Antibodies against factor VIII cause severe bleeding, requiring therapy that circumvents the normal coagulation pathway. In this issue of Blood, Waters and colleagues report that a novel RNA polymer can block an inhibitor of tissue factor, unleashing native procoagulant activity that stops the bleeding.1
Severe bleeding in normal subjects follows trauma such as gunshot wounds and blast injuries. In patients with hemophilia, severe bleeding can occur after minimal trauma such as climbing stairs, bumping against a friend, or twisting a leg while turning in bed. Medical intervention for severe bleeding includes delivery of blood coagulation proteins. Factor VIII is infused to patients with hemophilia A; factor IX into patients with hemophilia B. Normal subjects who have suffered trauma and hemophilia patients who have developed antibodies against factor VIII or factor IX require additional therapy to stop bleeding. Until now, activated coagulation proteins have provided the only option.
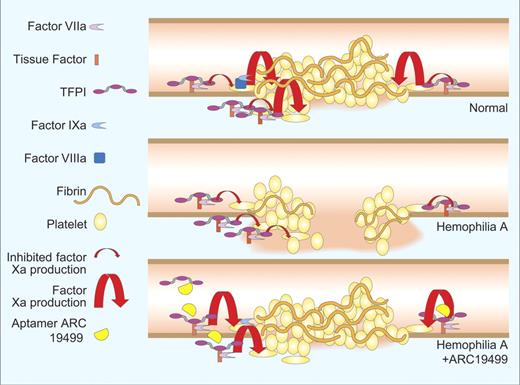
Bleeding is quenched by cooperative activity of blood platelets and coagulation proteins (see figure). The platelets recognize the vascular injury and adhere to proteins of the vascular matrix, to one another, and to endothelial cells. Tissue factor in subendothelial tissues binds to factor VIIa in blood producing a catalytic complex that activates factor X and factor IX. This leads to generation of the enzyme complexes of the coagulation cascade and thrombin production. Thrombin further stimulates platelets2,3 and endothelial cells as well as converting fibrinogen to fibrin. Fibrin provides a positive feedback signal to platelets4 and forms polymers that stabilize the platelet plug.
Bleeding from a normal vessel (top) is quenched by a platelet plug stabilized by fibrin strands. Bleeding continues from a hemophilia vessel (middle) because TFPI blocks factor Xa production leaving inadequate factor Xa to drive fibrin formation or platelet activation. Aptamer ARC19499 inhibits tissue factor pathway inhibitor (bottom) enabling the factor VIIa-tissue factor complex to produce sufficient factor Xa to stabilize the platelet plug. Professional illustration by Paulette Dennis.
Bleeding from a normal vessel (top) is quenched by a platelet plug stabilized by fibrin strands. Bleeding continues from a hemophilia vessel (middle) because TFPI blocks factor Xa production leaving inadequate factor Xa to drive fibrin formation or platelet activation. Aptamer ARC19499 inhibits tissue factor pathway inhibitor (bottom) enabling the factor VIIa-tissue factor complex to produce sufficient factor Xa to stabilize the platelet plug. Professional illustration by Paulette Dennis.
It is well known that severe bleeding in patients with hemophilia is caused by deficiency of either factor VIII or factor IX. Perhaps less well known is that severe hemophilia bleeding also requires the presence of a potent anticoagulant protein called tissue factor pathway inhibitor (TFPI). TFPI exerts its anticoagulant activity by simultaneously inhibiting factor VIIa and factor Xa.5 Thus, under normal conditions when TFPI is present, blood clotting proceeds through activation of factor IX by tissue factor-factor VIIa. Factor IXa then combines with factor VIIIa to activate factor X, bypassing the inhibitory activity of TFPI. Inhibitors of TFPI activity allow for blood clotting to occur via activation of factor X by tissue factor-factor VIIa without the need for factors VIII or IX and have been shown to restore thrombin generation and hemostasis in animal models of hemophilia.6,7
Here, Waters and coworkers describe a novel approach to the problem of excessive bleeding. Using state-of-the-art technology they have produced a polymer of chemically modified RNA units (aptamer) that binds specifically to TFPI. Thus, binding of this aptamer largely inhibits TFPI, increasing the production of factor Xa from the factor VIIa-tissue factor complex (bottom panel of figure). They have shown that enough factor Xa is produced to stop bleeding in animals lacking factor VIII function.
ARC19499 appears to be a promising candidate for human trials, particularly in hemophilia patients who have developed inhibitory antibodies to factor VIII or factor IX. Its ultimate success will depend on its effectiveness, safety, and convenience compared with the activated coagulation proteins that are currently infused into bleeding patients.
Conflict-of-interest disclosure: A.E.M. receives research grant funding from Novo Nordisk. G.E.G. declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal