Abstract
One of the main functions of A Disintegrin and Metalloproteinase 10 (ADAM10) is to regulate the bioavailability of adhesion molecules and ligands to various cellular-signaling receptors. Constitutive activation of ADAM10 has been implicated in the pathogenesis of several types of solid tumors. In this study, we found that mantle cell lymphoma (MCL) cell lines and all 12 patient samples examined expressed the active/mature form of ADAM10. In contrast, PBMCs from healthy donors (n = 5) were negative. Using immunohistochemistry, ADAM10 was readily detectable in 20 of 23 (87%) MCL tumors, but absent in 5 reactive tonsils. Knockdown of ADAM10 using short interfering RNA (siRNA) in MCL cells significantly induced growth inhibition and cell-cycle arrest, and these changes were correlated with down-regulation of cyclin D1, up-regulation of p21waf1, and significant reductions in the TNFα production/transcriptional activity of NFκBp65. The addition of recombinant ADAM10 to MCL cells led to the opposite biologic effects. Lastly, down-regulation of ADAM10 using siRNA enhanced the growth-suppressing effects mediated by the proteasome inhibitors MG132 and bortezomib. We conclude that constitutive activation of ADAM10 contributes to the growth of MCL and therefore inhibition of ADAM10 may be a useful strategy to enhance the response of MCL to other therapeutic agents.
Introduction
A Disintegrin and Metalloproteinase 10 (ADAM10), a member of the ADAM family of metalloproteinases, was discovered in the protein extract of brain myelin membranes and subsequently found to be a homolog of the Drosophila kuzbanian gene.1-3 ADAM10 is secreted as a precursor protein and consists of multiple functional domains, including a prodomain, catalytic domain, cysteine-rich domain, transmembranous domain, and cytoplasmic domain.4 To become the active/mature form, the precursor ADAM10 protein needs to be cleaved by proprotein convertase 7 and furin, both of which remove the ADAM10 prodomain.5 ADAM10 is biologically important, because ADAM10 knockout mice die on day 9 of embryogenesis due to multiple abnormalities in the nervous and cardiovascular systems.6 The key biologic function of ADAM10 appears to be attributed to its enzymatic activity as a metalloproteinase. Specifically, ADAM10 is involved in the intramembrane proteolysis process, whereby it mediates ectodomain shedding of various membrane-bound receptors, adhesion molecules, growth factors, and cytokines.7-9 For example, ADAM10 is involved in the regulation of the shedding of Notch, HER-2, CD44, IL-6 receptor, amyloid precursor protein, and cadherins.10 Directly relevant to our study, ADAM10 was recently found to be one of the enzymes responsible for cleaving TNFα and releasing its active form.11-13 Furthermore, it has been reported that ADAM10 is important for the development of the marginal zone B cells.14 By regulating the bioavailability of ligands to various cellular-signaling receptors, ADAM10 modulates the activation status of various cellular-signaling pathways that have an impact on cellular responses such as proliferation and migration.9 ADAM10 has been shown to be constitutively active in several solid tumors, and this biochemical defect is implicated in the pathogenesis of these tumors. For example, xenografting of colorectal cancer cells with enforced expression of ADAM10 in nude mice induced the formation of liver metastasis, unlike the negative control cells, and this effect was attributed to ADAM10-mediated cleavage and release of L1-CAM, a cell-adhesion molecule.15 In another study, ADAM10 expression detectable by immunohistochemistry in colorectal cancer patient samples was found to be correlated with a higher clinical stage.16 Using immunohistochemistry, it was also found that ADAM10 is overexpressed in squamous cell carcinomas of the oral cavity compared with the benign epithelial cells; knockdown of ADAM10 expression using short interfering RNA (siRNA) in the cell lines derived from these tumors was shown to induce a significant decrease in cell growth.17 Similar findings were made in pancreatic cancer, in which inhibition of ADAM10 expression in pancreatic carcinoma cell lines resulted in a significant decrease in invasiveness and migration.18 Lastly, ADAM10-mediated cleavage of N-cadherin was found to regulate the migratory properties of glioblastoma cells.19 Whereas the pathogenetic role of ADAM10 has been well documented in solid tumors, its role in hematologic malignancies is largely unknown. To our knowledge, there is only one published study that performed a survey of the expression of various ADAM family members in benign and malignant hematopoietic cells using RT-PCR, and the authors reported the expression of the ADAM10 mRNA in myeloma, erythroleukemia, and a subset of lymphoma cell lines.20 However, because only RT-PCR was used in this particular study, whether ADAM10 was in its active form could not be assessed and the biologic significance of ADAM10 in these malignancies also was not examined.20
Mantle cell lymphoma (MCL) is a specific subtype of aggressive B-cell lymphoma recognized by the World Health Organization Classification Scheme.21 The genetic hallmark of this disease is the recurrent chromosomal abnormality t(11;14)(q13;q32), which brings the cyclin D1 (CCND1) gene under the influence of the enhancer of the immunoglobulin heavy chain (IgH) gene, leading to CCND1 overexpression.22 Although it has been shown that CCND1 overexpression is not sufficient for the induction of lymphoma in animal models, this abnormality is considered to be the primary oncogenic event in MCL.23,24 Large-scale cDNA microarray studies using frozen MCL tumors have revealed a relatively large number of biochemical abnormalities in MCL, and these defects are frequently implicated in the regulation of apoptosis, survival, and DNA damage.25-28 An increasing number of cellular-signaling pathways have also been found to be abnormal. For example, NFκB has been reported to be constitutively active in MCL, and this biochemical defect is biologically important in the pathogenesis of MCL.29 The activation of NFκB in MCL can be partly attributed to TNFα,30,31 the bioavailability of which has been shown to be regulated by ADAM10.11-13
In the present study, we show for the first time that ADAM10 is constitutively activated in MCL cell lines and tumors. In view of the importance of TNFα in the biology of MCL and of a previous report that ADAM10 can increase the bioavailability of TNFα,11-13 we hypothesize that ADAM10 may be important in the pathogenesis of MCL.
