Abstract
The revived interest in (hematopoietic) stem cell (HSC) niches has highlighted the role of multiple cellular players found in the bone environment. Initially focused on the role of osteoblasts and sinusoid endothelial cells, the quest for HSC niche cells has recently focused on a unique role for osteoprogenitor cells (skeletal stem cells, mesenchymal stem cells). Strongly validated by observations of HSC dysregulation dictated by the dysregulation of osteoprogenitors, the role of osteoprogenitors in the HSC niche integrates data from different studies into a unified view. As preosteoblastic, periendothelial cells residing at the sinusoid wall, skeletal progenitors reconcile the notions of “osteoblastic” and “sinusoidal” niches with one another. In addition, they bring into focus the cross-regulation of skeletal and hematopoietic physiology as rooted into the interplay of two stem cells (hematopoietic and skeletal) sharing a single niche. As direct regulators of hematopoietic space formation, sinusoid development, and hematopoietic function(s), as well as direct progenitors of positive and negative regulators of HSCs such as osteoblasts and adipocytes, skeletal progenitors have emerged as pivotal organizers of a complex, highly plastic niche. This development seems to represents an evolutionary advance over the deterministic stem cell niches found in archetypal invertebrate systems.
Introduction
The concept of a “niche” for stem cells is one of several capital foundations that hematology has contributed to stem cell biology. Stated in a simple (but hopefully not simplistic) way, Schofield's original concept1 implies that lifelong hematopoiesis would not occur, despite an inherent potential of stem cells to self-renew for the lifetime of the organism, if the bone marrow structure would not be there. Hematopoietic stem cells (HSCs) may not necessarily behave as stem cells if placed in a specific context provided by something found in bone. As touching the very nature of “stemness,” the quest for that something, and the attention that the concept attracts well beyond hematopoiesis,2-4 is easily understood.
The last decade has witnessed a potent revival of interest in (hematopoietic) stem cell niches,3,5-8 fueled in part by the general significance gained by stem cell biology and in part by the introduction of novel in vivo experimental approaches. This renewed interest is nurtured by the increasing awareness of the major general implications of the niche concept for medicine—that the niche itself is relevant for disease biology and that manipulating the niche is, at least conceptually, a way of manipulating stem cells. The latter, obviously, provides a novel angle on targeting stem cells.9-13
The niche concept is, in and of itself, the concept of a physical entity that embodies and conveys exogenous functional instructions for stem cells, which keep them as stem cells or perhaps even turns cells into stem cells.1,14 Elucidating the code of signals encrypted in the niche is linked to the progressive narrowing of the focus from macroscopic structures (a Drosophila ovary, a mammalian bone) down to molecular effectors, a task facilitated by the definition of a specific instructor cell type that interacts with the pupil stem cell. This leads to postulate, perhaps subliminally, that the global “niche” effect in any given system can be traced to a niche-specific cell, in which to seek the ultimate effectors of extrinsic regulation of stemness. In a simplified view, one niche cell would interact with one stem cell. In a perspective more compliant with the inherent complexity of any stem cell–dependent tissue/lineage, this view can be amended to incorporate a variety, or a hierarchy, of “niche cells” (or microenvironments) matching a variety, or hierarchy, of progenitors and progenies, laid out in a defined spatial order. The Drosophila ovary is commonly seen as a paradigm of a reasonably characterized niche of this kind. Here, a specific cell type (terminal filament cell) in a specific anatomical region (anterior of the germarium) interacts with germline stem cells and keeps them in a stem cell state. Migration of the “stem cell niche” plausibly implies, in a different space, interaction with a different cell, exposure to a different cue, and specification of a cell's fate. The simple spatial order of the ovary and germarium of Drosophila and of their stable anatomy are a blessing to the pursuit of the functional determinants of its niche. In the Drosophila ovary, one might safely state that terminal filament cells (and cap cells) at the anterior of each germarium provide the niche for germline stem cells, which are found exactly and only there. In the mouse BM, the pursuit of a single cell type conveying a “niche” effect for hematopoiesis has uncovered, during the past decade, not one but three anatomical regions (endosteal surfaces, sinusoidal walls, and hematopoietic tissue proper) and four candidate cellular players (osteoblasts, sinusoidal endothelial cells, adipocytes, and stromal cells). The contention made in the following lines is twofold (1) that the apparently nonunivocal,15,16 current definition of a “niche cell,” which admittedly complicates the quest for functional determinants of the niche, can be sharpened by introducing a measure of anatomical clarity in the hematopoietic scene; and (2) that the apparent multiplicity of the landmarks of the scene can be profitably reinterpreted by assuming that the HSC niche does not have a fixed anatomy; that this is the product of a constantly adapting microenvironment; that this adaptation is made possible by a niche for stem cells that is peculiarly made by different stem cells; and that for these reasons the HSC niche is far more sophisticated and modern than any current, general but overreductionistic model of “stem cell niche.”
Anatomy matters
Lifelong hematopoiesis persists only in specific regions of the mammalian BM, not throughout the skeleton. If the niche is what dictates the life-long persistence of hematopoietic activity, it should then be found, in humans or in any mammal, where hematopoietic activity persists life-long. It should be lost, but not irreversibly, in all regions of bone/BM undergoing a timed depletion of hematopoiesis coupled to adipose conversion, such as a mouse tail or a human femoral shaft. In the mouse, the marrow of long (tubular) bones (made of compact cortical bone and medullary cavity) remains hematopoietically active for the animal's lifespan. In humans, in contrast, life-long hematopoiesis becomes restricted, in the third decade, to the axial skeleton and portions of long bone metaphyses, whereas it is reversibly lost in the rest of the marrow. Adipose conversion is reversible, and so is the block of hematopoiesis in yellow marrow. Regional adipose conversion of the marrow would per se suggest that physiologically, the HSC niche might be a flexible entity in mammals.
A number of studies17-20 have highlighted a local enrichment of hematopoietic progenitor and stem cells in mouse endosteal regions. Although this (not undisputed21 ) notion is often taken to surmise a functional role of osteoblasts in hematopoiesis, endosteal regions are unique in other respects as well. For example, they are home to bone-resorbing osteoclasts no less than to bone-forming osteoblasts. As the sites of the distal most ramification of marrow vascularity,22,23 endosteal regions are also enriched in microvessels. They are also the site in which a rich anastomotic network connects the bone and marrow circulation, which may contribute to define a specific ionic environment at the endosteum.24 Therefore, the endosteum is not only the bone surface but also a peculiar circulatory district. Regulation of, or injury to, local circulation might be relevant when interpreting, for example, the patterned homing of blood-borne hematopoietic progenitors in the context of myeloablation (which can induce major remodeling of medullary blood flow) or the lack of a pattern in the lack of myeloablation.25,26
Likewise, alkaline phosphatase–positive stromal cells are also enriched near the endosteum in small mammals,27 as are assayable stromal osteoprogenitors (CFU-fibroblasts [CFU-Fs]),28 which are found even within cortical bone.29 Given this promiscuity of the endosteum, a preferential endosteal localization of hematopoietic progenitors per se does not automatically signify a specific role of one putative “niche” cell over another in the mouse. In humans, the issue is made more complicated by the circumstance that all regions that remain lifelong hematopoietically active feature a cancellous bone architecture. In human cancellous bone, orderly spatial distributions of either hematopoietic or nonhematopoietic cell types, and even a clear-cut definition of endosteal as opposed to central regions, are difficult to grasp in 2-dimensional images because of the 3-dimensional complexity of cancellous bone and the adjacent marrow. This complicates perceiving a patterned distribution of hematopoietic progenitors with respect to histologic landmarks.30 Nonetheless, the distribution of hematopoietic progenitors seems to match, in human as in murine BM, a gradient of microvessel density, peaking close to trabecular surfaces.31
Osteoblasts
Two seminal studies32,33 published in 2003 drew attention to the specific role of osteogenic cells in regulating HSCs. Both studies documented, in vivo through a genetic approach, a correlation between the number of assayable HSCs and the size of a pool of bone cells identified as osteoblastic. The term osteoblast, introduced by Koelliker34 to denote the cell engaged in active bone deposition, has survived in bone biology ever since with precisely the same meaning. Like many cell types of critical physiologic relevance, osteoblasts are still defined by essentially the same original criteria. Markers suited to identify cells of osteoblastic lineage and also to distinguish osteoblasts from other cells of the same lineage are indeed available (reviewed in Gehron Robey and Bianco35 ). However, position, morphology, and bone deposition activity identify osteoblasts in vivo with the stringent accuracy required for clinical assessment (eg, in metabolic bone disease). The link between osteoblast identity and bone (collagen) deposition is reflected in the use, in mouse genetics, of a bone-specific COL1A1 promoter/enhancer (2.3 kb), which provides a reliable way of targeting transgenes specifically to bone-forming osteoblasts (reviewed in Wu et al36 ).
In adult cancellous bone, osteoblasts reside over forming bone surfaces only as long as a single event of bone apposition lasts. Bone apposition occurs throughout bone development and growth. In adult life, it is restricted to specific microscopic regions of bone, which undergo metabolic remodeling. In humans, only 0.1%-7.3% of adult endosteal surfaces are undergoing bone formation (are covered by osteoblasts) at any given time.37 A single remodeling event involves bone formation for a few weeks, then osteoblasts either die or turn into a functionally distinct cell type, no longer engaged in bone formation. Two such postosteoblastic cell types are known—osteocytes, entombed within mineralized bone, and elongated, flat cells called bone-lining cells that cover metabolically inert bone surfaces. Because osteoblasts are transient, if osteoblasts were the (only) key cell type in the HSC niche, then the niche would only exist, at any site at which osteoblasts are found, for the time that marks their very existence and function. After that, the niche would either disappear or migrate to the next distinct site of osteoblastic activity. Alternatively, a “niche” function would be taken over by cells other than osteoblasts at precisely the same site. In the first case, one would have to conclude that the HSC niche (unlike any other niche we know or conceptualize) is transient and migratory rather than fixed. In the second case, one would have to conclude that osteoblasts are not the only niche-making cells, and that the “niche” function is rather provided by a broader spectrum of osteogenic cells. Lending support to the latter view, both murine models that revealed an effect of osteoblast-targeted genes on HSC function also documented significant changes in nonosteoblastic compartments of the osteogenic lineage.32,33,38
Discerning the specific role of osteoblasts proper, as opposed to a broader spectrum of osteogenic cells, has inherent complexities. For example, ablation of osteoblasts by the use of a COL1A1 2.3-kb promoter driving Herpes Simplex Virus Thymidine Kinase39,40 would remove osteoblasts and spare osteoblast progenitors but not postosteoblastic cells; most in vitro studies of osteoblastic cells (reviewed in Taichman41 ) would necessarily include the evaluation of cells of genuine osteogenic lineage, which, however, may not fully duplicate, in culture, the in vivo properties of osteoblasts. Nonetheless, studies who focused on osteoblasts established the firm notion that cells of osteogenic lineage regulate HSC function and numbers (and also highlighted their specific involvement in B lymphopoiesis42,43 ), leaving, however, the identity of the specific differentiation/maturation or functional stages involved open to refinement.36
Osteoblasts are but one stage in the osteogenic lineage, which includes preosteoblastic and postosteoblastic stages (reviewed in Gehron Robey and Bianco35 ). Osteocytes and bone-lining cells are postosteoblastic. Skeletal progenitors found in BM are, of course, preosteoblastic. Clonogenic skeletal progenitors are able to give rise to osteoblasts, adipocytes, fibroblasts, and hematopoietic supportive stroma in vivo, with no need of previous BMP-mediated induction ex vivo. Stringently assayed in vivo by transplantation assays, this native osteogenic potential is restricted to skeletal progenitors found in bone and BM and not duplicated in cells with a similar in vitro phenotype derived from nonbone tissues. As reflected by the constitutive expression of the osteogenic master gene Runx2, postnatal skeletal progenitors are, in humans44 and mice,45 committed to skeletal lineages (skeletal stem cells46 ) and are identified in humans by a surface phenotype, including expression of MCAM/CD146, distinct from that of mature osteoblasts.44
Discrete steps in the osteoblast lineage are not as sharply defined as for other systems, notably hematopoiesis. Although commonly attributed to a lesser proficiency of the bone field to define and use specific markers, this is also due to the lack of a mitotic barrier between many distinct cell types within the osteogenic lineage. Many of these represent maturation or modulation of phenotypes rather than irreversible differentiation.47 Distinct cell phenotypes can share portions of differentiative programs and functions (eg, production of certain hematopoietic cytokines and chemokines), while diverging in others (eg, matrix production, adhesion molecules), to such an extent as to be perceived as distinct cell types. Plasticity of phenotypes within the bone cell lineage is extensive.
Sinusoids
While evidence was accumulating for a crucial role of osteogenic cells in regulating HSCs, strong evidence also pointed to sinusoidal walls as a niche for HSCs.48 BM sinusoids are > 50-μ wide, thin-walled blood vessels in which arterioles open directly. Lined by endothelial cells and covered by adventitial reticular cells on ∼60% of their abluminal surface, sinusoid walls are traversed by hematopoietic cells in both directions (blood to marrow and vice versa). A sinusoidal, rather than capillary, type of microcirculation is not unique to BM and is found in other hematopoietic organs, such as the liver and spleen. Sinusoids form in the nascent bone marrow cavity49 while the cavity is being excavated by osteoclasts. Osteogenic cells direct the formation of the cavity by driving the generation of osteoclasts through receptor activator of nuclear factor kappa-B ligand (reviewed in Boyle et al50 ). The creation of space by osteoclasts might be directly related to the conversion of nascent blood vessels into large sinusoids. Sinusoid formation is a crucial prerequisite for establishment of hematopoiesis, both in development and in heterotopic transplantation systems that recapitulate the ontogeny of the bone/BM organ in vivo.44,51,52 Interestingly, repair of radiation-induced damage to sinusoids is also a prerequisite for hematopoietic reconstitution after myeloablation and BM transplantation in mice.53 The mechanisms mediating the formation of sinusoids, rather than capillaries, in BM remain unknown and might be sought in the context of the close association of sinusoid formation with osteoclast activity. For example, proinflamamtory cytokines that regulate osteoclasts, also induce, in BM stromal cells, the production of NO,54 which mediates their immune modulatory functions,54 but likely also vasodilation. Intriguingly, NO also mediates the emergence of HSCs from the hemogenic endothelium of the embryonic aorta-gonad-mesonephron region, on establishment of circulation,55 and may be involved in stem cell mobilization.56 Blood flow is slow in the large sinusoids and permits the margination of blood-borne cells, which can then interact with endothelial cells via specific adhesion molecules,57-59 negotiate the sinus wall, and lodge into the extravascular, hematopoietic space. As seen by in vivo imaging of the intact BM, systemically injected HSCs or cancer cells rapidly lodge into the immediate perivascular space around specific domains of the BM sinusoidal network, noted for the high expression of stromal cell–derived factor-1 and E-selectin, where they are retained at least for several weeks.60
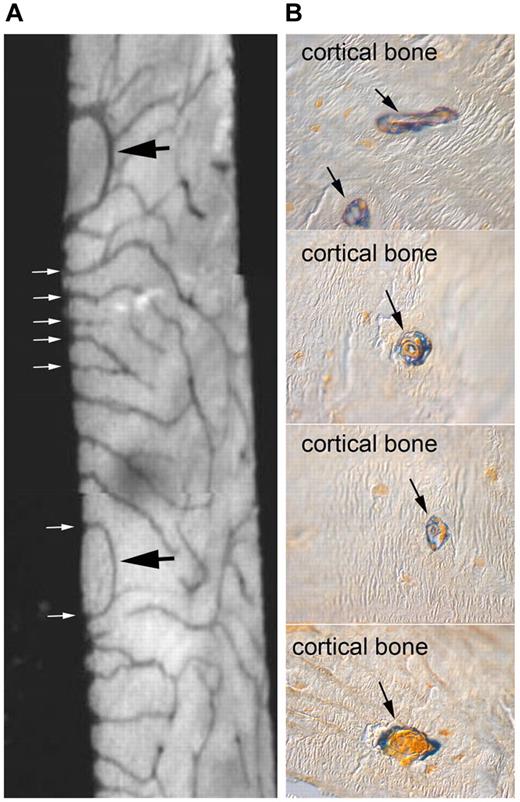
Per se, the notion of a sinusoidal niche would reconcile the bone niche with nonbone niches (such as liver and spleen) that operate in specific (developmental, pathologic, or experimental) circumstances. It would also preserve the flavor of a kinship between hematopoiesis and vascularity that echoes the very inception of blood cell formation in Wolff islands and the origin of HSCs from a hemogenic endothelium in the aorta-gonad-mesonephron.61 However, the apparent existence of two niches for HSCs in postnatal bone, each linked to a cell type of distinct lineage and function (osteoblasts vs endothelial cells), calls for a plausible reconciliation.15,16 Given the wealth of sinusoids running close to endosteal surfaces, viewing bone surfaces and sinusoidal surfaces as segregated anatomic sites could be misleading.62 Furthermore, bone and marrow vascularity are widely interconnected. Intraosseous blood vessels (which exist even within individual bone trabeculae) communicate with marrow vessels (Figure 1A); they run within spaces continuous with the marrow cavity, and yet not populated by hematopoiesis; and they are coated with quiescent osteogenic cells (Figure 1B), similar to, and continuous with, the bone-lining cells of endosteal surfaces.
Internal vascularity of rodent bone, its connection with the marrow cavity, and its association with osteogenic cells. (A) Contact microradiograph of a 50-μ section of an 8-week-old rat tibia demonstrating a rich network of intracortical vessels opening in the marrow at the endosteal surface (white arrows). Also note individual vascular spaces looping within bone and reentering the BM (black arrows). (B) Low-temperature processed, glycol methacrylate-embedded, 4-μ thick undecalcified sections of an 8-week-old wild-type mouse tibia stained for ALP activity (blue). Note that intracortical vessels are coated with an adventitial layer of ALP-positive cells.
Internal vascularity of rodent bone, its connection with the marrow cavity, and its association with osteogenic cells. (A) Contact microradiograph of a 50-μ section of an 8-week-old rat tibia demonstrating a rich network of intracortical vessels opening in the marrow at the endosteal surface (white arrows). Also note individual vascular spaces looping within bone and reentering the BM (black arrows). (B) Low-temperature processed, glycol methacrylate-embedded, 4-μ thick undecalcified sections of an 8-week-old wild-type mouse tibia stained for ALP activity (blue). Note that intracortical vessels are coated with an adventitial layer of ALP-positive cells.
Still, the wealth of HSCs residing over sinusoids, within marrow space proper, would seem difficult to reconcile with an exclusive endosteal niche even if incorporating endosteal sinusoids. By analogy with other systems (eg, the Drosophila ovary) one could then postulate a dual set of instructive cues embodied in a dual niche, each made by a specific cell type.16 Endosteal and sinusoidal niches would then be serially spaced microenvironments conveying distinct cues (eg, quiescence or self-renewal vs proliferation and differentiation). However, given the frequent close proximity of sinusoids and endosteal surfaces, one would then have to postulate that two opposing instructions could be delivered by spatially overlapping microenvironments. Alternatively, one could postulate a single type of niche, distributed either at endosteal surfaces, or away from them, and yet endowed, at either location, with a similar set of support cells.
Osteoprogenitors over sinusoids
BM sinusoids are partially coated with stromal cells classically known as adventitial reticular cells (ARCs)63 and long noted for the expression of alkaline phosphatase, a hallmark of osteogenic cells.27,64 ARCs are direct precursors of marrow adipocytes,23,63,65 which develop around sinusoids in the postnatal marrow. Recently, they were identified in humans as the in vivo counterpart of the clonogenic (CFU-F),44 multipotent stromal cells first identified by Friedenstein66 in a variety of species and later renamed “mesenchymal stem cells” by others (reviewed in Bianco et al67 ). More accurately referred to as skeletal stem cells (reviewed in Bianco et al67 ), phenotype-purified human CFU-F/ARCs are able to generate osteoblasts and adipocytes and to self-renew into new adventitial reticular cells and CFU-Fs in vivo, which substantiates a bona-fide stem cell nature of skeletal progenitor/stem cells found in BM (the “mesenchymal” stem cells of a copious literature44,67 ).
Notably, skeletal stem cells are also able to establish a functional hematopoietic microenvironment (HME) on transplantation at heterotopic sites.44 In skeletal stem cell–generated heterotopic ossicles, host blood vessels grow,44 and blood borne HSCs home,68,69 establishing a chimeric “ossicle.” In the same experimental system, mesenchymal cells from nonbone tissues fail to establish bone and hematopoiesis,44 whereas cell populations competent to establish osteoblasts and bone per se may not be sufficient to establish hematopoiesis.44,70 Because osteoprogenitors reside at the abluminal surface of sinusoids, the duality of sinusoidal and osteoblastic niches can be reconciled anatomically. The sinusoidal wall is not formed solely by endothelial cells. A nonosteoblastic, and yet preosteoblastic, and nonendothelial, and yet periendothelial cell resides at the abluminal surface of sinusoids in the human BM in vivo. These data make the sinusoidal wall the site of residence of two stem cells, and a crucial node of two interacting stem cell–dependent systems—hematopoiesis, as they host a putative niche for HSCs, and the skeletal scaffold in which hematopoiesis takes place, as they host a putative niche for skeletal progenitors.44
In addition to genes characteristic of microvascular cells and of osteogenic commitment, human skeletal stem cells (BM “mesenchymal stem cells”) express high levels of genes implicated in regulation of HSCs in the mouse, including JAGGED-1, N-CADHERIN, and CXCL 12.44 CXCL 12-abundant reticular (CAR) cells in the murine BM are involved in HSC regulation,71 and like human CFU-F/ARCs,44 represent direct progenitors of osteoblasts and adipocytes,72 suggesting that human ARCs44 and murine CAR cells71 represent equivalent populations. Like the human BM “mesenchymal stem cells,”44 “mesenchymal stem cells” (Nestin+) in the murine BM are clonogenic, multipotent, self-renewing, perivascular, and can regulate HSCs.73 Apparently, both in humans and mice, perisinusoidal “reticular” cells (human ARCs, murine CAR cells, murine Nestin+ cells) coincide with osteoprogenitors/“mesenchymal”/skeletal stem cells and play a significant role in regulating HSCs.
Why are osteogenic cells associated with blood vessels in bone and BM and not restricted to bone surfaces? In all growing tissues, mesenchymal cells are recruited to the neighboring nascent blood vessels as pericytes/mural cells via specific signaling loops.74,75 Endothelial cell–derived PDGF-BB recruits PDGF-Rβ–expressing mesenchymal cells to a pericyte fate.76 TGF-β, activated by localized protease activity at the endothelial/mural cell interface,74,77 induces mitotic quiescence and stabilizes both mural and endothelial cells.78 In developing bone and BM, blood vessels develop and grow within an atmosphere of committed osteogenic cells.79 The recruitment of presumptive mural cells to nascent blood vessels thus involves, in the developing bone/BM environment, committed osteoprogenitors, which associate with blood vessels and originate from the primitive perichondrium.49,80 Presumptive pericytes in every developing tissue also express angiopoietin-1, which signals through Tie-2 expressed in endothelial cells and is indispensable for vessel stabilization and integrity and for pericyte recruitment.81 Angiopoietin-1 is abundantly produced by perisinusoidal osteoprogenitors in human44 and murine73 BM and retains HSCs (which express Tie-2) in a quiescent state, contributing to a niche effect.82 The association of sinusoids with osteogenic cells recruited to a mural cell position is not merely anatomical. In heterotopic transplants in immunocompromised mice, human osteoprogenitors guide the formation of sinusoids from ingrowing host neovessels, mapping the sites where hematopoiesis will appear thereafter.44 Interestingly, when “mesenchymal stem cells” are cotransplanted83 with endothelial cells along with soft scaffolds, functional blood vessels are assembled in which mesenchymal stem cells are incorporated in a mural cell position.83
What do osteoprogenitors do in their existence as mural cells? First, they are themselves retained in a quiescent state, that is, they exploit the microvascular niche themselves.44,73 Second, they establish transvascular gradients of diffusible factors, such as CXCL 12, that facilitate the homing of blood-borne hematopoietic progenitors and hematopoiesis itself. Third, they represent the first-line extravascular environment seen by immigrant blood borne cells, with which they establish cell-cell contacts,46 which are integral to a niche effect.84 In every tissue, mural cells responsive to β-adrenergic signaling contribute to regulate vessel caliber. β-adrenergic signaling modulates both bone formation and osteoprogenitor-driven osteoclastogenesis and integrate endocrine and neural control of both functions.85 In addition, β-adrenergic signaling controls circadian changes in the mobilization of HSCs from BM in the mouse.86-88 In the mouse, the clonogenic osteoprogenitor cells associated with the marrow microvessels respond to β-adrenergic signaling and functionally interact with HSCs.73 Thus, β-adrenergic responses in sinusoidal osteoprogenitors could integrate the control of bone metabolism and hematopoiesis. Consistent with its general physiologic role, β-adrenergic responses in a mural cell in the bone vascularity would also control the local circulatory system, allowing for local changes in blood flow within and between medullary and intraosseous vascular domains.
Adipocytes
Uniformly distributed throughout the skeleton at birth, postnatal hematopoiesis in humans progressively restricts, upon a person's growth, to the cancellous bone of the axial skeleton, girdle bones, and some areas of the metaphyses89 (Neumann's law22,90,91 ). The rest of the BM undergoes adipose conversion (ie, “red” marrow turns into “yellow” marrow). Within red marrow, the volume taken up by adipocytes fluctuates in parallel to changes in hematopoiesis. Like a mouse's tail, specific regions of the human skeleton (short bones of hands and feet, epiphyses and apophyses of long bones) undergo adipose conversion early in the postnatal life. Depletion of hematopoiesis within yellow marrow would imply that either stem cells are retained in a quiescent state while the proliferative function of downstream progenitors is inhibited, or that stem cells are themselves depleted or inhibited. In the mouse, the latter circumstance does apply.92 Hence, if adipocytes are active inhibitors of HSC function,92 then adipose conversion of BM can be seen as a physiologic change in the “niche” that affects the physiology of HSC in defined regions of the skeleton.
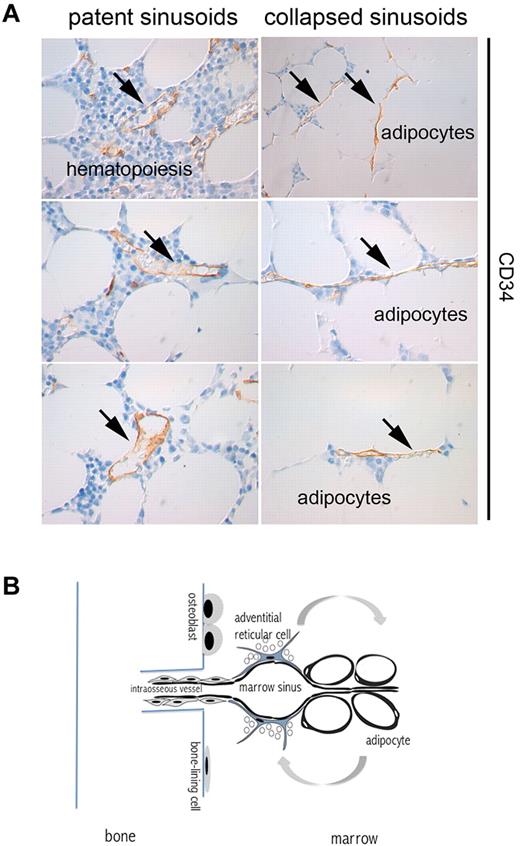
BM adipocytes develop postnatally from adventitial reticular cells.23,63 The ablation of hematopoiesis is followed by rapid accumulation of lipid within ARCs in red marrow, which turn into adipocytes.65 Because ARCs are physically associated with the ablumenal aspect of the sinusoid wall, the massive adipocytes that replace the slender reticular cells compress the sinus wall, resulting in a reduction of the sinus caliber. Individual sinusoids may thus collapse and be excluded from blood flow, while retaining an intact, complete endothelial wall (Figure 2). Outside of BM, primary microvascular networks are remodeled and simplified, following angiogenesis, by vascular pruning,93 and it is the recruitment of mural cells that specifies individual vessels that will be retained rather than irreversibly lost.
“Pruning” of sinusoids in adipose marrow. (A) CD34-immunolabeled sinusoids as seen in red and yellow areas of human iliac crest bone marrow. The lumen of sinusoids (arrows) running among hematopoietic areas is patent; the lumen of sinusoids running among adipocytes is virtual. (B) The connection of marrow vascularity to bone and fat. Marrow vascularity communicates with bone vascularity. Marrow adipogenesis involves adventitial stromal cells and is coupled with changes in sinusoid caliber.
“Pruning” of sinusoids in adipose marrow. (A) CD34-immunolabeled sinusoids as seen in red and yellow areas of human iliac crest bone marrow. The lumen of sinusoids (arrows) running among hematopoietic areas is patent; the lumen of sinusoids running among adipocytes is virtual. (B) The connection of marrow vascularity to bone and fat. Marrow vascularity communicates with bone vascularity. Marrow adipogenesis involves adventitial stromal cells and is coupled with changes in sinusoid caliber.
Adipogenesis at the sinusoid wall in BM can thus be seen as a unique kind of reversible microvascular pruning. With the loss of lipids through lipolysis, adipocytes would release space, sinusoids would dilate, and blood flow would resume in a given microscopic region of BM. On a larger scale, this would allow for conversion of yellow marrow into red marrow, as seen in response to hemolytic anemia or bleeding. Mechanisms mediating lipolysis specifically in marrow adipocytes have not been elucidated. In general, however, lipolysis in fat cells is mediated by cAMP and β-adrenergic signaling,94 which also regulates blood flow in microvessels in other tissues.
Osteoprogenitors and hematopoietic disorders
Prior to the dawning of the potential significance of osteoprogenitors proper for the “niche,” the significance of the “niche” for hematopoietic disease had not escaped attention. Even so, the dominant view of the HME has long remained that of an innocent bystander. Intuitively, the “niche” could be hijacked (or altered) by aliens such as leukemic or solid cancer (stem) cells,95-98 resulting in marrow substitution with leukemia or metastasis. Likewise, abnormal expansions of hematopoiesis would impress changes in the bone-BM structure, as classically recognized for congenital hemolytic anemias or idiopathic myelofibrosis.91 In primary myelofibrosis, expansion of the CD146+ osteoprogenitor pool correlates with neoangiogenesis and osteomyelosclerosis,99 highlighting the response of osteoprogenitors to abnormal hematopoiesis, and their role in organizing an abnormal microenvironment. However, changes in osteoprogenitors and the HME can be primary and cell-autonomous and extend their effects secondarily to hematopoiesis and HSC regulation. Human osteoprogenitors carrying activating mutations of the Gsα gene (which cause fibrous dysplasia, OMIM 174800) generate an abnormal HME on heterotopic transplantation in mice, recapitulating the changes (fibrosis, lack of adipocytes, localized absence of hematopoiesis) found in the natural disease.100 Consistent with the role of Gsα activity in parathyroid hormone/parathyroid hormone-related protein (PTH/PTHrP) signaling, these changes mimic the effects of excess PTH/PTHrP signaling in humans101 and mice.38 A crucial regulator of skeletal development and metabolism, PTH/PTHrP signaling regulates the organogenesis of BM, the organization of the HSC “niche,”32 and the population kinetics of both skeletal70 and HSCs.32 In mice, genetic changes in the HME can result in a myeloproliferative phenotype,102 and disruption of micro-RNA regulation in osteoprogenitors alters the conservative kinetics of HSC self-renewal, leading to a myelodysplastic phenotype.103 Thus, changes in the niche as a function, and in osteoprogenitors as its substrate, may be inscribed in a path to tumorigenesis in mammalian hematopoiesis. Consistent with earlier evidence for the development of germ cell “tumors” in Drosophila ovaries as a result of overexpression of decapentaplegic, a specific determinant of the specific niche,104 these data provide the strongest evidence for the role of osteoprogenitors as providers of a “niche” effect and for the significance of the latter in disease.
A stochastic niche and its evolutionary advantage
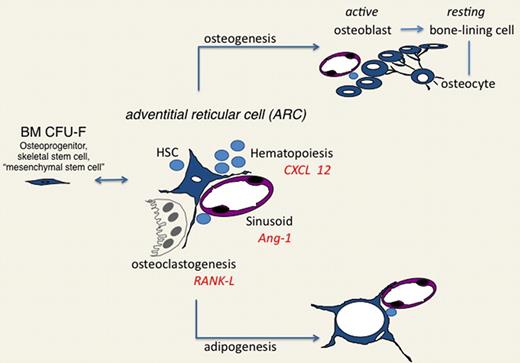
The notion that an osteoprogenitor cell rather than an osteoblast may establish an HSC “niche” in BM might, at a glance, seem to add just another candidate to the list of putative “niche” cells. In contrast, it allows reconciliation of the multiplicity of candidates into a unified view. Osteoprogenitors (skeletal stem cells, BM-mesenchymal stem cells) coincide with adventitial stromal cells, generate osteoblasts and adipocytes, and organize the BM sinusoidal network. Hence, all “niche” effects thus far ascribed to osteoblasts, stromal cells, adipocytes, and sinusoidal walls, respectively, could be seen as rooted into the pivotal role of a single cell type acting as a dynamic organizer of a complex system of effector cells, conveying diversified instructive cues to HSCs and their progeny but remaining dynamically connected to one another within a single regulated stromal lineage (Figure 3).
Adventitial reticular cell as the central organizer of the hematopoietic microenvironment/niche. The in situ counterpart of the BM CFU-F (also known as an osteoprogenitor, skeletal stem cell, or mesenchymal stem cell), ARCs, direct formation of cavities through regulation of osteoclastogenesis; contribute to the development of sinusoids, with which they associate; interact with HSC and other hematopoietic cells; and generate osteoblasts and adipocytes, which in turn embody different functional cues for HSCs. ARCs thus integrate the regulation of hematopoiesis with the local, hormonal, and neural control of skeletal growth, remodeling, and circulation.
Adventitial reticular cell as the central organizer of the hematopoietic microenvironment/niche. The in situ counterpart of the BM CFU-F (also known as an osteoprogenitor, skeletal stem cell, or mesenchymal stem cell), ARCs, direct formation of cavities through regulation of osteoclastogenesis; contribute to the development of sinusoids, with which they associate; interact with HSC and other hematopoietic cells; and generate osteoblasts and adipocytes, which in turn embody different functional cues for HSCs. ARCs thus integrate the regulation of hematopoiesis with the local, hormonal, and neural control of skeletal growth, remodeling, and circulation.
The interaction of different stem cells in “niche” systems portrayed in the mammalian BM might be more common and have broader general relevance than currently appreciated. In the Drosophila testis, for example, progenitors of the somatic cyst cells form a niche for germline stem cells.105 The notion that it is a skeletal progenitor that establishes the “niche” for hematopoiesis also seems to encourage a fresh look at the hematopoietic niche itself. A fixed anatomy is an a priori of our view of stem cell niches across the animal kingdom. This is the case not only for the archetypal model systems found in Drosophila104,105 or Caenorhabditis elegans,106 but also for mammalian systems (hair follicles, the intestinal crypts, the subventricular zone of mouse brain3 ). In addition, most defined niche systems are built as a multiplicity of units working in parallel, reflected in a multiplicity of units of renewing tissue.4 A fixed anatomy and a system of equivalent structural units are (so far at least) the specific missing elements in our way to conceive the HSC niche. Endosteal surfaces are either forming, resorbing, or quiescent; osteoblasts, adipocytes, and stromal cells can be perivascular or endosteal, and all wax and wane; sinusoids and bone cavities open and close, blood flow changes paths and routes and speed through bone and marrow. Bone is rigid, but the bone niche is plastic. Blood, itself, is fluid, and HSCs are mobile and blood borne. If no bone and no marrow cavity form at all, hematopoiesis is altered, but not blocked, and relocates to other organs, as illustrated in the osteoblast-free, boneless, marrowless Runx2−/− mice.107
Mobile HSCs choose bone, then, on a competitive basis. The competitive advantage of bone over other options relies on circulation, and rests in part, surprisingly, with its plasticity, which would not be there if a unique system of plastic cellular stroma had not evolved with it. BM hematopoiesis is evolutionarily recent: postnatal hematopoiesis settles in BM only in amphibians, becomes entirely extravascular only in mammals (although extravascular hematopoiesis first appears in Ganoid fishes,108 in meninges rather than bone), and is exclusive to BM only in primates. It is tempting to speculate that the deterministic nature of the stem cell niche portrayed in the beautiful fixed order of invertebrate systems may be superseded by the modern emergence, in vertebrate BM, of a stochastic niche blinking across volumes and surfaces as vast as the skeleton. This system meets the requirements of a blood-borne stem cell and a fluid tissue subjected to an extraordinarily complex interplay of local and systemic regulatory inputs. This kind of a niche would best suit the need to integrate the “niche” effect with the intrinsic determinants of HSC self-renewal, their own regulation, and the contribution of population-based mechanisms to HSC self-renewal.2,109 By emancipating the function of the HSC niche from a fixed anatomy and a single cell basis, the link between the stromal system progenitor and the HSC niche posits an attractive intellectual challenge, while changing the story of bone and blood as two strange bedfellows into a tale of two stem cells.
Acknowledgments
Personal work cited here was supported in part by grants from AIRC, Telethon GGP09227, the European Union, Fondazione Roma, and the Ministry for University and Research of Italy.
Authorship
Contribution: P.B. wrote the manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interest.
Correspondence: Professor Paolo Bianco, Dipartimento di Medicina Molecolare, Sapienza Universita' di Roma, Viale Regina Elena 324, 00161 Roma, Italy; e-mail: paolo.bianco@uniroma1.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal