Abstract
This study investigated the immature platelet fraction (IPF) in assessing treatment effects in immune thrombocytopenia (ITP). IPF was measured on the Sysmex XE2100 autoanalyzer. The mean absolute-IPF (A-IPF) was lower for ITP patients than for healthy controls (3.2 vs 7.8 × 109/L, P < .01), whereas IPF percentage was greater (29.2% vs 3.2%, P < .01). All 5 patients with a platelet response to Eltrombopag, a thrombopoietic agent, but none responding to an anti-FcγRIII antibody, had corresponding A-IPF responses. Seven of 7 patients responding to RhoD immuneglobulin (anti-D) and 6 of 8 responding to intravenous immunoglobulin (IVIG) did not have corresponding increases in A-IPF, but 2 with IVIG and 1 with IVIG anti-D did. This supports inhibition of platelet destruction as the primary mechanism of intravenous anti-D and IVIG, although IVIG may also enhance thrombopoiesis. Plasma glycocalicin, released during platelet destruction, normalized as glycocalicin index, was higher in ITP patients than controls (31.36 vs 1.75, P = .001). There was an inverse correlation between glycocalicin index and A-IPF in ITP patients (r2 = −0.578, P = .015), demonstrating the relationship between platelet production and destruction. Nonresponders to thrombopoietic agents had increased megakaryocytes but not increased A-IPF, suggesting that antibodies blocked platelet release. In conclusion, A-IPF measures real-time thrombopoiesis, providing insight into mechanisms of treatment effect.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disease affecting adults and children, in which most patients have autoantibodies that accelerate platelet destruction1,2 and may also impair megakaryocyte platelet production.3-5 Cytotoxic effects of CD8+ T lymphocytes are also thought to cause thrombocytopenia in an apparently small number of cases, perhaps by impairing megakaryocytopoiesis.6,7 Thrombopoietin levels are normal or only slightly elevated in patients with ITP, suggesting that the lack of compensatory stimulation of megakaryocytes may contribute to impaired platelet production.8 If thrombocytopenia is sufficiently profound, it can result in bleeding, which is infrequently severe.9,10
Traditional frontline treatments of ITP, including corticosteroids, intravenous immunoglobulin (IVIG), and intravenous anti-D, are effective but typically cause transient elevations in platelet counts. Second- and third-line therapies, including rituximab, splenectomy, thrombopoietin receptor agonists (TPO-A), and immunosuppressants, are often successful and, particularly rituximab and splenectomy, may cause long-term increases in the platelet count.11,12
This study focused in part on exploring the mechanisms of action of IVIG and anti-D. The primary immediate effect of IVIG in patients with ITP, first suggested by Imbach et al in 198113 and then Fehr in 1982,14 is thought to be inhibition of peripheral immune platelet destruction. This explanation was based largely on inferential data demonstrating slower clearance of antibody-coated, chromium-labeled red cells, rather than on direct studies of platelets.15 Studies in murine ITP have shown various effects of IVIG on ITP: protection against autoantibody-mediated immune destruction of platelets via up-regulation of FcγRIIB, the inhibitory Fcγ receptor16 ; decreased autoantibody production by B lymphocytes, via up-regulation of FcγRIIB17 ; and inhibition of antibody mediated but not cell-mediated platelet destruction.18 There is less information describing the mechanism of effect of anti-D, although it is presumed to inhibit platelet destruction via blocking FcγRIIA and FcγRIIIA activation, as also supported by an animal model.19,20 Nonresponse to these agents is presumed to be the result of underlying impaired platelet production, such that slowing the rate of platelet destruction has minimal to no impact on the platelet count.15
TPO-A increases the platelet count via stimulating megakaryocytopoiesis and thereby increasing thrombopoiesis to a level that overcomes the antiplatelet antibody effect in the majority of chronic ITP patients.21-23 The pathoetiology of nonresponse to TPO-A in patients with ITP has not been well studied, and the mechanisms are currently unknown. Theoretically, they could range from defects at the level of the TPO receptor, including its signaling pathway, to increased thrombopoiesis insufficient to overcome peripheral platelet destruction.
Overall, the mechanisms of effect of treatments for ITP have proven difficult to investigate.24,25 Antiplatelet antibody assays, such as the monoclonal antibody-specific immobilization of platelet antigens, are relatively specific but not very sensitive and appear to be only semiquantitative.26-28 This has not been systematically investigated in a large prospective study. Radiolabeled, platelet-kinetic studies early on suggested that platelet production may be reduced, rather than increased, in patients with ITP.29,30 However, these studies are cumbersome in that they are technically challenging in marked thrombocytopenia, require multiple patient visits, and involve exposure to radioactivity. Furthermore, the mathematical assumptions used in modeling the raw data to derive the platelet half-life are not precise because the exact extent of “random” platelet consumption via interaction with the vessel wall is unknown.1 Despite this uncertainty, these kinetic studies are supported by morphologic assessments of megakaryocytes demonstrating cell damage consistent with reduced platelet production.31,32 Another approach to estimate platelet turnover is to measure plasma glycocalicin, an extramembranous portion of the α-subunit of platelet membrane glycoprotein Ib released during platelet destruction. Plasma glycocalicin levels and glycocalicin indices (GCIs) have been shown to be a measure of platelet destruction and turnover.33-35 The technical difficulties of the assay have thus far impeded its widespread use.
A more recent technique for estimating platelet production and hence turnover has been to measure the number of newly produced platelets (ie, the platelet reticulocyte count). Immature platelets can be distinguished from mature platelets by their content of RNA, allowing a direct assessment, in real time, of thrombopoiesis to be made using flow cytometry with an RNA-binding fluorochrome, such as thiazole orange, to assess platelet maturity.36,37 However, flow cytometry of reticulated platelets has proven difficult to standardize. The Sysmex XE-2100 uses a proprietary RNA staining fluorescent dye containing polymethine and oxazine and a gating system in the fluorescent reticulocyte/platelet channel, which reliably quantifies the immature platelet fraction (IPF).38 The major advantage of the Sysmex XE-2100, an automated, laser-based, hematologic analyzer, is that it can be set to routinely measure the IPF as part of a standard complete blood count. The IPF and platelet reticulocytes measured with thiazole orange, are thought to be equivalent. They are derived using similar principles but have never been formally compared.36
The IPF is most commonly reported as the percentage IPF (the percentage of platelets with above-threshold RNA), but it can also be expressed as the absolute-IPF (A-IPF), which is the actual number of immature platelets per unit volume (% IPF × the platelet count).37,39 A high IPF percentage is indicative of consumptive or recovering thrombocytopenic disorders in contrast to a low IPF percentage seen in aplastic states.37,39 A-IPF specifically reflects the number of immature platelets in circulation (ie, platelet production).
The basic tenet of these studies was that, if the platelet count increased in response to treatment and the A-IPF did not increase, then the platelet increase was mediated by interfering with platelet destruction, as seen with GMA161. Conversely, if the platelet count increased and there was a substantial increase in the A-IPF, then the mechanism would include stimulation of platelet production, as seen with thrombopoietic agents. The latter effect was observed in a study using platelet reticulocyte percentage as a marker of platelet production in 4 patients with refractory ITP treated with pegylated megakaryocyte growth and development factor.40
There were 3 aims of this multipart study: (1) to explore the usefulness of A-IPF as a tool to assess dynamic platelet production in patients with ITP; (2) to use the A-IPF to elucidate the mechanisms of effect of anti-D and IVIG; and (3) to preliminarily investigate patients who did not have platelet responses to TPO-A.
Methods
Study design
Patients with ITP managed at Weill Cornell Medical College were enrolled into the study after providing written, informed consent in accordance with the Declaration of Helsinki. A separate Institutional Review Board-approved protocol was used to obtain oral consent from the healthy controls. Patients receiving experimental treatments (Eltrombopag and GMA161) had consented to these treatment studies previously; consents for IPF assessment were obtained separately for this study. Permission to use the study data for the Eltrombopag-, AKR501-, and GMA161-treated patients was obtained from GlaxoSmithKline (Julien Jenkins), MGI Pharma (Akhil Baranwahl), and Genzyme (Dan Magilauay), respectively. Consents for inclusion of the bone marrow examinations in the report were obtained separately.
Peripheral blood samples for measuring IPF were obtained from 100 healthy adult controls and 24 patients with ITP and collected into ethylenediaminetetraacetic acid tubes, which were run at the Platelet Disorders Center at Weill Cornell Medical College University campus of New York Presbyterian Hospital, on Sysmex XE-2100, within 8 hours of venesection. (IPF is stable for 24 hours after venesection.37 ) The first part of this study explored the use of percentage IPF and A-IPF. First, baseline percentage IPF, A-IPF, and platelet counts were compared between ITP patients and healthy controls. Second, the use of A-IPF as a measure of thrombopoiesis was explored in ITP patients before and after treatment with Eltrombopag, a TPO-A known to increase platelet production,41,42 and with GMA161, a monoclonal anti-FcγRIII antibody that inhibits the destruction of antibody-coated platelets.43,44 Third, simultaneous blood samples from 17 additional patients with chronic ITP and 8 healthy adult controls were analyzed for platelet counts and IPF values, in conjunction with plasma glycocalicin levels measured at the Laboratory for Thrombosis Research, Kantonsspital Baden, Switzerland.
In the second part of this study, the A-IPF was used to investigate the mechanisms of treatment effect of anti-D and IVIG; therefore, only ITP patients who responded to treatment were included in the analysis.
In the third part of this study, a preliminary, retrospective analysis of patients who did not have platelet responses to TPO-A was performed by integrating A-IPF responses and, when available, bone marrow examinations for these selected patients.
Patients and treatments
All patients included in this Institutional Review Board-approved study had a diagnosis of ITP according to the international consensus guidelines.11 The eligibility criteria for “Support for the use A-IPF as a measure of thrombopoiesis” and for “A-IPF to assess the mechanisms of treatment effect of anti-D and IVIG” were patients who had a platelet count and IPF measurement before treatment and within 10 days after treatment. To determine the mechanisms of response to treatment, 24 patients were included in the analysis of these 2 sections, selected on the basis of a defined platelet response to treatment as more than or equal to 30 × 109/L peak platelet count and at least 2-fold increase from baseline.45 There was one exception, a patient who initially received placebo in an Eltrombopag-placebo, blinded study and later responded to Eltrombopag in the open label Extend study (Table 1). All but 1 treatment episode was initiated at a baseline platelet count less than 30 × 109/L.
Results of platelet count and IPF values for ITP patient episodes before and up to 10 days after therapeutic interventions
| Treatment . | Platelet count, × 109/L . | Absolute IPF, × 109/L . | IPF, % . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretreatment . | Maximum posttreatment . | Change* . | Pretreatment . | Maximum posttreatment . | Change* . | Pretreatment . | Maximum posttreatment . | Change* . | |
| Eltrombopag | 13 | 113 | 100 | 7.4 | 66.4 | 59‖ | 57.2 | 58.8 | 1.6 |
| Eltrombopag | 16 | 565 | 549 | 5.9 | 29.4 | 23.5‖ | 36.6 | 5.2 | −31.4 |
| Eltrombopag | 29 | 173 | 144 | 3.4 | 18.2 | 14.8‖ | 11.6 | 10.5 | −1.1 |
| Eltrombopag | 2 | 47 | 45 | 1.0 | 17.4 | 16.4‖ | 52.2 | 37.1 | −15.1 |
| Eltrombopag | 17 | 634 | 617 | 3.9 | 23.5 | 19.6‖ | 22.8 | 3.7 | −19.1 |
| Placebo† | 8 | 14 | 6 | 3.5 | 4.1 | 0.6 | 43.7 | 29.4 | −14.3 |
| GMA161 | 14 | 60 | 46 | 3.4 | 13 | 9.6 | 24.2 | 21.6 | −2.6 |
| GMA161 | 13 | 45 | 32 | 1.2 | 3.8 | 2.6 | 9.2 | 17.1 | 7.9 |
| Anti-DX‡ | 9 | 79 | 70 | 2 | 5.5 | 3.5 | 21.9 | 6.9 | −15 |
| Anti-DX‡ | 14 | 115 | 101 | 2.2 | 2.8 | 0.6 | 15.8 | 2.4 | −13.4 |
| Anti-D | 13 | 167 | 154 | 2 | 5 | 3 | 15.7 | 3 | −12.7 |
| Anti-DA§ | 7 | 335 | 328 | 2.8 | 8.4 | 5.6 | 39.8 | 2.5 | −37.3 |
| Anti-DA§ | 10 | 175 | 165 | 2.1 | 6.8 | 4.7 | 21.1 | 3.9 | −17.2 |
| Anti-D | 20 | 163 | 143 | 1.8 | 5.2 | 3.4 | 9 | 3.2 | −5.8 |
| Anti-D | 16 | 421 | 405 | 2.2 | 7.6 | 5.4 | 14 | 1.8 | −12.2 |
| Anti-D | 9 | 108 | 99 | 2.4 | 5 | 2.6 | 26.7 | 4.6 | −22.1 |
| Anti-D | 12 | 409 | 397 | 2.5 | 5.3 | 2.8 | 21.2 | 1.3 | −19.9 |
| Anti-D + IVIG | 3 | 160 | 157 | 1.8 | 17.3 | 15.5‖ | 60.9 | 10.8 | −50.1 |
| IVIGY‡ | 1 | 121 | 120 | 0.7 | 33.5 | 32.8‖ | 67.2 | 27.2 | −40 |
| IVIGY‡ | 1 | 79 | 78 | 0.3 | 15.2 | 14.9‖ | 26.2 | 19.2 | −7 |
| IVIGY‡ | 1 | 73 | 72 | 0.3 | 18.4 | 18.1‖ | 29.3 | 25.2 | −4.1 |
| IVIGB§ | 1 | 210 | 209 | 0.4 | 12 | 11.6‖ | 38.5 | 5.7 | −32.8 |
| IVIGB§ | 11 | 197 | 186 | 2.8 | 16.7 | 13.9‖ | 25.8 | 8.5 | −17.3 |
| IVIG | 48 | 113 | 65 | 13 | 17.5 | 4.5 | 27 | 15.5 | −11.5 |
| IVIG | 10 | 143 | 133 | 1.7 | 8 | 6.3 | 17.3 | 5.6 | −11.7 |
| IVIG | 15 | 58 | 43 | 2.1 | 2.3 | 0.2 | 14 | 4 | −10 |
| IVIG | 9 | 46 | 37 | 1.7 | 9.8 | 8.1 | 19 | 21.3 | 2.3 |
| IVIG | 15 | 159 | 144 | 4.3 | 5.4 | 1.1 | 28.9 | 3.4 | −25.5 |
| IVIG | 7 | 171 | 164 | 1.5 | 6.0 | 4.5 | 21.3 | 3.5 | −17.8 |
| Treatment . | Platelet count, × 109/L . | Absolute IPF, × 109/L . | IPF, % . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretreatment . | Maximum posttreatment . | Change* . | Pretreatment . | Maximum posttreatment . | Change* . | Pretreatment . | Maximum posttreatment . | Change* . | |
| Eltrombopag | 13 | 113 | 100 | 7.4 | 66.4 | 59‖ | 57.2 | 58.8 | 1.6 |
| Eltrombopag | 16 | 565 | 549 | 5.9 | 29.4 | 23.5‖ | 36.6 | 5.2 | −31.4 |
| Eltrombopag | 29 | 173 | 144 | 3.4 | 18.2 | 14.8‖ | 11.6 | 10.5 | −1.1 |
| Eltrombopag | 2 | 47 | 45 | 1.0 | 17.4 | 16.4‖ | 52.2 | 37.1 | −15.1 |
| Eltrombopag | 17 | 634 | 617 | 3.9 | 23.5 | 19.6‖ | 22.8 | 3.7 | −19.1 |
| Placebo† | 8 | 14 | 6 | 3.5 | 4.1 | 0.6 | 43.7 | 29.4 | −14.3 |
| GMA161 | 14 | 60 | 46 | 3.4 | 13 | 9.6 | 24.2 | 21.6 | −2.6 |
| GMA161 | 13 | 45 | 32 | 1.2 | 3.8 | 2.6 | 9.2 | 17.1 | 7.9 |
| Anti-DX‡ | 9 | 79 | 70 | 2 | 5.5 | 3.5 | 21.9 | 6.9 | −15 |
| Anti-DX‡ | 14 | 115 | 101 | 2.2 | 2.8 | 0.6 | 15.8 | 2.4 | −13.4 |
| Anti-D | 13 | 167 | 154 | 2 | 5 | 3 | 15.7 | 3 | −12.7 |
| Anti-DA§ | 7 | 335 | 328 | 2.8 | 8.4 | 5.6 | 39.8 | 2.5 | −37.3 |
| Anti-DA§ | 10 | 175 | 165 | 2.1 | 6.8 | 4.7 | 21.1 | 3.9 | −17.2 |
| Anti-D | 20 | 163 | 143 | 1.8 | 5.2 | 3.4 | 9 | 3.2 | −5.8 |
| Anti-D | 16 | 421 | 405 | 2.2 | 7.6 | 5.4 | 14 | 1.8 | −12.2 |
| Anti-D | 9 | 108 | 99 | 2.4 | 5 | 2.6 | 26.7 | 4.6 | −22.1 |
| Anti-D | 12 | 409 | 397 | 2.5 | 5.3 | 2.8 | 21.2 | 1.3 | −19.9 |
| Anti-D + IVIG | 3 | 160 | 157 | 1.8 | 17.3 | 15.5‖ | 60.9 | 10.8 | −50.1 |
| IVIGY‡ | 1 | 121 | 120 | 0.7 | 33.5 | 32.8‖ | 67.2 | 27.2 | −40 |
| IVIGY‡ | 1 | 79 | 78 | 0.3 | 15.2 | 14.9‖ | 26.2 | 19.2 | −7 |
| IVIGY‡ | 1 | 73 | 72 | 0.3 | 18.4 | 18.1‖ | 29.3 | 25.2 | −4.1 |
| IVIGB§ | 1 | 210 | 209 | 0.4 | 12 | 11.6‖ | 38.5 | 5.7 | −32.8 |
| IVIGB§ | 11 | 197 | 186 | 2.8 | 16.7 | 13.9‖ | 25.8 | 8.5 | −17.3 |
| IVIG | 48 | 113 | 65 | 13 | 17.5 | 4.5 | 27 | 15.5 | −11.5 |
| IVIG | 10 | 143 | 133 | 1.7 | 8 | 6.3 | 17.3 | 5.6 | −11.7 |
| IVIG | 15 | 58 | 43 | 2.1 | 2.3 | 0.2 | 14 | 4 | −10 |
| IVIG | 9 | 46 | 37 | 1.7 | 9.8 | 8.1 | 19 | 21.3 | 2.3 |
| IVIG | 15 | 159 | 144 | 4.3 | 5.4 | 1.1 | 28.9 | 3.4 | −25.5 |
| IVIG | 7 | 171 | 164 | 1.5 | 6.0 | 4.5 | 21.3 | 3.5 | −17.8 |
The change in the platelet count and IPF indicates the difference between that parameter on the day of treatment and on the day of maximum observed count after treatment.
This patient received placebo during the Eltrombopag/placebo randomized controlled study.
Data were available for the same patient on multiple, consecutive treatment episodes with the same agent for patient X with anti-D and patient Y with IVIG.
Data were available for the same patient on multiple, consecutive treatment episodes with the same agent for patient A with anti-D and patient B with IVIG.
Greater than 10 × 109/L increase in absolute IPF.
In “Comparison of A-IPF and glycocalicin,” 17 ITP patients and 8 healthy controls had A-IPF and plasma glycocalicin levels determined simultaneously. In “A-IPF and bone marrow morphology in nonresponders to TPO-A,” 11 nonresponding patients were identified among a larger group of patients treated with several TPO-As. The eligibility criteria of nonresponse were arbitrarily defined as less than 20 × 109/L peak platelet count or less than 50% increase from baseline after a minimum of 2 months of continuous TPO-A treatment.
Assessments and outcome measures
The initial part of the study measured platelet count, percentage IPF, and A-IPF for patients and controls. In the second part of the study, the mechanisms of treatment effects were assessed. A peak A-IPF increase more than 10 × 109/L was arbitrarily chosen to define a substantial thrombopoietic effect of the therapeutic intervention (eg, an increase in platelet production). The maximum observed A-IPFs and the corresponding platelet counts after therapeutic interventions were compared with the pretreatment baselines for the patients.
In “Comparison of A-IPF and glycocalicin,” plasma glycocalicin levels were measured using 2 monoclonal IgG murine antibodies directed against a peripheral 45-kDa fragment of glycocalicin and evaluated in a standardized, specific and sensitive enzyme-linked immunosorbent assay, as previously described.33 GCI (plasma glycocalicin normalized for the individual platelet count) was calculated for every subject and control, using the following formula: GCI = plasma glycocalicin level μg/mL × (250 × 109 platelets/L)/individual platelet count × 109/L.33
In “A-IPF and bone marrow morphology in nonresponders to TPO-A,” nonresponse to TPO-A was assessed in 11 patients via evaluation of their platelet counts and A-IPF data and integrating the available bone marrow morphologies from 7 of these patients. Bone marrow cellularity, myeloid to erythroid ratio, and the morphologic features of the megakaryocytes were evaluated. The number of megakaryocytes was quantified per 10 high-power fields and subsequently scored as 0 (normal number), 1+ (mild increase, up to 2 times normal), 2+ (moderate increase, 3-4 times normal), or 3+ (marked increase, > 4 times normal). Reticulin stain was performed on all cases and was graded according to a modified Bauermeister/Manoharan scale as follows: 1+ (focal fine fibers), 2+ (diffuse fine fibers), 3+ (diffuse coarse fibers), and 4+ (diffuse fiber network with collagenization).
Statistical analysis
Analysis was largely descriptive, based on means and SDs as well as medians and ranges. Student t test was used to compare percentage IPF, A-IPF, glycocalicin, and GCI values of normal controls with patients with ITP. Correlations were assessed with 2-tailed Spearman rank, and P < .05 was considered statistically significant. For comparison of patients with ITP with normal controls, each of the 24 patients was used only once. The evaluation of pretreatment and posttreatment platelet counts and IPF values included 5 additional treatment episodes where an individual patient received consecutive repeated treatments. For these patients, only the episode with the greatest A-IPF change was included in the statistical analyses.
Results
ITP versus normal controls
Patient demographics and previous treatments for 24 patients with chronic ITP are outlined in Table 2. All percentage IPFs for the 24 patients with ITP were greater than any of the 100 normal controls (Figure 1A), and the mean percentage IPF of the ITP patients was substantially higher than that of the controls (29.16% ± 16.80% vs 3.2% ± 1.4%, P < .01). In distinction, the mean A-IPF was lower for patients with ITP than controls (3.02 ± 2.66 × 109/L vs 7.8 ± 3.1 × 109/L; P < .01) and only 4 of 24 (16.7%) ITP patients had an A-IPF pretreatment more than 4 × 109/L, whereas 93 of 100 (93%) controls had an A-IPF more than 4 × 109/L (Figure 1B).
Patient demographics and previous and current treatments for chronic ITP patients
| Demographic . | Value . |
|---|---|
| IPF (n = 24) | |
| Mean age, y (range) | 42 (0.5-82) |
| Male, no. (%) | 11 (46) |
| Previous treatment | 16 (67) |
| Steroids, no. (%) | |
| IVIG, no. (%) | 15 (63) |
| Intravenous anti-D, no. (%) | 14 (58) |
| Rituximab, no. (%) | 10 (42) |
| Other agents,* no. (%) | 9 (38) |
| Splenectomy, no. (%) | 8 (33) |
| Comparison of A-IPF and glycocalicin (n = 17) | |
| Mean age, y (range) | 53 (31-81) |
| Males, no. (%) | 8 (47) |
| Current treatment | |
| TPO-A, no. (%) | 9 (53) |
| Prednisone, no. (%) | 2 (12) |
| IVIG, no. (%) | 2 (12) |
| Prednisone and IVIG, no. (%) | 1 (6) |
| Rigel R788, no. (%) | 1 (6) |
| Danazol and MMF,† no. (%) | 1 (6) |
| No treatment, no. (%) | 1 (6) |
| Demographic . | Value . |
|---|---|
| IPF (n = 24) | |
| Mean age, y (range) | 42 (0.5-82) |
| Male, no. (%) | 11 (46) |
| Previous treatment | 16 (67) |
| Steroids, no. (%) | |
| IVIG, no. (%) | 15 (63) |
| Intravenous anti-D, no. (%) | 14 (58) |
| Rituximab, no. (%) | 10 (42) |
| Other agents,* no. (%) | 9 (38) |
| Splenectomy, no. (%) | 8 (33) |
| Comparison of A-IPF and glycocalicin (n = 17) | |
| Mean age, y (range) | 53 (31-81) |
| Males, no. (%) | 8 (47) |
| Current treatment | |
| TPO-A, no. (%) | 9 (53) |
| Prednisone, no. (%) | 2 (12) |
| IVIG, no. (%) | 2 (12) |
| Prednisone and IVIG, no. (%) | 1 (6) |
| Rigel R788, no. (%) | 1 (6) |
| Danazol and MMF,† no. (%) | 1 (6) |
| No treatment, no. (%) | 1 (6) |
Other agents included danazol, MMF, and azathioprine.
MMF indicates mycophenolate mofetil.
Immature platelet fraction (IPF) for ITP patients and controls. (A) Baseline IPF percentage in healthy controls (n = 100) and ITP patient treatment episodes (n = 29). The y-axis represents percentage IPF; and the x-axis, individual treatment episodes. Every pretreatment percentage IPF for all 29 patient episodes with ITP was greater than any of the 100 normal controls. Mean percentage IPF for ITP patients, 28.2% ± 15.5% was greater than controls, 3.2% ± 1.4% (P < .01). (B) Baseline A-IPF in healthy controls (n = 100) and ITP patient treatment episodes (n = 29). The y-axis represents the A-IPF values; and the x-axis, individual treatment episodes. Only 4 of 29 ITP patient episodes before treatment had A-IPF greater than 4 × 109/L, to contrast to 93 of 100 controls. Mean A-IPF for ITP patients (n = 29), 2.8 ± 2.5 × 109/L was less than the controls (n = 100), 7.8 ± 3.1 × 109/L (P < .01).
Immature platelet fraction (IPF) for ITP patients and controls. (A) Baseline IPF percentage in healthy controls (n = 100) and ITP patient treatment episodes (n = 29). The y-axis represents percentage IPF; and the x-axis, individual treatment episodes. Every pretreatment percentage IPF for all 29 patient episodes with ITP was greater than any of the 100 normal controls. Mean percentage IPF for ITP patients, 28.2% ± 15.5% was greater than controls, 3.2% ± 1.4% (P < .01). (B) Baseline A-IPF in healthy controls (n = 100) and ITP patient treatment episodes (n = 29). The y-axis represents the A-IPF values; and the x-axis, individual treatment episodes. Only 4 of 29 ITP patient episodes before treatment had A-IPF greater than 4 × 109/L, to contrast to 93 of 100 controls. Mean A-IPF for ITP patients (n = 29), 2.8 ± 2.5 × 109/L was less than the controls (n = 100), 7.8 ± 3.1 × 109/L (P < .01).
The percentage IPF and A-IPF for this study were generally congruent with those of the 6 previously reported studies, except that the mean percentage IPF of patients in the ITP group was marginally higher in this study (Table 3).37,39,46-49
Comparison of percentage IPF and A-IPF for ITP patients and controls in this study and other studies reported in the literature
| . | Controls . | ITP patients . | ||
|---|---|---|---|---|
| IPF, %, mean (range) . | A-IPF, × 109/L, mean (range) . | IPF, %, mean (range) . | A-IPF, × 109/L, mean (range) . | |
| Current study (control, n = 108; ITP, n = 41) | 3.3 (0.5-7.9) | 8.0 (2.6-17.2) | 25.5 (3.5-67.2) | 5.0 (0.3-32.3) |
| Briggs et al37 (control, n = 50; ITP, n = 22) | 3.4 (1.1-6.1) | 8.6 (3.1-16.4) | 19.5 (2.3-52.1)* | 8.1 (1.6-38.6)* |
| Pons I et al39 (control, n = 14; ITP, n = 20) | 2.6 (95% CI, 1.7-3.4) | NA | 16.8 (95% CI, 12.2-21.4) | NA |
| Abe et al46 (control, n = 129; ITP, n = 46) | 3.3 (1.0-10.3) | 7.5 (1.8-25.2) | 17.4 (1.2-53.2) | 4.0 (0.3-19.7) |
| Cannavo et al47 | 2.2 (1.0-4.5) | NA | Median, 11.8 (5.3-54.3) | NA |
| Jung et al48 (control, n = 2039; ITP, n = 150) | 1.1 Male (0.5-3.2) | NA | 7.7 (1.0-33.8) | NA |
| Female (0.4-3.0) | ||||
| Cho et al49 (control, n = 142; ITP, n = 14) | 1.7 (0.4-5.4) | NA | 12.5 | NA |
| . | Controls . | ITP patients . | ||
|---|---|---|---|---|
| IPF, %, mean (range) . | A-IPF, × 109/L, mean (range) . | IPF, %, mean (range) . | A-IPF, × 109/L, mean (range) . | |
| Current study (control, n = 108; ITP, n = 41) | 3.3 (0.5-7.9) | 8.0 (2.6-17.2) | 25.5 (3.5-67.2) | 5.0 (0.3-32.3) |
| Briggs et al37 (control, n = 50; ITP, n = 22) | 3.4 (1.1-6.1) | 8.6 (3.1-16.4) | 19.5 (2.3-52.1)* | 8.1 (1.6-38.6)* |
| Pons I et al39 (control, n = 14; ITP, n = 20) | 2.6 (95% CI, 1.7-3.4) | NA | 16.8 (95% CI, 12.2-21.4) | NA |
| Abe et al46 (control, n = 129; ITP, n = 46) | 3.3 (1.0-10.3) | 7.5 (1.8-25.2) | 17.4 (1.2-53.2) | 4.0 (0.3-19.7) |
| Cannavo et al47 | 2.2 (1.0-4.5) | NA | Median, 11.8 (5.3-54.3) | NA |
| Jung et al48 (control, n = 2039; ITP, n = 150) | 1.1 Male (0.5-3.2) | NA | 7.7 (1.0-33.8) | NA |
| Female (0.4-3.0) | ||||
| Cho et al49 (control, n = 142; ITP, n = 14) | 1.7 (0.4-5.4) | NA | 12.5 | NA |
NA indicates not applicable because this value was not measured in the study.
The means were taken as an average of those reported (platelet > 50 × 109/L: IPF% 16.8%; A-IPF 7.8 × 109/L and platelet < 50 × 109/L: IPF% 22.3%; A-IPF 8.1 × 109/L).
Support for the use A-IPF as a measure of thrombopoiesis
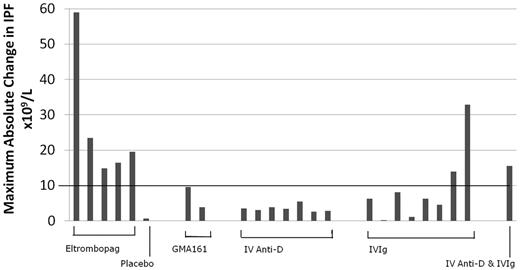
The A-IPF, percentage IPF, and platelet count for the responding ITP patients treated with either Eltrombopag (including 1 with placebo) or GMA161 are included in Table 1. Eltrombopag resulted in A-IPF responses (peak increase ≥ 10 × 109/L) in all 5 of the treated patients, 2 of which were more than 20 × 109/L. The patient who received placebo did not mount either a platelet or an A-IPF response (6.0 and 0.6 × 109/L, respectively). The 2 patients with platelet responses to GMA161, an anti-FcγRIII antibody, did not have A-IPF responses (Figure 2; Table 1).
Maximum observed change in the A-IPF within 10 days after treatment in patients with ITP (n = 24). The y-axis represents the maximum change in A-IPF after treatment, with an A-IPF response line threshold drawn at 10 × 109/L; and the x-axis, those patients who had a platelet response to different treatments. All patients responding to Eltrombopag, none of the patients responding to GMA161, and 0 of 7 patients responding to intravenous anti-D had an increase in A-IPF more than the 10 × 109/L threshold. Two of 8 patients treated with IVIG and 1 treated with IVIG and intravenous anti-D had an A-IPF increase of at least 10 × 109/L.
Maximum observed change in the A-IPF within 10 days after treatment in patients with ITP (n = 24). The y-axis represents the maximum change in A-IPF after treatment, with an A-IPF response line threshold drawn at 10 × 109/L; and the x-axis, those patients who had a platelet response to different treatments. All patients responding to Eltrombopag, none of the patients responding to GMA161, and 0 of 7 patients responding to intravenous anti-D had an increase in A-IPF more than the 10 × 109/L threshold. Two of 8 patients treated with IVIG and 1 treated with IVIG and intravenous anti-D had an A-IPF increase of at least 10 × 109/L.
Comparison of A-IPF and glycocalicin
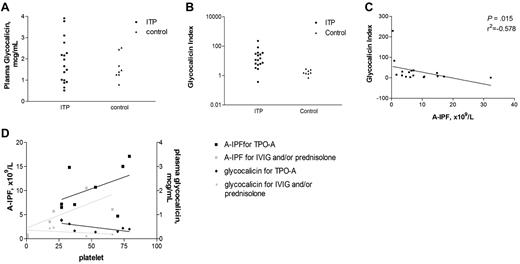
The results from the separate investigations assessing plasma glycocalicin and IPF measurements of patients with chronic ITP on various treatment regimens are listed in Table 4. Mean plasma glycocalicin levels did not differ significantly between ITP patients (n = 17) and controls (n = 8): 1.86 ± 1.04 μg/mL versus 1.60 ± 0.51 μg/mL (P = .529, Figure 3A; Table 4). However, ITP patients had a markedly greater mean GCI than the healthy controls: 31.36 ± 56.96 versus 1.75 ± 0.687 (P = .001). GCI values for 15 of 17 ITP patients were greater than all of the GCI values of the controls (Figure 3B; Table 4). In these patients, there was a strong inverse correlation between GCI and A-IPF (r2 = −0.578, P = .015), illustrating the expected equivalence between platelet production and platelet destruction as measured by A-IPF and GCI, respectively (Figure 3C). Furthermore, the patients treated with TPO-A, compared with those receiving IVIG and/or steroids, tended to have higher A-IPF and plasma glycocalicin values, implying a higher rate of platelet turnover with these agents (Figure 3D; Table 4).
Platelet, A-IPF, plasma glycocalicin level, and GCIs for ITP patients treated with thrombopoietic agonists, IVIG and/or prednisone, other (danazol + mycophenolate mofetil and Rigel R788), no treatment, and healthy controls
| Treatment . | Platelet count, × 109/L . | A-IPF, × 109/L . | Glycocalicin level, μg/mL . | GCI . | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD . | Median (range) . | Mean ± SD . | Median (range) . | Mean ± SD . | Median (range) . | Mean ± SD . | Median (range) . | |
| Thrombopoietic agonist (n = 9) | 44.78 ± 25.75 | 45.00 | 9.33 ± 5.41 | 7.15 | 2.27 ± 1.07 | 1.97 | 23.81 ± 13.21 | 11.19 |
| (3-79) | (0.9-17.1) | (1.00-3.91) | (5.31-83) | |||||
| IVIG and/or prednisone (n = 5) | 30.40 ± 25.58 | 21.00 | 5.24 ± 3.71 | 5.70 | 1.35 ± 0.81 | 0.92 | 58.43 ± 96.20 | 27.05 |
| (1-66) | (0.4-10.5) | (0.51-2.27) | (2.80-229) | |||||
| Others (n = 2) | 245.00 ± 280.00 | 245.00 | 16.95 ± 21.71 | 16.95 | 1.72 ± 1.49 | 1.72 | 7.53 ± 10.13 | 7.53 |
| (47-443) | (1.6-32.3) | (0.65-2.76) | (0.37-14.7) | |||||
| Nil (n = 1) | 22.00 | 22.00 | 3.40 | 3.40 | 1.02 | 1.02 | 11.64 | 11.64 |
| Control (n = 8) | 238.33 ± 29.91 | 223.00 | 10.55 ± 3.77 | 8.50 | 1.60 ± 0.51 | 1.43 | 1.76 ± 0.69 | 1.56 |
| (202-275) | (7.7-12.5) | (0.79-2.53) | (0.72-2.83) | |||||
| Treatment . | Platelet count, × 109/L . | A-IPF, × 109/L . | Glycocalicin level, μg/mL . | GCI . | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD . | Median (range) . | Mean ± SD . | Median (range) . | Mean ± SD . | Median (range) . | Mean ± SD . | Median (range) . | |
| Thrombopoietic agonist (n = 9) | 44.78 ± 25.75 | 45.00 | 9.33 ± 5.41 | 7.15 | 2.27 ± 1.07 | 1.97 | 23.81 ± 13.21 | 11.19 |
| (3-79) | (0.9-17.1) | (1.00-3.91) | (5.31-83) | |||||
| IVIG and/or prednisone (n = 5) | 30.40 ± 25.58 | 21.00 | 5.24 ± 3.71 | 5.70 | 1.35 ± 0.81 | 0.92 | 58.43 ± 96.20 | 27.05 |
| (1-66) | (0.4-10.5) | (0.51-2.27) | (2.80-229) | |||||
| Others (n = 2) | 245.00 ± 280.00 | 245.00 | 16.95 ± 21.71 | 16.95 | 1.72 ± 1.49 | 1.72 | 7.53 ± 10.13 | 7.53 |
| (47-443) | (1.6-32.3) | (0.65-2.76) | (0.37-14.7) | |||||
| Nil (n = 1) | 22.00 | 22.00 | 3.40 | 3.40 | 1.02 | 1.02 | 11.64 | 11.64 |
| Control (n = 8) | 238.33 ± 29.91 | 223.00 | 10.55 ± 3.77 | 8.50 | 1.60 ± 0.51 | 1.43 | 1.76 ± 0.69 | 1.56 |
| (202-275) | (7.7-12.5) | (0.79-2.53) | (0.72-2.83) | |||||
ITP patients had similar glycocalicin levels but higher glycocalicin indicies (GCI) than controls with an inverse correction between GCI and A-IPF, and higher glycocalicin and A-IPF values for patients treated with TPO-A. (A) Plasma glycocalicin levels for patients with ITP compared with healthy controls. The y-axis represents plasma glycocalicin levels; and the x-axis, individual samples. There was no statistically significant difference between the mean (± SE) glycocalicin levels for ITP patients (n = 17) and controls (n = 8): 1.86 ± 0.25 versus 1.60 ± 0.21 μg/mL (P = .144). (B) GCIs for patients with ITP and healthy controls. The y-axis represents GCIs; and the x-axis, individual samples. There was a significant and large difference between the mean (± SE) GCI for ITP patients (n = 17) and controls (n = 8): 31.36 ± 13.28 versus 1.75 ± 0.24 (P = .001). (C) Correlative analysis of GCI and A-IPF for ITP patients. The y-axis represents GCI; and the x-axis, A-IPF. There was a negative correlation between GCI and A-IPF (r2 = −0.578, P = .015). This demonstrates that platelet destruction is equivalent to platelet production. (D) Paired correlative analyses of plasma glycocalicin levels and A-IPF with platelet counts for ITP patients receiving thrombopoieitin receptor agonists and IVIG and/or prednisone. One line graph has a y-axis of A-IPF, and the other represents plasma glycocalicin levels, divided into those patients treated with TPO-A and those treated with IVIG and/or prednisone. The x-axis represents the platelet count. There were positive trends for A-IPF with platelet count for those patients treated with TPO-A (r2 = 0.503, P = .216) and IVIG and/or prednisone (r2 = 0.829, P = .058). This is shown in conjunction with negative trends for plasma glycocalicin levels with platelet count for those treated with TPO-A (r2 = −0.611, P = .115) and for those treated with IVIG and/or prednisone (r2 = −0.543, P = .297). Patients treated with TPO-A had greater A-IPF and plasma glycocalicin levels than those treated with IVIG and/or prednisone.
ITP patients had similar glycocalicin levels but higher glycocalicin indicies (GCI) than controls with an inverse correction between GCI and A-IPF, and higher glycocalicin and A-IPF values for patients treated with TPO-A. (A) Plasma glycocalicin levels for patients with ITP compared with healthy controls. The y-axis represents plasma glycocalicin levels; and the x-axis, individual samples. There was no statistically significant difference between the mean (± SE) glycocalicin levels for ITP patients (n = 17) and controls (n = 8): 1.86 ± 0.25 versus 1.60 ± 0.21 μg/mL (P = .144). (B) GCIs for patients with ITP and healthy controls. The y-axis represents GCIs; and the x-axis, individual samples. There was a significant and large difference between the mean (± SE) GCI for ITP patients (n = 17) and controls (n = 8): 31.36 ± 13.28 versus 1.75 ± 0.24 (P = .001). (C) Correlative analysis of GCI and A-IPF for ITP patients. The y-axis represents GCI; and the x-axis, A-IPF. There was a negative correlation between GCI and A-IPF (r2 = −0.578, P = .015). This demonstrates that platelet destruction is equivalent to platelet production. (D) Paired correlative analyses of plasma glycocalicin levels and A-IPF with platelet counts for ITP patients receiving thrombopoieitin receptor agonists and IVIG and/or prednisone. One line graph has a y-axis of A-IPF, and the other represents plasma glycocalicin levels, divided into those patients treated with TPO-A and those treated with IVIG and/or prednisone. The x-axis represents the platelet count. There were positive trends for A-IPF with platelet count for those patients treated with TPO-A (r2 = 0.503, P = .216) and IVIG and/or prednisone (r2 = 0.829, P = .058). This is shown in conjunction with negative trends for plasma glycocalicin levels with platelet count for those treated with TPO-A (r2 = −0.611, P = .115) and for those treated with IVIG and/or prednisone (r2 = −0.543, P = .297). Patients treated with TPO-A had greater A-IPF and plasma glycocalicin levels than those treated with IVIG and/or prednisone.
In addition, there was a significant direct correlation between A-IPF and mean platelet volume (r2 = 0.615, P = .0086), substantiating the association between A-IPF and the number of larger (immature) platelets (data not shown).50
A-IPF to assess the mechanisms of treatment effect of intravenous anti-D and IVIG
The peak A-IPF increases in patients with platelet count responses to the therapeutic interventions are shown in Figure 2. In all 7 patients treated with anti-D alone, there were considerable increases in the platelet counts (mean 231 × 109/L, range 70-405 × 109/L) with no response in A-IPF (all < 10 × 109/L; Figure 2; Table 1).
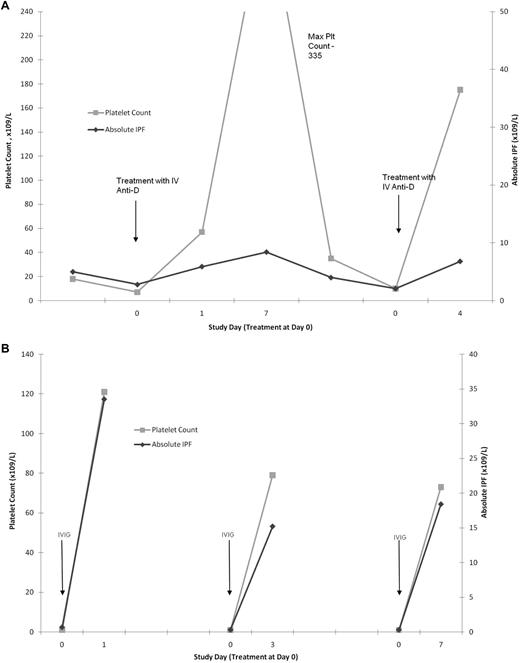
The effect of IVIG treatment on the A-IPF was more heterogeneous. For 6 of 8 patients treated with IVIG alone, the results were similar to those with anti-D: substantial platelet increases (mean, 98 × 109/L; range, 37-164 × 109/L) with no response in A-IPF (mean, 4.12 × 109/L; range, 0.20-8.10 × 109/L; Figure 2; Table 1). However, for 2 of 8 IVIG-treated patients and 1 treated with IVIG anti-D combined, the substantial platelet count increases (mean, 154 × 109/L; range, 120-186 × 109/L) were accompanied by marked A-IPF responses (mean, 20.7 × 109/L; range, 13.9-32.8 × 109/L; Figure 2; Table 1). There were no significant differences between the patients with and without A-IPF responses to IVIG, probably partly because of the small numbers of patients analyzed. However, patients with A-IPF responses to IVIG tended to have lower initial platelet counts and higher peak platelet counts after treatment. Furthermore, the A-IPF responses (or lack thereof) seemed to be characteristic of the individual patient, whose results with intravenous anti-D and IVIG were reproducible on consecutive treatment episodes (Figure 4A-B; Table 1).
Intrapatient consistency for A-IPF and platelet count responses to anti-D and IVIG. (A) A-IPF and platelet count responses on 2 consecutive treatment episodes with intravenous anti-D in the same ITP patient. One line graph has a y-axis representing platelet count, and the other represents A-IPF, against an x-axis representing time. Dramatic increases in platelet counts with minimal corresponding increase in A-IPF, in response to anti-D treatment, are shown. This response was consistent for 2 consecutive treatment episodes in the same patient. (B) A-IPF and platelet count responses on 3 consecutive IVIG treatment episodes in the same ITP patient. One line graph has a y-axis representing platelet count, and the other represents A-IPF, against an x-axis representing time. The platelet count and A-IPF increased substantially after each IVIG administration. The platelet count and A-IPF responses to IVIG for this patient were consistent for all 3 treatment episodes.
Intrapatient consistency for A-IPF and platelet count responses to anti-D and IVIG. (A) A-IPF and platelet count responses on 2 consecutive treatment episodes with intravenous anti-D in the same ITP patient. One line graph has a y-axis representing platelet count, and the other represents A-IPF, against an x-axis representing time. Dramatic increases in platelet counts with minimal corresponding increase in A-IPF, in response to anti-D treatment, are shown. This response was consistent for 2 consecutive treatment episodes in the same patient. (B) A-IPF and platelet count responses on 3 consecutive IVIG treatment episodes in the same ITP patient. One line graph has a y-axis representing platelet count, and the other represents A-IPF, against an x-axis representing time. The platelet count and A-IPF increased substantially after each IVIG administration. The platelet count and A-IPF responses to IVIG for this patient were consistent for all 3 treatment episodes.
A-IPF and bone marrow morphology in nonresponders to TPO-A
The demographics and results of 11 patients without platelet count responses to TPO-A are listed in Table 5. Seven of these patients had bone marrow examinations after treatment. Four of 7 patients had a normal cellularity for age, and 3 had hypercellular marrows. All patients had an increased number of megakaryocytes with focal megakaryocytic clustering and many apoptotic nuclei. Six patients had no significant increase in reticulin fibers (grades 0-1+), and 1 patient had moderate (2+) reticulin fibrosis. The 2 older patients had slightly atypical morphology with marked marrow hypercellularity and an increased number of small, hypolobated megakaryocytes. One patient went on to develop a marked reticulin fibrosis (3+), after a 6-year follow-up. The mean duration of TPO-A treatment was 6.5 months (range, 2-13 months) and the mean platelet count was 11.36 ± 9.68 × 109/L with a corresponding mean A-IPF 2.59 ± 1.69 × 109/L. One patient who required weekly IVIG and intravenous methylprednisolone in addition to TPO-A later maintained a substantial platelet count (116 × 109/L) with azathioprine and TPO-A combined. This suggests that a decrease in the platelet antibody levels allowed the TPO-A to increase the platelet count.
Demographics and platelet counts, A-IPF, and bone marrow histology at a point in time in patients without a platelet response to treatment with TPO-A for a given duration
| . | Age, y . | Sex . | Splenectomy, yes/no . | Duration of TPO-A treatment, mo . | Platelet count, × 109/L . | A-IPF, × 109/L . | Megakaryocyte quantity . | Megakaryocyte clustering . | Reticulin fibrosis grade . |
|---|---|---|---|---|---|---|---|---|---|
| 63 | Female | Yes | 6 | 12* | 1.44* | 1+ | + | 0 | |
| 35 | Male | Yes | 13 | 3* | 1.79* | 2+ | + | 1+ | |
| 55 | Female | Yes | 3 | 17* | 5.61* | 2+ | + | 1+ | |
| 5 | Male | No | 10 | 2* | 0.71* | 1+ | + | 0 | |
| 34 | Female | No | 9 | 11* | 3.23* | 1+ | + | 2+ | |
| 7 | Male | Yes | 2 | 2* | 0.61* | 3+ | + | 1+ | |
| 75 | Male | No | 8 | 2* | 0.77* | 2+ | + | 1+ | |
| 5 | Male | No | 9 | 12 | 3.00 | ||||
| 52 | Male | Yes | 3 | 19 | 2.85 | ||||
| 7 | Female | No | 3 | 34 | 4.76 | ||||
| 12 | Female | No | 6 | 11 | 3.78 | ||||
| Mean (SD) | 32 | 6.55 (± 3.56) | 11.36 (± 9.68) | 2.60 (± 1.69) |
| . | Age, y . | Sex . | Splenectomy, yes/no . | Duration of TPO-A treatment, mo . | Platelet count, × 109/L . | A-IPF, × 109/L . | Megakaryocyte quantity . | Megakaryocyte clustering . | Reticulin fibrosis grade . |
|---|---|---|---|---|---|---|---|---|---|
| 63 | Female | Yes | 6 | 12* | 1.44* | 1+ | + | 0 | |
| 35 | Male | Yes | 13 | 3* | 1.79* | 2+ | + | 1+ | |
| 55 | Female | Yes | 3 | 17* | 5.61* | 2+ | + | 1+ | |
| 5 | Male | No | 10 | 2* | 0.71* | 1+ | + | 0 | |
| 34 | Female | No | 9 | 11* | 3.23* | 1+ | + | 2+ | |
| 7 | Male | Yes | 2 | 2* | 0.61* | 3+ | + | 1+ | |
| 75 | Male | No | 8 | 2* | 0.77* | 2+ | + | 1+ | |
| 5 | Male | No | 9 | 12 | 3.00 | ||||
| 52 | Male | Yes | 3 | 19 | 2.85 | ||||
| 7 | Female | No | 3 | 34 | 4.76 | ||||
| 12 | Female | No | 6 | 11 | 3.78 | ||||
| Mean (SD) | 32 | 6.55 (± 3.56) | 11.36 (± 9.68) | 2.60 (± 1.69) |
1+ indicates mild increase; 2+, moderate increase; 3+, severe increase; and 0, no increase.
Platelet counts and A-IPF values were measured on the day of the bone marrow examination.
Discussion
Thrombocytopenia in ITP is caused by accelerated platelet destruction and impaired platelet production.1,3,8 Studying the mechanisms of therapeutic effects in chronic ITP involves assessing changes in the rates of platelet destruction and/or production before and after treatment administration. In this report, the IPF, based on its near identity with platelet reticulocytes, was used to assess platelet production in ITP patients before and after treatment. This provided a novel approach to the investigation of therapeutic effect and contributed to an understanding of how patients responded to different therapies and why certain patients did not respond to TPO-A.
Comparison of IPF for ITP patients and controls
Previous reports have focused on the IPF as a percentage of total platelet count to represent the immature platelet population.37,39 However, because the A-IPF measures the number of immature platelets circulating in the periphery, it may be more useful in measuring platelet production in thrombocytopenic states. In “ITP versus normal controls,” ITP patients had markedly lower baseline A-IPF values than controls, supporting the widely accepted hypothesis that there is an impairment of platelet production in many patients with ITP (Figure 1B; Table 1).
Support for A-IPF as a measure of thrombopoiesis
The initial investigation used 2 model treatments, Eltrombopag and GMA161, to support A-IPF as a useful tool to measure real time, in vivo thrombopoiesis in patients with ITP. Eltrombopag has been shown to increase peripheral platelet counts via activation of JAK/STAT and MAPK signaling pathways, resulting in proliferation and differentiation of bone marrow precursor cells and increased platelet production.22,24 3G8 and GMA161, the latter a humanized monoclonal antibody derived from 3G8, have been shown to increase platelet counts by blocking ligand binding to FcγRIII, thereby inhibiting platelet destruction.43,44 Change, or lack thereof, of A-IPF was tested in patients who had platelet responses to Eltrombopag or GMA-161. Treatment with Eltrombopag caused substantial increases in A-IPF (≥ 10 × 109/L) in all 5 patients who had platelet responses (increases ≥ 30 × 109/L and achieving at least a 2-fold increase45 ). In contrast, both patients who had platelet responses to GMA161 did not have corresponding A-IPF responses. One patient had a larger than expected, although less than 10 × 109/L, peak A-IPF increase, presumably as a result of concurrent high-dose intravenous steroid and/or the fever-chill reaction for which it was administered (Figure 2). The validity of A-IPF was further enhanced by the correlation between the measures of platelet production (A-IPF) and platelet destruction (glycocalicin index; Figure 3C).
A-IPF in assessing mechanisms of treatment response to IVIG and intravenous anti-D
A-IPF was then used to investigate mechanisms of treatment effect for IVIG and anti-D by comparing the A-IPF before and after platelet responses. Although the mechanisms of action of IVIG and anti-D continue to be explored, both are thought to primarily interact with Fcγ receptors (FcγR) on mononuclear cells, and thereby reduce reticuloendothelial clearance of opsonized platelets.19,20 Anti-D apparently interacts with the activating FcγRs (FcγRIIA, FcγRIIIA),20 whereas IVIG is thought to up-regulate the inhibitory FcγRIIB.16,17,19
The pretreatment and posttreatment A-IPF results verified that the primary effect of anti-D and IVIG is inhibition of platelet destruction, rather than increased platelet production. No patients with platelet responses to anti-D treatment alone had an A-IPF response. However, 2 patients treated with IVIG alone and 1 with combined IVIG and anti-D had substantial A-IPF increases, suggesting that IVIG may also augment platelet production in certain patients (Figure 2).
Two anecdotal patients with ITP had Indium111 oxine radiolabeled, autologous platelet life spans, determined at another center, before and after IVIG treatment. The platelet counts increased after therapy, but platelet survivals were not dramatically increased (Table 6). The calculated increase in platelet turnover suggests that enhanced platelet production was an important mechanism of the increased platelet count in these 2 IVIG-treated patients.1 IPF was not simultaneously measured in these 2 patients.
Indium111 oxine-labeled platelet kinetic studies in 2 patients at baseline and after intravenous immunoglobulin therapy
| Platelet count, × 109/L . | Survival, h . | Platelet turnover, × 109/L/d . | ||||
|---|---|---|---|---|---|---|
| Baseline . | Posttreatment . | Increase . | Baseline . | Posttreatment . | Baseline . | Posttreatment . |
| 31 | 157 | 126 | 52.8 | 60.0 | 18 | 35 |
| 6 | 35 | 29 | 26.4 | 9.6 | 14 | 104 |
| Platelet count, × 109/L . | Survival, h . | Platelet turnover, × 109/L/d . | ||||
|---|---|---|---|---|---|---|
| Baseline . | Posttreatment . | Increase . | Baseline . | Posttreatment . | Baseline . | Posttreatment . |
| 31 | 157 | 126 | 52.8 | 60.0 | 18 | 35 |
| 6 | 35 | 29 | 26.4 | 9.6 | 14 | 104 |
IVIG has many potential mechanisms of effect. Further investigation is necessary to understand how IVIG may increase platelet production, to distinguish the cases in which this occurs, and to explain why this effect was not seen with anti-D.
Nonresponse to TPO-A
The focus of this study was to assess mechanisms of platelet response to treatment in patients with chronic ITP, using serial measurements of A-IPF to estimate changes in platelet production. Subsequently, the use of A-IPF to provide an insight into the mechanisms of nonresponse to TPO-A was explored. Three hypothetical categories could be considered: (1) reduced megakaryocytes in the bone marrow (failure of TPO agents to have their biologic effect); (2) increased megakaryocytes in the bone marrow but little or no increase in A-IPF (platelet antibodies, or activated CD8+ T cells preventing the megakaryocytes from making or releasing platelets); and (3) increased megakaryocytes in the bone marrow and increased A-IPF but no platelet increase (substantial peripheral platelet destruction preventing an increased A-IPF from resulting in a platelet count increase).
All 11 patients not responding to TPO-A had A-IPFs less than 6 × 109/L: none of the nonresponding patients had any A-IPF response. All 7 patients with simultaneous bone marrow histology had increased (often very increased) megakaryocytes and megakaryocyte clustering, which is a TPO-specific finding in patients with ITP (Table 5); all patients except 1 had less than moderate fibrosis (Table 5). Overall, the bone marrow findings in these patients appeared similar to those in TPO-responsive ITP patients. This shows that, in these cases of TPO-A nonresponse, defined by platelet count, TPO-A had its expected biologic effect to stimulate megakaryocytopoiesis. These patients had no increases in A-IPF, despite increased megakaryocytes, suggesting that the megakaryocytes were somehow blocked from producing or releasing platelets. The hypothesis that antibody-mediated inhibition of platelet production or release prevented response in these patients is supported by the anecdotal additive effect of azathioprine in 1 patient. Studies focusing on the pathophysiologic differences between responders and nonresponders may further clarify the mechanisms of nonresponse to TPO-A.
Implications
Patients treated with TPO-A tended to have higher plasma glycocalicin levels and the higher A-IPF values compared with patients treated with IVIG and/or prednisone (Figure 3D; Table 4). This is consistent with increased platelet turnover in the TPO-A–treated patients. It supports preliminary data that, by increasing blood levels of transforming growth factor-β,7 TPO-A may be able to induce CD4+ regulatory T cells, which may in turn ameliorate ITP in longitudinally treated patients over time.6
Limitations
The primary limitation of this manuscript is the relatively small size of the patient groups because of both the eligibility criteria of only patients responding to treatment and the use of experimental agents in certain cases. A mitigating factor was that the results for each treatment in responders were relatively consistent (Figure 2). Prospective studies with larger number of patients are needed to verify the mechanisms of treatment effects using A-IPF.
A second limitation was the substantial variation in IPF values between patients with ITP, both in published reports and in this study37,39,46-49 (Figure 1; Table 1 and Table 3). Similarly, there was considerable interpatient variability in responses to treatments. In contrast, there was high intrapatient consistency of the A-IPF because the results for paired platelet and A-IPF changes were remarkably similar between different treatment episodes within an individual patient (Figure 4). In addition, by comparing A-IPF in the same patient before and after treatment, any individual variability was minimized in the assessment of treatment effects (Table 1).
Another limitation was that the follow-up of patients was short (10 days); therefore, long-term mechanisms of action were not ascertained. Finally, the life span of the reticulated platelets remains unclear and may be shorter in ITP.
In conclusion, this study highlights that reduced platelet production, as measured by A-IPF, contributes to the thrombocytopenia of ITP, albeit to a greater extent in certain patients than others. Furthermore, the differences in the mechanisms of several treatments to augment or leave unchanged platelet production while increasing the platelet count are well illustrated in these studies. The ability to use A-IPF as an indicator of the thrombopoietic state in real time allows it to provide insight into the mechanisms of treatment effect in patients with ITP and why certain patients may not respond to these treatments. In the future, A-IPF could be used diagnostically (not explored in this study) to identify subgroups of ITP patients with particularly decreased platelet production as a contributing factor to their thrombocytopenia and potentially facilitate individualized treatment selection.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Skerrett and the Cellular Therapy staff at Platelet Disorders Center, Department of Pediatrics, Weill-Cornell Medical College, for their assistance with the Sysmex XE2100 machine.
This work was supported in part by Sysmex XE2100 Corporation, Dana Hammond Stubgen and the Children's Cancer and Blood Foundation (S.J.B., B.P., L.K.P., J.B.B.), and the National Institutes of Health (grant U01 HL072186; J.B.B.).
National Institutes of Health
Authorship
Contribution: S.J.B., B.P., and J.B.B. wrote the manuscript; J.B.B. designed and coordinated the research; S.J.B. and B.P. analyzed the patient data; P.A.S. performed data analysis; L.K.P. and M.M.R. recruited patients and coordinated their sample collection and laboratory investigations; J.T.G. reviewed the bone marrow morphologies and provided background information to enable integration into the study; J.H.B. and M.F. performed plasma glycocalicin investigations; G.O.V. performed the Sysmex XE-2100 studies; T.B.G. performed the platelet kinetic studies in the 2 IVIG-treated patients; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: J.B.B. received research support from Amgen, GlaxoSmithKline, Eisai, Shionogi, Ligand, Cangene, and Sysmex; is a consultant or on the advisory board for Amgen, GlaxoSmithKline, Eisai, Shionogi, Ligand, and Baxter; and is a stockholder in Amgen and GlaxoSmithKline. The remaining authors declare no competing financial interests.
Correspondence: Sarah J. Barsam, Weill-Cornell Medical College, Division of Pediatric Hematology/Oncology, 525 East 68th St, P-695, New York, NY 10021; e-mail: sarahbarsam@gmail.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal