with 5-year survivals of 20% or less in patients with advanced stage disease. Yong et al treated 18 patients who were refractory to CHOP with L-asparaginase, vincristine, dexamethasone, and involved field radiotherapy and reported a response rate and 5-year OS of 55%.115 A phase 1 study escalating methotrexate and etoposide was completed and reported a response rate of 67%. A prospective phase 2 trial has been reported with the SMILE regimen in patients with newly diagnosed stage IV or relapsed refractory NK-/T-cell lymphomas.116 Of 39 enrolled patients, 29 (74%) completed the planned treatment. The responses were complete remission (CR) in 15, partial remission in 14, and early death because of infection in 4. ORR and CR were 74% (95% CI, 58%-87%) and 38%, respectively. The most common grade 3 nonhematologic toxicity was infection (41%).
For patients with ATL, results with conventional chemotherapy regimens have been uniformly poor. A phase 3 Japanese study evaluated a combination regimen of VCAP (vincristine, cyclophosphamide, doxorubicin, and prednisone), AMP (doxorubicin, ranimustine, and prednisone), and VECP (vindesine, etoposide, carboplatin, and prednisone) against CHOP-14 in ATL.117 The study demonstrated superiority for VCAP-AMP-VECP for newly diagnosed aggressive ATL patients. A phase 2 study is in preparation to investigate the ability of allogeneic stem cell transplantation after induction with the VCAP-AMP-VECP regimen to prolong the median survival time, which is currently 13 months with the VCAP-AMP-VECP regimen.
The use of interferon and zidovudine has been shown to induce responses in up to 50% of patients with acute or lymphomatous ATL.118 In a recent meta-analysis, 116 patients with acute ATL, 18 patients with chronic ATL, 11 patients with smoldering ATL, and 100 patients with ATL lymphoma were evaluated.119 Five-year OS rates were 46% for 75 patients who received first-line antiviral therapy (P = .004), 20% for 77 patients who received first-line chemotherapy, and 12% for 55 patients who received first-line chemotherapy followed by antiviral therapy. The authors claimed that patients with acute, chronic, and smoldering ATL significantly benefited from first-line antiviral therapy, whereas patients with ATL lymphoma experienced a better outcome with chemotherapy. In acute ATL, 82% of patients were alive at 5 years with antiviral therapy, and 100% of patients with chronic and smoldering ATL were alive at 5 years. Multivariate analysis showed that first-line antiviral therapy significantly improved OS (hazard ratio = 0.47; 95% CI, 0.27-0.83; P = .021). However, considering the potential selection bias in this retrospective study, future prospective studies are needed.
Finally, a humanized anti-CCR4 antibody, KW-0761, has shown promise as a single agent in Japan for the treatment of ATL. KW-0761 was used for relapsed patients with CCR4-positive ATL and PTCL in a phase 1 study.120 The ORR was 31% (5 of 16; 95% CI, 11%-59%). There were no dose-limiting toxicities, and no anti–KW-0761 antibodies were detected.120 A phase 2 trial for relapsed ATL patients was recently completed. Of 27 enrolled patients (14 acute, 6 lymphomatous, 7 chronic ATL), the ORR was 54% with 7 CR. Toxicities included cytopenias (lymphopenia 96%, neutropenia 33%), skin rash (52%), and mild transaminitis.
Transplantation
Several retrospective studies suggest that there are populations of patients with PTCL that will benefit from transplantation. The National Cancer Consortium Network guideline includes transplantation as an option for consolidation after first remission in patients with histologies other than ALK+ ALCL and in patients with intermediate or high IPI scores. Disease status at transplantation is a major predictor of success, particularly for autologous transplantation.121 Results are better for patients who are in chemosensitive remission, whereas only 25% to 30% of refractory patients benefit.122,123 In the studies where 5-year outcomes are reported, OS and FFS average 34% and 18%, respectively.124 The single-center experience at Stanford reported only a modest benefit after autologous transplantation (5-year OS of 36%) for patients with relapsed disease and a 5-year OS of 76% in patients transplanted in first remission.125
A prospective study from Germany using chemotherapy and up-front autologous transplantation for PTCL has been reported. The treatment regimen consisted of 4 to 6 cycles of CHOP, followed by mobilizing therapy with either dexaBEAM (dexamethasone, carmustine, melphalan, etoposide, and cytarabine) or ESHAP (etoposide, methylprednisolone, cytarabine, and cisplatin). Patients in complete or partial remission then underwent myeloablative chemoradiotherapy and ASCT. Two-thirds (66%) of the patients had a disease response to chemotherapy and thus went on to receive ASCT. At a median follow-up time of 33 months, the estimated 3-year OS and PFS for patients in CR were 48% and 36%, respectively. Patients who did not experience a response to chemotherapy and therefore did not undergo ASCT had a very poor outcome, with a median survival of less than 2 years.126 The role of autologous transplantation in second or subsequent remission is less well defined.
There are currently no randomized studies comparing outcomes between autologous and allogeneic transplantation in the United States. The Nordic and German lymphoma groups are launching a large, prospective randomized trial to compare the different strategies as consolidation after first-line therapy. In addition, the Center for International Blood & Marrow Transplant Research is currently conducting a database review comparing allogeneic and autologous transplants in PTCL patients. In a retrospective single-institution study that compared autologous and allogeneic transplantation, outcomes for autologous transplantation were best when conducted in first remission, and allogeneic transplantation was better for patients with resistant or relapsed disease.127 Further prospective studies are needed to define which subsets of PTCL patients will optimally benefit from allogeneic or ASCT.
Evidence-based treatment approaches for PTCL
Because of the inferior outcomes with CHOP-based regimens, novel strategies are needed for patients with aggressive T-cell lymphomas. The National Cancer Consortium Network has established evidence-based treatment approaches for T-cell lymphoma and stratifies patients based on stage. For early-stage patients with localized disease, chemotherapy should be followed by involved field radiotherapy. It is recommended that all patients except for those with low IPI be consolidated with ASCT. ALK+ ALCL is identified as the one subtype that has an excellent outcome and should not be transplanted in first remission. Recent data suggest that ALK+ patients with high IPI could be an exception to this rule. In prospective trials where up to 40% of patients do not undergo a complete remission and therefore cannot be consolidated with transplantation, new approaches are necessary.
Selection of first-line therapy based on histopathologic features has not yet been widely used but should be considered. For nodal T-cell lymphomas (PTCL-NOS, AITL, and ALCL), the standard regimen used is a CHOP-based therapy. For extranodal subtypes, regimens may be individualized. For panniculitis-like T-cell lymphoma, distinction should be made between the αβ type and the γδ type, which is now included in the category of cutaneous γδ T-cell lymphoma. The αβ patients may be treated with single-agent therapies or combination chemotherapy and generally have an excellent outcome. The cutaneous γδ T-cell lymphomas overall do poorly and should be treated with aggressive chemotherapy followed by transplantation. Likewise, hepatosplenic and intestinal T-cell lymphomas have a poor outcome. In one study, 26 enteropathy-associated T-cell lymphoma patients were treated with CHOP and then methotrexate alternating with ifosfamide, etoposide, and epirubicin.128 Patients who achieved CR went on to transplantation (n = 33). For the transplanted enteropathy-associated patients, the PFS and OS were 52% and 60%, respectively. NK-/T-cell lymphoma patients have also had inferior outcomes with CHOP-based regimens, and consideration of alternative regimens, such as SMILE and asparaginase combinations, should be strongly considered for these patients.
The role of autologous versus allogeneic stem cell transplantation in patients with poor prognosis subtypes, such as NK-/T-cell and γδ T-cell lymphomas, has not been ascertained. In retrospective series, results with these subtypes are inferior to those of the more common PTCL-NOS and AITL subtypes after autologous transplantation. Therefore, consolidation with allogeneic stem cell transplantation should be considered in patients who have appropriate donors.
In conclusion, from this summary of the current state of classification, prognosis, and therapeutic approaches in PTCL, it is apparent that much progress has been made. Although the problem with PTCL treatment in the past was a lack of available therapies, the development of novel therapies necessitates the development of paradigms to combine these agents to improve response rates and durability of responses. Based on the success of the chronic lymphocytic leukemia and the mantle cell lymphoma consortiums, investigators from North America have formed a North American PTCL Collaborative Group, which will collaborate with the International PTCL Project to develop new treatment strategies to improve outcome in PTCL. An international registry is currently open and registering patients with newly diagnosed PTCL. In addition, an international tissue bank has been initiated to capture sufficient quantities of clinically annotated and well-characterized specimens.
Authorship
Contribution: F.M.F. prepared the manuscript for publication; and all authors wrote and reviewed the manuscript.
Conflict-of-interest disclosure: F.M.F. was a consultant for Eisai and Celgene and received research funding from Merck and Bayer. J.M.V. received research funding from Allos. S.T.R. was on the Scientific Advisory Board of Celgene and Genzyme, the Speakers Bureau of Merck, and the Advisory Panel of Eisai and Allos. K.T. received research funding from Kyowa Kirin, Mundipharma, and Merck. The remaining authors declare no competing financial interests.
Correspondence: Francine M. Foss, Yale Cancer Center, 333 Cedar St, FMP 112, PO Box 208032, New Haven, CT 06520-8032; e-mail: francine.foss@yale.edu.