Methods
Cell lines and tissue culture
The characteristics of the 3 MCL cell lines Jeko-1, Mino, and SP53 have been described previously.32 All 3 cell lines are negative for the Epstein-Barr virus nuclear antigen. Cells were grown at 37°C and 5% CO2 and maintained in RPMI medium (Sigma-Aldrich). The culture medium was enriched with 10% fetal bovine serum (Life Technologies). A Ficoll-Paque gradient (GE Healthcare) was used to isolate PBMCs from healthy donors (n = 5) and leukemic MCL patients (n = 3). Optimal cutting temperature medium–embedded frozen tumors from classic MCL (n = 9), chronic lymphocytic leukemia (n = 5), follicular lymphoma (n = 4), diffuse large B-cell lymphoma (n = 4), and marginal zone lymphoma (n = 4) were included for comparison. The use of these patient samples was reviewed and approved by our institutional ethics committee.
Subcellular protein fractionation, Western blots, and antibodies
For subcellular protein fractionation, we used a kit purchased from Active Motif and followed the manufacturer's instructions. Preparation of cell lysates for Western blots was done as described previously.33 Antibodies used in this study included those reactive with ADAM10 and phospho-NFκBp65 (Chemicon); with NFκBp65, CCND1, tubulin, histone deacetylase, and β-actin (Santa Cruz Biotechnology); and with p21Waf1, p27Kip1, p15, p16, cleaved poly(adenosine diphosphate)-ribose polymerase, and cleaved caspases 3, 7, and 9 (Cell Signaling Technology).
siRNA
Two siRNA species for ADAM10 from 2 different commercial sources were used in this study: siRNA #1 was a pool of 4 siRNA species from Dharmacon and siRNA #2 was from Invitrogen. Scrambled siRNA was purchased from Dharmacon. Transfection of siRNA was carried out using an electrosquare electroporator (BTX ECM 800) at 225 V, 8.5 ms, and 3 pulses. The concentration of siRNA used was 200 pmol/1 × 106 cells, and cells were harvested at 48 hours after transfection. The ADAM10 protein levels were assessed using Western blot to evaluate the efficiency of inhibition.
Cell viability
A total of 100 000 cells suspended in 1 mL of culture medium were plated in triplicate. To assess cell viability, the trypan blue exclusion assay (Sigma-Aldrich) was performed every 24 hours for up to 3 days after transfection of ADAM10 siRNA or scrambled siRNA. The MTS assay was also performed during this time frame following the manufacturer's instructions. The measurements were obtained at a wavelength of 450 nm using a microplate reader (Bio-Rad). The absorbance values were normalized to the wells with medium only using Microplate Manager 5.2.1 software (Bio-Rad). All experiments were performed in triplicate.
Recombinant ADAM10, TNFα assay, bortezomib, and MG132
Human recombinant ADAM10 was purchased from BD Biosciences and corresponded to the segment spanning from Thr214 to Glu672, which represents the active/mature form of ADAM10, and added at a concentration of 100 ng/mL to 1 × 106 cells. After 24 hours, cell lysates were prepared and cells were counted. TNFα secretion was monitored using a commercially available ELISA kit (R&D Systems). Aliquots of the culture medium from MCL cell lines collected 48 hours after transfection with either scrambled siRNA or ADAM10 siRNA were centrifuged at 15 000g, and the supernatant was assayed for the TNFα levels as per the manufacturer's instructions. Bortezomib (LC Laboratories), a proteasome inhibitor, was added at a concentration of 5nM to MCL cells 24 hours after siRNA transfection, and cells were counted using the trypan blue exclusion assay 48 hours after the initiation of the experiment. Another proteasome inhibitor, MG132 (Calbiochem) was added at a concentration of 1μM to MCL cells 24 hours after siRNA transfection, and cells were counted using the trypan blue exclusion assay 48 hours after the initiation of the experiment.
NFκB transcriptional activity
To assess the transcriptional activity of NFκB in MCL cell lines, we used the NFκB-responsive firefly luciferase reporter plasmid and the Renilla reporter plasmid (Promega). After 48 hours of transfection of the reporter plasmid (with the Renilla luciferase plasmid as an internal control), together with either scrambled siRNA or ADAM10 siRNA, MCL cells were harvested and cell extracts were prepared using a lysis buffer purchased from Promega. The firefly luciferase activity and Renilla luciferase activity were assessed using a dual-luciferase reagent (Promega).
Cell-cycle analysis by flow cytometry
Cells transfected with either scrambled siRNA or ADAM10 siRNA were fixed with ice-cold 70% ethanol 24 hours after gene transfection. These cells were then subjected to RNase treatment and propidium iodide staining. DNA content was determined using a FACSCalibur flow cytometer (BD Biosciences). Data acquisition was gated to exclude cell doublets, and the cell-cycle phase distribution was determined using the CellQuest program (20 000 events were counted).
Assessment of CCND1 expression using qRT-PCR
The expression of CCND1 in MCL cells treated with ADAM10 siRNA was assessed using quantitative RT-PCR (qRT-PCR). The assay was performed using the Applied Biosystems 7900 HT, and the SYBR GreenER qPCR SuperMix from Invitrogen. The primer sequence for ADAM10 was as follows: forward 5′-AGCAACATCTGGGGACAAAC-3′; reverse 5′-CTTCCCTCTGGTTGATTTGC-3′. The primer sequence for CCND1 was as follows: forward 5′-CAAATGGAGCTGCTCCTGGTG-3′; reverse 5′-TGGCACCAGCCTCGGCATTTC-3′.34 Triplicate experiments were performed, and the statistical significance of the differences was assessed using the Student t test.
Immunohistochemistry and archival MCL tumors
Formalin-fixed, paraffin-embedded tumors from a cohort of 23 patients with classic MCL were retrieved from the files at the Cross Cancer Institute (Edmonton, AB). All MCL primary tumors were diagnosed at the Cross Cancer Institute and the diagnostic criteria were based on those described in the World Health Organization Classification Scheme.21 All cases were confirmed to express CCND1 by immunohistochemistry. The use of these tissues was approved by our institutional ethics committee. Immunohistochemistry was performed using standard techniques. Briefly, formalin-fixed, paraffin-embedded tissue sections of 4-μm thickness were deparaffinized and hydrated. Heat-induced epitope retrieval was performed in a pressure cooker using citrate buffer (pH = 6) in a microwave. After antigen retrieval, tissue sections were incubated with 3% hydrogen peroxide for 10 minutes to block endogenous peroxidase activity. Tissue sections were then incubated with anti-ADAM10 antibody (1:200; the same antibody used in Western blots) overnight in a humidified chamber at 4°C. Immunostaining was visualized with a labeled streptavidin-biotin method using 3,3′-diaminobenzidine as a chromogen (Dako), and counterstained with hematoxylin. A colon carcinoma case served as the positive control, and the lymphoid cells of the mantle zone in benign tonsils served as the negative control.
Statistical analysis
Data are expressed as the means ± SD. Unless stated otherwise, statistical significance was determined using a 2-tailed Student t test and was achieved when P < .05. In experiments in which we needed to determine the statistical significance between more than 2 groups, ANOVA was applied.
Results
The active/mature form of ADAM10 is expressed in MCL cells and other B-cell–lineage malignancies
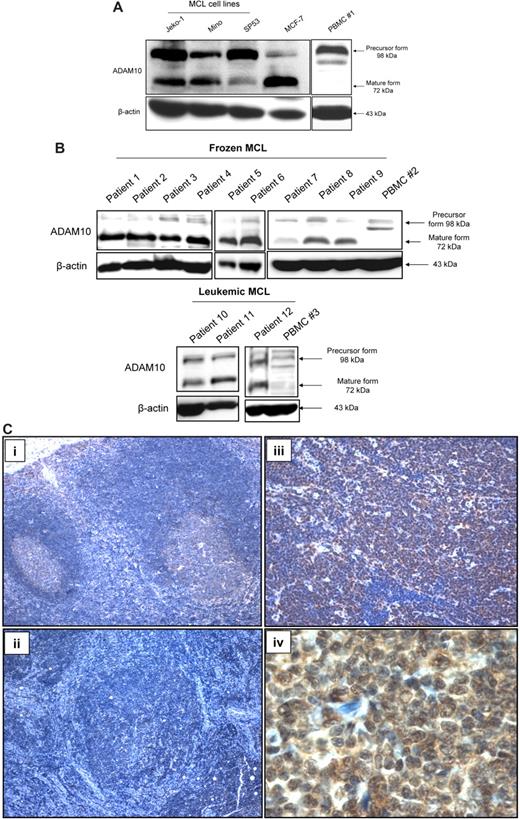
The expression of ADAM10 in 3 MCL cell lines was assessed using Western blots, and the results are illustrated in Figure 1A. Whereas the precursor form of ADAM10 at 98 kDa was highly expressed in all 3 cell lines examined, the expression levels of the active/mature form of ADAM10 at 72 kDa were variable among these 3 cell lines. Specifically, although the active/mature form of ADAM10 was readily detectable in Jeko-1 and Mino cells, it was barely detectable in SP53 cells. MCF-7, a breast cancer cell line previously reported to have a high level of the active/mature form of ADAM10,35 served as the positive control. In contrast to MCL cells, PBMCs from 5 healthy donors expressed no detectable active/mature form of ADAM10 at 72 kDa, although the precursor form was readily detectable in all cases. The results from 3 of these 5 healthy individuals are illustrated in Figure 1A and B.
ADAM10 expression in MCL cell lines and patient samples. (A) Western blots showing the expression of the precursor and active/mature forms of ADAM10 in 3 MCL cell lines. Whereas the precursor was highly expressed, the active/mature form was readily detectable only in Jeko-1 and Mino cells. A faint band at 72 kDa, representing the active/mature form of ADAM10, was also detectable in SP53. MCF-7 cells were used as the positive control. PBMCs from a healthy individual (PBMC #1) showed abundant precursor ADAM10 protein but no detectable active/mature ADAM10. (B) Western blots showing the presence of the precursor and active/mature form of ADAM10 in all MCL patient samples (both frozen #1-9 and leukemic #10-12); PBMCs from 2 healthy individuals (#2 and #3) showed no detectable active/mature ADAM10. (C) Immunohistochemistry showing no detectable signal of ADAM10 in the mantle zone of a reactive tonsil. At 100× magnification, the germinal centers showed faint staining (i), one case of MCL tumor showed no detectable ADAM10 (ii), and another MCL tumor showed a relatively high level of ADAM10 expression (iii). On high magnification (1000×), the ADAM10 immunostaining pattern was detectable in both of the nucleus and cytoplasm (iv).
ADAM10 expression in MCL cell lines and patient samples. (A) Western blots showing the expression of the precursor and active/mature forms of ADAM10 in 3 MCL cell lines. Whereas the precursor was highly expressed, the active/mature form was readily detectable only in Jeko-1 and Mino cells. A faint band at 72 kDa, representing the active/mature form of ADAM10, was also detectable in SP53. MCF-7 cells were used as the positive control. PBMCs from a healthy individual (PBMC #1) showed abundant precursor ADAM10 protein but no detectable active/mature ADAM10. (B) Western blots showing the presence of the precursor and active/mature form of ADAM10 in all MCL patient samples (both frozen #1-9 and leukemic #10-12); PBMCs from 2 healthy individuals (#2 and #3) showed no detectable active/mature ADAM10. (C) Immunohistochemistry showing no detectable signal of ADAM10 in the mantle zone of a reactive tonsil. At 100× magnification, the germinal centers showed faint staining (i), one case of MCL tumor showed no detectable ADAM10 (ii), and another MCL tumor showed a relatively high level of ADAM10 expression (iii). On high magnification (1000×), the ADAM10 immunostaining pattern was detectable in both of the nucleus and cytoplasm (iv).
Western blot analysis was performed using 9 frozen MCL tumor samples (patients 1-9) and 3 fresh leukemic MCL blood samples (patients 10-12). The active/mature form of ADAM10 was readily detectable in all patient samples, although the level was relatively low in patient 7 (Figure 1B). Again, PBMCs from healthy donors 2 and 3 had no convincing band at 72 kDa. In contrast to the MCL cell lines (Figure 1A), most MCL patient samples expressed the active/mature form of ADAM10 at a higher level than its precursor form. We also analyzed the expression of both the precursor and active/mature forms of ADAM10 in frozen tissues derived from other B-cell–lineage malignancies (5 chronic lymphocytic leukemia cases, 4 diffuse large B-cell lymphoma cases, 4 follicular lymphoma cases, and 4 marginal zone lymphoma cases). As shown in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article), the expression of ADAM10 was readily detectable in all of these B-cell neoplasms, with a pattern similar to that seen in MCL patient samples.
We then performed immunohistochemistry applied to 23 MCL formalin-fixed/paraffin-embedded tumor samples and 5 cases of benign reactive tonsils, and found that the mantle zones in benign reactive tonsils were negative for ADAM10, whereas the germinal centers showed only faint cytoplasmic staining (Figure 1C). The ADAM10 immunostaining in MCL was generally homogenous within the same tumor, and we considered a MCL tumor to be positive when the majority of the tumor cells showed a staining intensity higher than that of germinal center cells. With this criterion, ADAM10 was assessed as positive in 20 of 23 (87%) cases. On higher magnification, the immunostaining was predominantly cytoplasmic in all cases, whereas a small subset of cases also showed some degree of nuclear staining (Figure 1C). Five of these MCL cases used for immunohistochemical studies were also included in the experiment illustrated in Figure 1B (Western blot studies). All 5 cases were positive as shown by immunohistochemistry and carried the active form of ADAM10 as shown by Western blots.
ADAM10 promotes cell growth in MCL cells
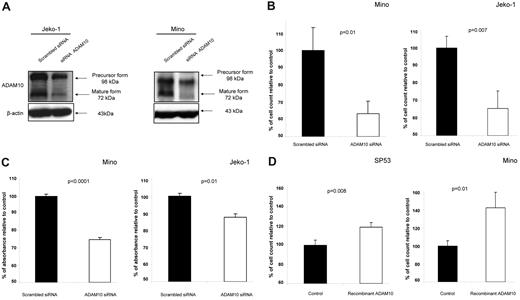
To assess the functional importance of ADAM10 in MCL cells, we evaluated whether siRNA knockdown of ADAM10 had any impact on the growth of MCL cells. As shown in Figure 2A, transfection of siRNA into Jeko-1 and Mino cells resulted in a reduction of both the precursor and the active/mature form of ADAM10. With the knockdown of ADAM10, the cell growth (assessed by the trypan blue exclusion assay) was significantly decreased compared with cells transfected with scrambled siRNA (P = .01 and P = .007 for Mino and Jeko-1, respectively) 3 days after transfection (Figure 2B). The decrease in the viable cell count was not associated with any substantial increase in trypan blue–positive dead cells. Similar experiments were performed using the MTS assay, and we found comparable results (P < .0001 and P = .01 for Mino and Jeko-1, respectively; Figure 2C). To further strengthen the conclusion that ADAM10 promotes cell growth in MCL cells, we investigated how MCL cells may respond to recombinant ADAM10. SP53 and Mino cells were used for this experiment because both cell lines had a lower level of active ADAM10 than Jeko-1 cells, and therefore were expected to show a more dramatic response to recombinant ADAM10. As shown in Figure 2D, the addition of recombinant ADAM10 induced a significant increase in cell growth (P = .008 and P = .01, respectively).
ADAM10 promotes cell growth in MCL. (A) Western blots showing down-regulation of the precursor and active/mature form of ADAM10 in Jeko-1 and Mino cells with the use of siRNA. (B) ADAM10 knockdown induced significant inhibition of cell growth in Mino and Jeko-1 cells 3 days after transfection, as assessed by the trypan blue exclusion assay (P = .01 and P = .007, respectively). Triplicate experiments were performed. (C) ADAM10 knockdown induced significant inhibition of cell growth in Mino and Jeko-1 cells 3 days after transfection, as assessed by MTS assay (P < .0001 and P = .01, respectively). Triplicate experiments were performed. (D) Addition of human recombinant ADAM10 (100 ng/mL) to SP53 and Mino cells induced a significant increase in their growth at 24 hours (P = .008 and P = .01, respectively).
ADAM10 promotes cell growth in MCL. (A) Western blots showing down-regulation of the precursor and active/mature form of ADAM10 in Jeko-1 and Mino cells with the use of siRNA. (B) ADAM10 knockdown induced significant inhibition of cell growth in Mino and Jeko-1 cells 3 days after transfection, as assessed by the trypan blue exclusion assay (P = .01 and P = .007, respectively). Triplicate experiments were performed. (C) ADAM10 knockdown induced significant inhibition of cell growth in Mino and Jeko-1 cells 3 days after transfection, as assessed by MTS assay (P < .0001 and P = .01, respectively). Triplicate experiments were performed. (D) Addition of human recombinant ADAM10 (100 ng/mL) to SP53 and Mino cells induced a significant increase in their growth at 24 hours (P = .008 and P = .01, respectively).
Down-regulation of ADAM10 induces cell-cycle arrest but not apoptosis
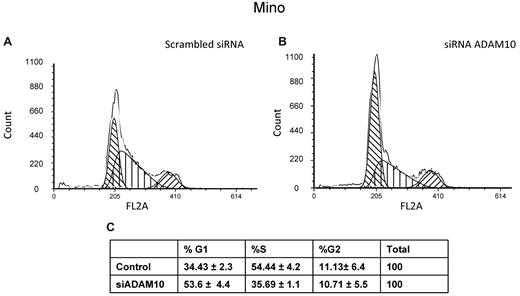
We performed cell-cycle analysis to characterize the mechanism by which ADAM10 promotes cell growth in MCL. Transfection of ADAM10 siRNA into Mino and Jeko-1 cells induced a significant G0/1 arrest and reduced the proportion of cells in the S phase compared with cells transfected with scrambled siRNA (P = .02 and P = .05 for Mino and Jeko-1 cells, respectively). No appreciable increase in the proportion of cells in the sub-G0/1 phase was found. All experiments were performed in triplicate and representative results are shown in Figure 3. To further exclude the occurrence of apoptosis, we performed Western blots and found no detectable cleavage of caspases 3, 7, 9 and poly(adenosine diphosphate)-ribose polymerase (supplemental Figure 2). Cells treated with MG132 served as positive controls.
ADAM10 induces cell-cycle arrest. Cell-cycle analysis by flow cytometry using propidium iodide showed significant G0/1 cell-cycle arrest in Mino cells after ADAM10 down-regulation using siRNA. No appreciable increase in the fraction of cells in the sub-G0/1 phase was noted.
ADAM10 induces cell-cycle arrest. Cell-cycle analysis by flow cytometry using propidium iodide showed significant G0/1 cell-cycle arrest in Mino cells after ADAM10 down-regulation using siRNA. No appreciable increase in the fraction of cells in the sub-G0/1 phase was noted.
ADAM10 regulates the CCND1 expression level in MCL cells
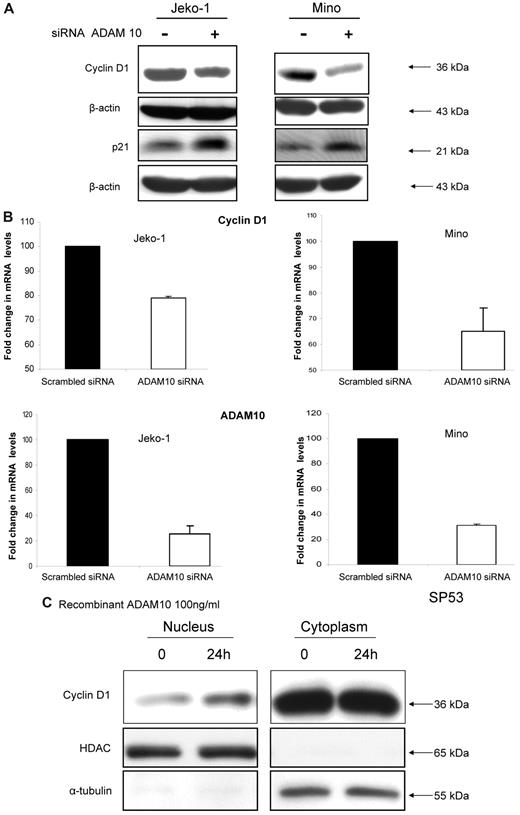
Because CCND1 overexpression is believed to play an important pathogenetic role in MCL,22 we investigated whether ADAM10 mediates any effects on the expression of CCND1 in MCL cell lines. As shown in Figure 4A, down-regulation of ADAM10 using siRNA appreciably reduced the CCND1 protein expression level in Jeko-1 and Mino cells. This decrease was correlated with our observations that the CCND1 mRNA levels were significantly reduced 24 hours after treatment of ADAM10 siRNA (Figure 4B). Specifically, the CCND1 mRNA levels detected by qRT-PCR were reduced by 21% and 35% in Jeko-1 and Mino cells, respectively. The opposite effects were observed when recombinant ADAM10 (100 ng/mL) was added to SP53 cells (not shown). Using subcellular fractionation, we found that the increase in the CCND1 protein expression level was largely attributed to an increase in CCND1 in the nuclear fraction (Figure 4C). We also investigated whether down-regulation of ADAM10 using siRNA modulates several other cell-cycle regulators, including p21Waf1, p27Kip1, p15, and p16, and found that there was an up-regulation of p21Waf1, an inhibitor of cyclin-dependent kinase 4 (Figure 4A). The other 3 proteins showed no appreciable change (data not shown).
ADAM10 regulates CCND1 expression in MCL. (A) Western blots showing down-regulation of CCND1 and up-regulation of p21Waf1 in Jeko-1 and Mino cells after ADAM10 down-regulation using siRNA. (B) qRT-PCR showing down-regulation of the CCND1 mRNA in Jeko-1 and Mino cells 24 hours after down-regulation of ADAM10 using siRNA. (C) Western blots showing up-regulation of the nuclear level of CCND1 protein in SP53 cells 24 hours after the addition of human recombinant ADAM10 (100 ng/mL); histone deacetylase was used as the loading control for the nuclear extract.
ADAM10 regulates CCND1 expression in MCL. (A) Western blots showing down-regulation of CCND1 and up-regulation of p21Waf1 in Jeko-1 and Mino cells after ADAM10 down-regulation using siRNA. (B) qRT-PCR showing down-regulation of the CCND1 mRNA in Jeko-1 and Mino cells 24 hours after down-regulation of ADAM10 using siRNA. (C) Western blots showing up-regulation of the nuclear level of CCND1 protein in SP53 cells 24 hours after the addition of human recombinant ADAM10 (100 ng/mL); histone deacetylase was used as the loading control for the nuclear extract.
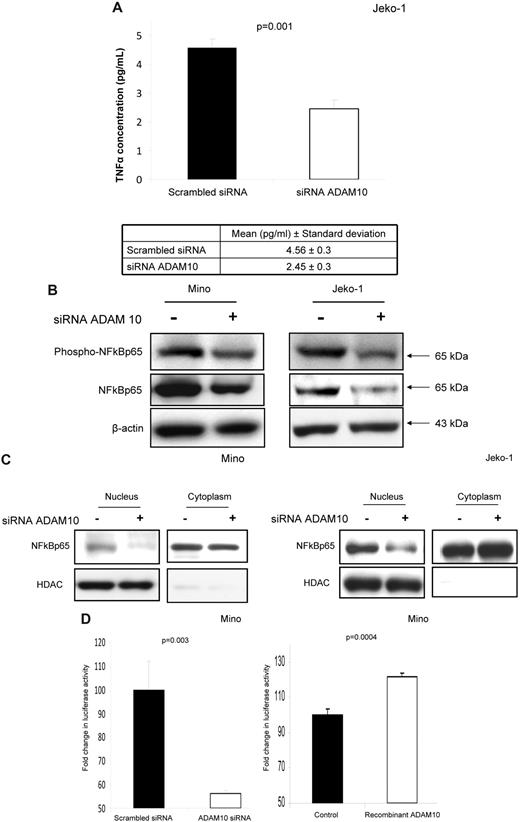
ADAM10 activates the TNFα/NFκB axis
In view of previous studies showing that ADAM10 regulates the production of TNFα in murine cell lines and immortalized human cell lines,11,13 as well as the importance of the TNFα in the biology of MCL,29-31 we hypothesized that ADAM10 may promote the growth of MCL via up-regulation of TNFα. In keeping with this concept, we found that down-regulation of ADAM10 using siRNA in Jeko-1 cells resulted in a significant decrease in the levels of TNFα present in the culture medium (P = .001; Figure 5A). Because TNFα is an activator of NFκB,36 a signaling protein also strongly implicated in the pathogenesis of MCL,29 we then assessed the relationship between ADAM10 and this signaling protein. We performed subcellular fractionation experiments in which we found a correlation among the expression levels of phosphorylated NFκB (active form), total NFκBp65, and ADAM10 in 3 MCL cell lines (supplemental Figure 3). Specifically, we found that Jeko-1 cells, which have a relatively high level of the active/mature form of ADAM10, carried a higher level of phosphorylated NFκBp65 than SP53 cells, which have a much lower level of the active/mature form of ADAM10. As shown in Figure 5B, there was a significant down-regulation in both the phosphorylated and total NFκBp65 level in Mino and Jeko-1 cells after ADAM10 siRNA treatment. Using subcellular fractionation, we found that the protein level of NFκBp65 in the nuclear fractions was decreased, more so than that in the cytoplasmic fractions (Figure 5C). Lastly, we assessed the transcriptional activity of NFκB using a commercially available reporter construct. As shown in Figure 5D, there was significant down-regulation of NFκB transcriptional activity after ADAM10 down-regulation in Mino cells. In contrast, the addition of human recombinant ADAM10 (100 ng/mL) resulted in a significant increase in the NFκB transcriptional activity in these cells (Figure 5E).
ADAM10 activates the TNFα/NFκB axis. (A) ELISA showing a significant down-regulation of TNFα in Jeko-1 cells 48 hours after the transfection of ADAM10 siRNA compared with cells transfected with scrambled siRNA. (B) Western blots showing a down-regulation of phospho-NFκBp65 and total NFκBp65 after ADAM10 down-regulation using siRNA, both in the total lysates harvested from Jeko-1 and Mino cells. (C) Western blots showing a down-regulation of total NFκBp65 in nuclear extracts from Jeko-1 and Mino cells. (D) Using a NFκB reporter vector dual luciferase assay, we showed a significant down-regulation of the NFκB transcriptional activity in Mino cells after down-regulation of ADAM10 using siRNA. (E) Using a NFκB reporter vector dual luciferase assay, we showed a significant up-regulation of the NFκB transcriptional activity in Mino cells after the addition of human recombinant ADAM10 (100 ng/mL).
ADAM10 activates the TNFα/NFκB axis. (A) ELISA showing a significant down-regulation of TNFα in Jeko-1 cells 48 hours after the transfection of ADAM10 siRNA compared with cells transfected with scrambled siRNA. (B) Western blots showing a down-regulation of phospho-NFκBp65 and total NFκBp65 after ADAM10 down-regulation using siRNA, both in the total lysates harvested from Jeko-1 and Mino cells. (C) Western blots showing a down-regulation of total NFκBp65 in nuclear extracts from Jeko-1 and Mino cells. (D) Using a NFκB reporter vector dual luciferase assay, we showed a significant down-regulation of the NFκB transcriptional activity in Mino cells after down-regulation of ADAM10 using siRNA. (E) Using a NFκB reporter vector dual luciferase assay, we showed a significant up-regulation of the NFκB transcriptional activity in Mino cells after the addition of human recombinant ADAM10 (100 ng/mL).
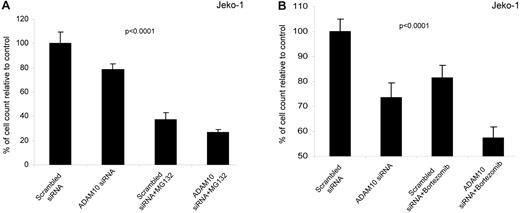
ADAM10 inhibition enhanced the growth-suppressing effect of the proteasome inhibitors MG132 and bortezomib
Proteasome inhibitors have been shown to induce apoptosis and cell-cycle arrest in MCL cells, and these effects are believed to be mediated via an inhibition of the NFκB-signaling pathway.37 Because we have shown that ADAM10 mediates its effects via the NFκB pathway, we hypothesized that ADAM10 inhibition may enhance the growth-suppressing effects of proteasome inhibitors such as MG132 in MCL cells. In our initial experiment, we determined that the inhibitory concentration at 50% (ie, the IC50) for MG132 was in the range of 1μM for Jeko-1 cells (data not shown). Using this concentration of MG132, we found that Jeko-1 cells transfected with ADAM10 siRNA had a significantly higher reduction in the number of viable cells compared with cells transfected with the scrambled siRNA (P < .0001 by ANOVA; Figure 6A). We also performed similar experiments using bortezomib, a proteasome inhibitor currently used in various clinical trials for the treatment of MCL.38 We used a 5nM concentration for this experiment, because cell growth inhibition was approximately 15% at this drug concentration. As shown in Figure 6B, the combination of bortezomib and ADAM10 siRNA resulted in a significant reduction in the number of viable cells compared with bortezomib or ADAM10 siRNA alone (P < .0001 by ANOVA)
ADAM10 inhibition enhances the growth-suppressing effects of the proteasome inhibitors bortezomib and MG132 in Jeko-1 cells. (A) Jeko-1 cells transfected with either scrambled or ADAM10 siRNA were treated with MG132 (1μM). At 24 hours after the MG132 treatment, the trypan blue exclusion assay was performed, and we found that ADAM10 siRNA enhanced the growth-suppressing effect of MG132 in Jeko-1 cells (P < .0001). (B) Jeko-1 cells transfected with either scrambled or ADAM10 siRNA treated with bortezomib (5nM). At 24 hours after the bortezomib treatment, the trypan blue exclusion assay was performed, and we found that ADAM10 siRNA enhanced the growth-suppressing effect of bortezomib in Jeko-1 cells (P < .0001 by ANOVA).
ADAM10 inhibition enhances the growth-suppressing effects of the proteasome inhibitors bortezomib and MG132 in Jeko-1 cells. (A) Jeko-1 cells transfected with either scrambled or ADAM10 siRNA were treated with MG132 (1μM). At 24 hours after the MG132 treatment, the trypan blue exclusion assay was performed, and we found that ADAM10 siRNA enhanced the growth-suppressing effect of MG132 in Jeko-1 cells (P < .0001). (B) Jeko-1 cells transfected with either scrambled or ADAM10 siRNA treated with bortezomib (5nM). At 24 hours after the bortezomib treatment, the trypan blue exclusion assay was performed, and we found that ADAM10 siRNA enhanced the growth-suppressing effect of bortezomib in Jeko-1 cells (P < .0001 by ANOVA).
Discussion
ADAM10 has recently been implicated in the pathogenesis of several types of malignant solid tumors, such as those arising from the colon, pancreas, ovary, uterus, and oral cavity.15,17,18,39 For the first time, we have demonstrated that constitutive activation of ADAM10 is a very frequent finding in MCL. Our findings also suggest that ADAM10 is biologically significant in MCL. Specifically, ADAM10 promotes cell-cycle progression and increased cell growth, which are associated with modulations of 2 important cell-cycle regulators (CCND1 and p21waf1) and with activation of the TNFα/NFκB-signaling pathway. Whereas MCL was the focus of this study, we also found evidence of constitutive activation of ADAM10 in other types of B-cell nonHodgkin lymphomas, although the functional significance of ADAM10 in these tumors needs to be further examined.
Whereas ADAM10 has been reported previously to be an important sheddase for TNFα, all of those studies were done using murine fibroblasts or other immortalized human cell lines, such as the human kidney cell line 293A and human articular chondrocytes.11,13 In this study, inhibition of ADAM10 expression with the use of siRNA led to down-regulation of TNFα secretion. To our knowledge, this is the first study directly linking ADAM10 and TNFα in a human cancer model. Furthermore, this is also the first report describing the link between ADAM10 and NFκB. The pathogenetic importance of NFκB in MCL has been reported previously.29,31 Specifically, NFκB appears to confer an antiapoptotic signal in MCL, because blockade of this signaling protein in MCL cells induces down-regulation of several antiapoptotic proteins such as bcl-2 and bcl-xL.29 The link between TNFα and NFκB in MCL was also established in one of our previous studies.31 It appears that ADAM10 up-regulates the autocrine production of TNFα, thereby activating the NFκB-signaling pathway. Interestingly, although pharmacologic inhibition of NFκB in MCL has been previously shown to result in significant apoptosis in MCL cells,29,31 we did not observe any detectable evidence of apoptosis as a result of ADAM10 knockdown using siRNA. This may have been due to the fact that residual NFκB activity is sufficient to prevent the activation of the apoptotic pathway.
In the present study, we have shown that ADAM10 is frequently active in MCL cell lines and tumors, but not in PBMCs from healthy donors. Interestingly, in the majority of the patient samples (n = 12), the active/mature form of ADAM10 was expressed at a higher level than the precursor form of ADAM10. This pattern is in contrast to that of MCL cell lines, in which the precursor form of ADAM10 was expressed at a higher level than the active/mature form of ADAM10. Whereas we cannot provide definitive explanations regarding this discrepancy between cell lines and tumors, we have considered the possibility that the tumor microenvironment may be involved in the regulation of ADAM10.
Our immunohistochemical data revealed that ADAM10 was readily detectable in most of the MCL tumors examined. This is in contrast to benign mantle zones, which did not show detectable ADAM10 immunoreactivity. These findings suggest that ADAM10 is “overexpressed” in MCL cells compared with benign mantle zone cells. We used a commercially available anti-ADAM10 antibody that recognizes this protein regardless of its activation status. The “overexpression” of ADAM10 in MCL tumors per se may be biologically important, because one previous report showed that overexpression of ADAM10 in colorectal cancer was significantly correlated with a higher clinical stage.16 In this study, we demonstrated that 5 of 5 cases of MCL showing ADAM10 immunoreactivity also expressed the active/mature form of ADAM10. It is likely that most MCL tumors overexpress ADAM10 and carry constitutive activation of this protein. Regarding our observation that ADAM10 can be localized to the nuclei of MCL cells, a previous publication suggested that nuclear accumulation of ADAM10 in prostate cancer was correlated with a higher Gleason score.40 Nevertheless, the biologic and/or clinical significance of the nuclear localization of ADAM10 in MCL needs to be further studied.
CCND1 overexpression due to t(11;14)(q13;q32), which brings the CCND1 gene under the influence of the enhancer of the IgH gene, is considered the hallmark for MCL.22 CCND1 has been previously shown to be involved in the regulation of MCL-cell proliferation.41 In the context of the role of ADAM10 in regulating cell-cycle progression in MCL cells, we investigated whether ADAM10 regulates the expression of CCND1 and other cell-cycle regulatory proteins, including p21waf1. We observed that ADAM10 down-regulation indeed resulted in a substantial down-regulation of CCND1 and up-regulation of p21Waf1, which correlated well with cell-cycle arrest. Based on our observation that the down-regulation of the CCND1 transcripts occurred within 24 hours after siRNA treatment, we believe that this change in the CCND1 expression was caused directly by the down-regulation of ADAM10, rather than being a consequence of cell-cycle arrest. In view of the established link between the NFκB pathway and CCND1, as well as p21Waf1, 42,43 it is possible that ADAM10 modulates the expression of CCND1 and p21Waf1 via activating the NFκB-signaling pathway.
ADAM10 appears to enhance the growth-suppressing effect of bortezomib and MG132, 2 proteasome inhibitors. This observation is in keeping with the concept that proteasome inhibitor–induced apoptosis in MCL is at least partly mediated via suppression of the NFκB-signaling pathway.37 Overall, our findings suggest that inhibition of ADAM10 may be a therapeutically useful strategy in treating MCL patients; specifically, inhibition of ADAM10 used in combination with proteasome inhibitors can enhance the overall tumor-suppressive effects in MCL. Pharmacologic inhibitors of ADAM10 are available, one of which is being tested in a clinical trial for patients with breast cancer.10
Different mechanisms have been shown to regulate the ADAM10 protein expression level. For example, β-catenin has been shown to modulate the expression of ADAM10 in colon cancer.44 Interestingly, β-catenin is known to be activated in a subset of MCL.34 We investigated whether blockade of β-catenin using siRNA in MCL cell lines can result in a down-regulation of ADAM10, and no detectable change was observed (unpublished findings). Therefore, the mechanism by which ADAM10 expression is modulated may well be cell type specific. Several enzymes are known to activate ADAM10, including proprotein convertase 7 and furin, which remove the prodomain of ADAM10.5 The overexpression and/or aberrant activation of these enzymes in MCL may be involved in the activation of ADAM10. Further studies are needed to investigate the role of these proteins in MCL.
In conclusion, our study shows for the first time that constitutive activation of ADAM10 is a consistent finding in MCL. We have provided evidence that ADAM10 contributes to the pathogenesis of MCL by activating the TNFα/NFκB–signaling pathway, and therefore that inhibition of ADAM10 may be a useful approach to enhance the therapeutic effects of other agents (such as proteasome inhibitors) in treating MCL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by research operating grants from the Canada Institute of Health Research and the Canadian Cancer Society (awarded to R.L.). H.A. is a recipient of a doctoral scholarship from the Egyptian government. M.A. is a clinical research fellow of the Terry Fox Foundation through an award from the Canadian Cancer Research Institute (18019).
Authorship
Contribution: H.A. and P.G. designed research, performed experiments, analyzed data, and wrote the manuscript; M.A. performed experiments; A.B. reviewed the manuscript and contributed to patient sample collection; and R.L. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raymond Lai, MD, PhD, Department of Laboratory Medicine and Pathology, Cross Cancer Institute and University of Alberta, 11560 University Ave, Edmonton, Alberta, Canada T6G 1Z2; e-mail: rlai@ualberta.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal