Abstract
A girl presented during childhood with a single course of extensive chickenpox and moderate albeit recurrent pneumonia in the presence of idiopathic CD4+ T lymphocytopenia (ICL). Her clinical condition remained stable over the past 10 years without infections, any granulomatous disease, or autoimmunity. Immunophenotyping demonstrated strongly reduced naive T and B cells with intact proliferative capacity. Antibody reactivity on in vivo immunizations was normal. T-cell receptor-Vβ repertoire was polyclonal with a very low content of T-cell receptor excision circles (TRECs). Kappa-deleting recombination excision circles (KRECs) were also abnormal in the B cells. Both reflect extensive in vivo proliferation. Patient-derived CD34+ hematopoietic stem cells could not repopulate RAG2−/−IL2Rγc−/− mice, indicating the lymphoid origin of the defect. We identified 2 novel missense mutations in RAG1 (p.Arg474Cys and p.Leu506Phe) resulting in reduced RAG activity. This report gives the first genetic clue for ICL and extends the clinical spectrum of RAG mutations from severe immune defects to an almost normal condition.
Introduction
Selective depletion of T lymphocytes is common in both primary and secondary immunodeficiencies. Idiopathic CD4+ T lymphocytopenia (ICL) is defined by an unexplained persistent CD4+ T lymphocyte count of < 300 cells/μL or < 20% of the total T-cell count.1
Since the discovery of human retroviruses, sporadic ICL patients were recognized with a CD4+ lymphocytopenia not infected by HIV or HTLV-1.2-7 Smith et al reviewed 230179 cases from the CDC AIDS Reporting System and described 47 ICL patients.2,3 Of these cases, only 3 (6%) were asymptomatic. Screening of healthy blood donors confirmed a low prevalence of ICL of 0.2%-0.6%.4,5
The disease may have a transient nature over the years but mostly persists.3 The immunologic parameters of ICL consist of a prolonged decrease in CD4+ T cell numbers, sometimes with a concomitant decrease in CD8+ T cells and B cells as well. Immunoglobulin levels are normal,2-5 which helps to distinguish ICL from Common Variable Immunodeficiency (CVID).1,6
Although function declines with age, thymic output is well maintained into late adulthood.7-9 Thymic size correlates with numbers of CD4+CD45RA+ naive T cells. At very young age T lymphocytopenia is often caused by congenital defects resulting in severe combined immunodeficiency syndromes (SCID).10
Null mutations in RAG1 or RAG2 account for 70% of SCID cases with the classic T−B−SCID phenotype.10 Hypomorphic mutations with residual RAG activity occur in typical Omenn syndrome,10 or rare cases with lympocytopenia, hypogammaglobulinemia, granulomas in the skin, mucosa, internal organs and viral complications, including EBV-related lymphomas.11,12 In the present paper, we describe hypomorphic RAG1 mutations that corresponds with mild CD4+ T lymphocytopenia, normal in vitro lymphocyte function and in vivo vaccination responses.
Methods
Subjects and blood samples
Heparinized venous blood was collected from healthy (age-matched) donors, patient and family members. The study was approved by the institutional medical ethical committee and informed consent for the research purpose described was obtained from the parents of the child and age-matched controls in accordance with standards of the 1964 declaration of Helsinki.
Lymphocyte phenotyping
Lymphocyte activation, determination of T-cell receptor CDR3 spectratype, and TRECs
KREC assay and Vκ3-20–specific IgκREHMA
RAG gene analysis and in vitro V(D)J recombination assay
The RAG1 and RAG2 genes were amplified by PCR and sequenced.18 The level of recombination activity of the RAG1 mutant proteins were compared with the wt RAG1.
Case
The patient was the first child of healthy, nonconsanguineous Dutch parents, born in May 1992. At the age of 5, she contracted chickenpox with numerous large hemorrhaghic skin lesions and VZV-associated pneumonia. Skin lesions healed slowly with cicatriciation. Until the age of approximately 8 years she has suffered from recurrent episodes of fever and (viral) pneumonia for which she regularly received antibiotics. High-resolution CT scanning of neck, thorax, and abdomen around that age showed mild bronchiectasis without enlargement of perihilar lymph nodes or granulomatous lesions in lungs, liver, or spleen; a thymus was not detectable (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Dysmorphic features were not observed by clinical geneticists. Chromosomal abnormalities (including 22q11 hemizygosity) were excluded.
She remained disease-free for the past 10 years using oral cotrimoxazole as prophylaxis.
Results and discussion
Lymphocyte subpopulations and humoral immune responses
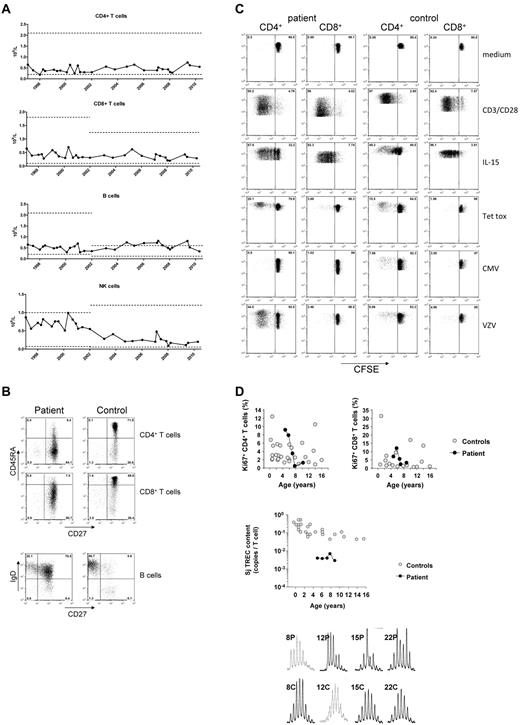
Immunophenotyping of the patient's PBMCs showed low numbers of CD4+ T cells and to a lesser extent, CD8+ T cells. The CD4+ lymphocytopenia was persistent over time with some fluctuations (Figure 1A). The percentage of CD4+ and CD8+ T cells with a CD45RO+ memory phenotype was strongly increased as compared with the naive T cells (Figure 1B).
Absolute numbers of CD4+ and CD8+ T cells, CD19+ B cells and CD3−CD16/56+ NK cells over time. (A) Numbers of the patient's lymphocytes are indicated in closed circles; the age-matched control levels for different age categories (ie, 4-10 years; 10-18 years) are indicated in a range of ± 2SD (dotted lines). (B) Immunophenotyping of CD4+ and CD8+ T cells and CD19+CD20+ B cells, according to CD45RA/CD27 and sIgD/CD27, respectively, as compared with a healthy, age-matched control. Proliferative capacity of the patient's T cells. T-cell proliferation of the patient and a healthy control is shown by CFSE dilution after 6 days of culture. Polyclonal proliferation was induced by a combination of CD3/CD28 or IL-15, whereas antigen-specific proliferation was assessed by stimulation with tetanus toxoid (Tet Tox), cytomegalovirus (CMV) or varicella zoster virus (VZV). (C) As expected in the presence of negative serology, CMV antigen did not activate her T cells. Increased proliferation of the peripheral CD4+ T-cell compartment as demonstrated by nuclear Ki67 staining in naive (CD45RA+CD27+) CD4+ and CD8+ T cells, compared with healthy age-matched naive control T cells, as described.13 TRECs in patient's T cells for the early, so-called signal joint (Sj) over a period of 5 years. (D) For T-cell repertoire analysis CDR3 spectratyping in the patient's T cells was analyzed, being representative for 2 separate experiments in triplicate, more than 2 years apart.
Absolute numbers of CD4+ and CD8+ T cells, CD19+ B cells and CD3−CD16/56+ NK cells over time. (A) Numbers of the patient's lymphocytes are indicated in closed circles; the age-matched control levels for different age categories (ie, 4-10 years; 10-18 years) are indicated in a range of ± 2SD (dotted lines). (B) Immunophenotyping of CD4+ and CD8+ T cells and CD19+CD20+ B cells, according to CD45RA/CD27 and sIgD/CD27, respectively, as compared with a healthy, age-matched control. Proliferative capacity of the patient's T cells. T-cell proliferation of the patient and a healthy control is shown by CFSE dilution after 6 days of culture. Polyclonal proliferation was induced by a combination of CD3/CD28 or IL-15, whereas antigen-specific proliferation was assessed by stimulation with tetanus toxoid (Tet Tox), cytomegalovirus (CMV) or varicella zoster virus (VZV). (C) As expected in the presence of negative serology, CMV antigen did not activate her T cells. Increased proliferation of the peripheral CD4+ T-cell compartment as demonstrated by nuclear Ki67 staining in naive (CD45RA+CD27+) CD4+ and CD8+ T cells, compared with healthy age-matched naive control T cells, as described.13 TRECs in patient's T cells for the early, so-called signal joint (Sj) over a period of 5 years. (D) For T-cell repertoire analysis CDR3 spectratyping in the patient's T cells was analyzed, being representative for 2 separate experiments in triplicate, more than 2 years apart.
Although B-cell numbers were normal, the distribution of the B-cell subsets was severely disturbed (Figure 1B). The frequency of class-switched CD27+ memory B cells was low (3.4%-9.2%) whereas the frequency of nonswitched sIgD+CD27+ memory B cells showed a high and stable percentage between 58%-72% (controls between 5-15 years: 18.4 ± 7.2%, n = 40). Humoral immunity was intact (supplemental Table 1).
In vitro functionality of T cells, thymic function, and TCR repertoire
T-cell proliferation to general stimuli (ie, CD3/CD28, cytokines), and specific antigens was intact (Figure 1C; data not shown). Cytokine release, CTL or spontaneous NK-cell killing of target cells were normal (data not shown).
In the absence of a detectable thymus, peripheral in vivo T-cell proliferation was expected to be increased to sustain normal T-cell numbers. This was indeed demonstrated in 2 ways. First, lymphocyte expression of the nuclear proliferation marker Ki67 was not different from age-matched control samples (Figure 1D; data not shown). Second, the content of TRECs was reduced compared with controls (Figure 1D), indicative for increased T-cell proliferation. However, despite extensive peripheral T-cell expansion to sustain the number of T cells, no restriction in the TCR repertoire was demonstrated (Figure 1D).
Cellular and molecular properties of the circulating B-cell compartment
B-cell proliferation was found to be normal on BcR-dependent and BcR-independent stimulation (supplemental Figure 2A). The number of cell divisions of the transitional, naive-mature, and nonswitched B cells was strongly increased as compared with healthy controls (supplemental Figure 2B).16,17 Naive B cells were not somatically mutated and the mutation frequency in the expanded fraction of nonswitched natural effector B cells was only 4.7% (healthy controls 15%; n = 5; supplemental Figure 2C). These results indicate that the number of total peripheral B cells is normal because of extensive (antigen-independent) proliferation of the transitional, naive and nonswitched B cells.
Lymphoid origin of the defect and genetic immunodeficiency screening
T-cell maturation data suggested an intrinsic T-cell developmental defect with thymus hypoplasia from early age onward. Using a humanized RAG2−/−IL2Rγc−/− mouse model,19 normal human T-cell reconstitution on intrahepatic injection of 4 × 105 patient-derived CD34+CD38− HSCs from the bone marrow of the patient failed, in contrast to control HSCs. In the blood, no patient T cells could be detected at 6 and 9 weeks, nor in the thymus at 10 weeks (supplemental Table 2), confirming the lymphoid background of the thymus hypoplasia.
RAG defect
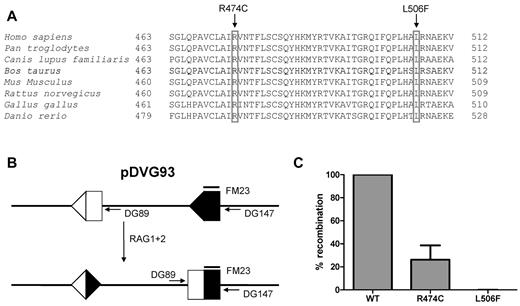
Sequence analysis demonstrated 2 novel heterozygous missense mutations in the RAG1 gene (c.1420C > T and c.1516C > T), affecting the evolutionary conserved amino acids Arg474 and Leu506 (Figure 2A). The RAG1 p.Arg474Cys recombinant protein had 25% recombinase activity compared with wild-type RAG1, whereas the p.Leu506Phe mutant did not have any residual activity (Figures 2B-C).
RAG1 gene mutations and residual recombination activity. Sequence alignment of RAG1 protein of different species. Both the arginine (R) at amino acid position 474 and the leucine (L) at 506 are conserved residues. (A) The parents were heterozygous for each of the mutation. (B) Transfection of pDVG93, RAG1 and RAG2 in 3T3 fibroblasts results in an inversion rearrangement of pDVG93, which can be detected by the primers DG89 and DG147. (C) Only the R474C mutation results in residual recombination activity, as assessed by the in vitro recombination assay using pDVG93.
RAG1 gene mutations and residual recombination activity. Sequence alignment of RAG1 protein of different species. Both the arginine (R) at amino acid position 474 and the leucine (L) at 506 are conserved residues. (A) The parents were heterozygous for each of the mutation. (B) Transfection of pDVG93, RAG1 and RAG2 in 3T3 fibroblasts results in an inversion rearrangement of pDVG93, which can be detected by the primers DG89 and DG147. (C) Only the R474C mutation results in residual recombination activity, as assessed by the in vitro recombination assay using pDVG93.
Hypomorphic mutations may result in typical Omenn patients with T cells showing an oligoclonal TCR repertoire, and poor development of precursor B cells,18,20 which may contribute to hyperinflammation/autoimmunity because of low-affinity autoantibody production.21,22 These features were absent in our patient.
Epicrise
V(D)J recombination activity of the RAG1 mutant proteins was very low. Even this residual RAG1 activity provides a low level of T- and B-cell production for effective host immunity. The functional immune system in terms of the mild clinical course in the absence of a detectable thymus could be explained by cytokine-driven proliferation, as reflected by the maintenance of a polyclonal repertoire.23 The persistent lymphocyte activation could be compatible with cytokine-driven homeostasis (Figure 2A; supplemental Table 3).
The thymus hypoplasia associated with an immune defect similar to ICL and few clinical symptoms was identified to be caused by novel heterozygous RAG1 mutations with very low RAG activity. Instead of SCID, Omenn syndrome or extensive granulomatous disease, our case study adds to the complexity of hypomorphic mutations in the RAG genes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Paul Baars for technical support and Prof Jacques van Dongen for the help in genetic analyses. We are grateful to Profs Hergen Spits and Frank Miedema for critically reading the manuscript and the useful comments and research suggestions over the years.
Authorship
Contribution: T.W.K. performed the diagnosis and treatment, designed research, and wrote the paper; H.I. performed research, cloning and expression of RAG variants, and analysis of data; E.M.M.v.L. contributed to the design of research and data analysis; M.H.J. and M.D.H. performed research and data analysis; K.C.W. performed vital mouse studies; R.A.W.v.L. contributed to the design of research and wrote the manuscript; and M.v.d.B. contributed to the design of study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: T. W. Kuijpers, AMC (location G8-205), Meibergdreef 9, 1105 AZ Amsterdam; The Netherlands; e-mail: t.w.kuijpers@amc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal