Abstract
Adhesive interactions between circulating sickle red blood cells (RBCs), leukocytes, and endothelial cells are major pathophysiologic events in sickle cell disease (SCD). To develop new therapeutics that efficiently inhibit adhesive interactions, we generated an anti–P-selectin aptamer and examined its effects on cell adhesion using knockout-transgenic SCD model mice. Aptamers, single-stranded oligonucleotides that bind molecular targets with high affinity and specificity, are emerging as new therapeutics for cardiovascular and hematologic disorders. In vitro studies found that the anti–P-selectin aptamer exhibits high specificity to mouse P-selectin but not other selectins. SCD mice were injected with the anti–P-selectin aptamer, and cell adhesion was observed under hypoxia. The anti–P-selectin aptamer inhibited the adhesion of sickle RBCs and leukocytes to endothelial cells by 90% and 80%, respectively. The anti–P-selectin aptamer also increased microvascular flow velocities and reduced the leukocyte rolling flux. SCD mice treated with the anti–P-selectin aptamer demonstrated a reduced mortality rate associated with the experimental procedures compared with control mice. These results demonstrate that anti–P-selectin aptamer efficiently inhibits the adhesion of both sickle RBCs and leukocytes to endothelial cells in SCD model mice, suggesting a critical role for P-selectin in cell adhesion. Anti–P-selectin aptamer may be useful as a novel therapeutic agent for SCD.
Introduction
Sickle cell disease (SCD) is caused by a point mutation of the β-globin chain, but its pathophysiology is extremely complex and heterogeneous. A salient clinical feature of this disorder is vaso-occlusive crisis, which is a major cause of morbidity and mortality in SCD patients; repetitive crises could eventually lead to multiorgan damage in the long term.1 Adhesive interactions between circulating sickle red blood cells (RBCs), leukocytes, and endothelial cells have been implicated as critical pathologic events for the development of vaso-occlusion. Much attention has been directed to identifying adhesion molecules involved in cell-cell interactions.
Endothelial cell P-selectin, a member of the selectin family of cell adhesion molecules,2 plays a key role in leukocyte recruitment as well as the adhesion of sickle RBCs to the endothelium.3,4 Presynthesized P-selectin is stored in the Weibel-Palade bodies in endothelial cells and rapidly translocated to the cell surface in response to extracellular stimuli such as hypoxia.5 Expression levels of P-selectin are elevated in patients with SCD.6,7 The interactions between P-selectin and its ligands are likely to contribute to cell adhesion between multiple types of cells, which results in the impairment of microvascular circulation presumably involved in the development of painful vaso-occlusive episodes.4,8 Several antiadhesion compounds have been tested for their ability to inhibit sickle RBC adhesion to endothelial cells, however, no agents are currently used for the treatment of patients with SCD.9
Aptamers are short, single-stranded oligonucleotides that are capable of binding to proteins and small-molecule targets by complementary shape interaction with high affinity and specificity.10 Aptamers can be isolated by an automated in vitro selection process known as systemic evolution of ligands by exponential enrichment procedure (SELEX).11 Rapid discovery and optimization of therapeutic properties (eg, manufacturability, potency, metabolic stability, and pharmacokinetics) makes aptamers a very efficient and cost-effective technology among inhibitor-based approaches to various disorders. Moreover, aptamers have low toxicities, do not elicit immunogenic response, and have half-lives ranging from very short to very long, allowing them to be used for both acute and chronic indications.10 Recently, an RNA aptamer directed against vascular endothelial growth factor was approved for the therapy of age-related macular degeneration.10,12 Other aptamers are under clinical trials for patients with hematologic and cardiovascular diseases.12,13
In this study, we generated an anti–mouse P-selectin aptamer and investigated its effects on cell adhesion in SCD model mice using intravital microscopy. More specifically, we examined whether the anti–P-selectin aptamer can inhibit the adhesion of sickle RBCs and leukocytes to vascular endothelial cells in the bone marrow microvasculature of SCD model mice that were exposed to hypoxic stress.
Methods
Aptamers
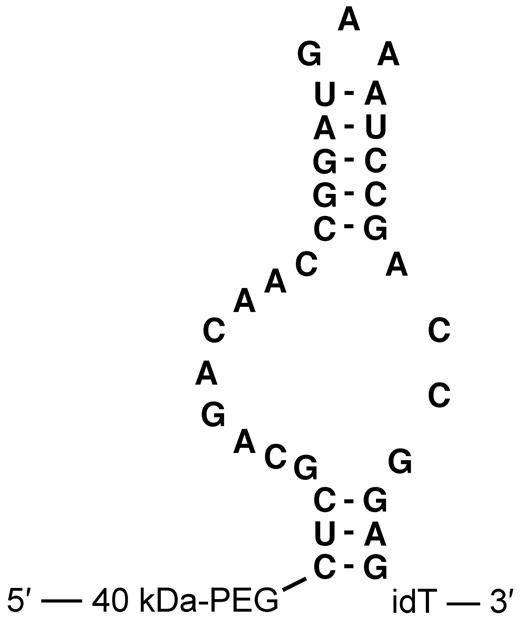
The anti–mouse P-selectin aptamer ARC5690 was generated by systemic evolution of ligands by exponential enrichment procedure11 using recombinant mouse P-selectin as a target for selection. ARC5690 is a 33-mer oligonucleotide conjugated at the 5′-terminus with a 40-kDa branched polyethylene glycol (PEG) group to inhibit renal filtration and extend the aptamer's plasma half-life. The proposed secondary structure of ARC5690 is shown in Figure 1. The target binding domain of ARC5690 for P-selectin consists of a 32-residue oligonucleotide with 2′-fluoro pyrimidine and 2′-methoxy purine monomer units that are resistant to nuclease-mediated attack. The molecule was synthesized with an additional nucleotide at the 3′-terminus, an “inverted” deoxy-thymidine conjugated by a 3′-3′ linkage, to further stabilize the sequence against 3′-5′ exonucleases. A “scrambled” version of the aptamer, ARC5694, was designed for the use as a negative control molecule with 10 nucleotide substitutions incorporated to disrupt the murine P-selectin recognition site while preserving the putative aptamer secondary structure. The nucleotide sequences of ARC5690 and ARC5694 are shown in supplemental Figure 1 (available on the Blood Web site; see the supplemental materials link at the top of the online article). ARC5690, including 5′-hydroxyl and 5′-biotin derivatives lacking the PEG group, which were used for nitrocellulose filtration assay and surface plasmon resonance assay, respectively as stated below, and scrambled aptamer ARC5694, were synthesized, purified and dissolved in 0.9% saline.
Primary sequence and proposed secondary structure of anti–mouse P-selectin aptamer ARC5690. ARC5690 is with 40-kDa PEG. idT, inverted 2′-deoxy-thymidine.
Primary sequence and proposed secondary structure of anti–mouse P-selectin aptamer ARC5690. ARC5690 is with 40-kDa PEG. idT, inverted 2′-deoxy-thymidine.
SCD mice
Knockout-transgenic SCD model mice (SJL, 129, C57BL/6J background) were kindly provided by Dr. Thomas Ryan (University of Alabama at Birmingham, Birmingham, AL).14 Genetically, homozygous SCD mice are deleted for the murine α- and β-globin alleles and carry transgenes encoding the human α-, β-, and γ-globin gene sequences. Specifically, one transgene construct consisted of a 22-kb human β-globin locus control region (LCR) that is linked to a 9.7-kb DNA fragment containing the Aγ-globin and βS-globin genes. The other construct included the locus control region linked to a 3.8-kb fragment that contains the human α1-globin gene. SCD mice were bred with C57BL/6J mice through multiple rounds to maintain the C57BL/6J genetic background and housed at the animal facility at the Medical College of Georgia. All mouse studies were approved by the Institutional Animal Care and Use Committee of the Medical College of Georgia.
Nitrocellulose filtration assay
Recombinant protein chimeras consisting of the extracellular domains of mouse P-, E- or L-selectin fused to the Fc domain of human immunoglobulin G1 (IgG1) were purchased from R&D Systems. Recombinant human P-selectin fused to human Fc was also purchased from R&D Systems. ARC5690 and ARC5694 prepared with 5′-terminal hydroxyl groups (5-OH) were 5′-end–labeled with [γ-32P] adenosine-5′-triphosphate (ATP) using T4 polynucleotide kinase (New England BioLabs) by standard protocols. Conjugation with 40 kDa PEG precluded 5′-end-labeling and interfered with the nitrocellulose filtration assay, so the PEG component of ARC5690 and ARC5694 was not included in this analysis. 32P-labeled aptamers were combined with excess mouse P-selectin (1pM-100nM), E-selectin (8pM-500nM), L-selectin (8pM-500nM), or human P-selectin (8pM-500nM) and allowed to equilibrate at 37°C for 30 minutes in Dulbecco phosphate-buffered saline (DPBS) containing 0.9mM CaCl2 and 0.5mM MgCl2 (Invitrogen), 0.1 mg/mL of bovine serum albumin (New England BioLabs), and 0.01 mg/mL of tRNA (Sigma-Aldrich). Protein-aptamer complexes were separated from free aptamer oligonucleotide using an acrylic mini-dot blot apparatus (Schleicher & Schuell) and a nitrocellulose membrane as previously described.15 Dissociation constant (KD) estimates were determined by fitting the following equation describing a 1:1 aptamer:protein complex to the resulting data (XLfit v.4.1.1; ID Business Solutions): fraction aptamer bound = amplitude*[protein]/(KD + [protein]).
Surface plasmon resonance assay
ARC5690 was synthesized with a 5′-biotin group in place of PEG. The biotinylated aptamer was immobilized on a research-grade streptavidin-coated (SA) biosensor chip (Biacore) to measure binding by recombinant mouse P-selectin (R&D Systems). Biotinylated aptamer was diluted to 25nM in DPBS, injected manually over the flow cell surface at a rate of 10 μL/min, and stopped when a net increase of surface response units reached 500. In a separate flow cell, a no-aptamer negative control surface was prepared by injecting 50μM biotin at 10 μL/min for 2 minutes. Recombinant mouse P-selectin was serially diluted into DPBS (31.6, 10.0, 3.16, 1.00 and 0.32nM) and run through the aptamer-coated streptavidin-coated biosensor chip at 20 μL/min for 5 minutes (association phase) followed by 5 minutes of buffer only (dissociation phase). Estimates of association and dissociation rate constants were calculated using BiaEvaluation v4.1 software (Biacore).
Blood cells preparation and labeling
Blood from SCD mice anesthetized with ketamine/xylazine (0.1 mg/0.015 mg/g body weight [bw]) was drawn into a heparinized syringe by cardiac puncture. RBC isolation and labeling were carried out as described.16 Briefly, 0.5 mL of blood was washed with phosphate-buffered saline (PBS), resuspended in 6 mL of PBS, and layered onto Histopaque 1077 (Sigma-Aldrich). The RBC pellet was resuspended in 10 mL of Dulbecco modified Eagle medium (Mediatech) supplemented with 1 g/L D-glucose, and 70 μL of 2,7-bis-(carboxyethyl)-5-(and-6) carboxyfluorescein (Molecular Probes) was added to label RBCs. The mixture was incubated at 37°C for 40 minutes. After washing once with PBS, RBCs were resuspended in Dulbecco Modified Eagle Medium to a hematocrit of 25% for infusion.8 Leukocytes were labeled in vivo with phycoerythrin (PE) rat anti–mouse CD45 antibody (0.12 mg/kg bw; BD Biosciences), as described.17 Labeled RBCs and PE rat anti–mouse CD45 antibody were infused via the carotid artery.
Concentration of aptamers and route of administration
The dose of aptamer and the route of administration were based on the results of preliminary experiments shown in supplemental Figures 2 and 3. ARC5690 and ARC5694 were injected intraperitonially into SCD mice at the dose 20 mg/kg of mouse bw. Saline (0.9%) was administered intraperitoneally to control SCD mice at 10 μL/g bw. Anti–P-selectin monoclonal antibody (RB40.34 rat anti–mouse CD625; BD Biosciences) was infused via carotid artery at a dose of 4 mg/kg bw.
Hypoxia/normoxia protocol for SCD mice
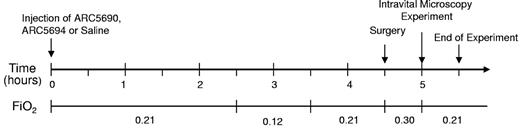
The hypoxia/normoxia protocol is shown in Figure 2. ARC5690 or scrambled aptamer ARC5694 at a dose of 20 mg/kg bw, or saline (10 μL/g bw) was injected intraperitoneally into SCD mice. Two and one-half hours after injection, SCD mice were subjected to 1 hour of hypoxia (fraction of inspired oxygen [FiO2] = 0.12) followed by 1 hour normoxia (FiO2 = 0.21). Intravital microscopy experiments were initiated after 30 minutes surgery, during which mice were supplemented with 30% oxygen (FiO2 = 0.30). In separate experiments where SCD mice were subjected to the hypoxia/normoxia, anti–mouse P-selectin antibody was infused via the carotid artery before intravital microscopy experiments.
Hypoxia/normoxia protocol to study effects of ARC5690 on sickle RBC and leukocyte adhesion. SCD mice were injected with saline, ARC5690 or ARC5694. Two and one-half hours after injection, mice were subjected to 1 hour of hypoxia (FiO2 = 0.12) followed by 1 hour of normoxia in room air (FiO2 = 0.21), and intravital studies were then initiated. During surgery, mice were supplemented with 30% O2. FiO2 indicates fraction of inspired oxygen in a gas mixture.
Hypoxia/normoxia protocol to study effects of ARC5690 on sickle RBC and leukocyte adhesion. SCD mice were injected with saline, ARC5690 or ARC5694. Two and one-half hours after injection, mice were subjected to 1 hour of hypoxia (FiO2 = 0.12) followed by 1 hour of normoxia in room air (FiO2 = 0.21), and intravital studies were then initiated. During surgery, mice were supplemented with 30% O2. FiO2 indicates fraction of inspired oxygen in a gas mixture.
Surgical procedures for intravital microscopy
Mice were prepared for intravital microscopy experiments as previously described.16 Briefly, mice were anesthetized with ketamine/xylazine (0.1 mg/0.015 mg/g intraperitoneally), given a tracheotomy, and connected to a small animal respirator (SAR-830; CWE Inc.). During surgery, mice were supplemented with oxygen (FiO2 = 0.30). A polyethylene catheter PE-10 was inserted into the right common carotid artery to inject 2,7-bis-(carboxyethyl)-5-(and-6) carboxyfluorescein-labeled RBCs and antibody. The mouse head was immobilized using a stereotaxic holder (Stoelting Co), hair was removed, the scalp incised in the midline, and the fronto-parietal skull exposed. Mouse body temperature was monitored and maintained in the range of 36.5-37.5°C using a TCAT-2 DF temperature control unit (Physitemp Instruments).
Intravital microscopy, cell adhesion analysis, and mortality rate associated with experimental procedures
Observations of the skull bone marrow microvascular network were performed using an intravital microscope (IV 500; Mikron Instruments) equipped with water-immersion objectives (Carl Zeiss). Fifteen minutes after completing the surgical procedures stated above, several 3-minute images of the microvasculature from a preselected area in the left fronto-parietal skull at the intersection of the sagittal and coronal sutures were recorded using a SIT camera (VE 1000-SIT, Dage-MTI), a time-base generator (For-A), and DVCAM digital videocassette recorder (Sony DSR-20MD). Fluorescent cells were visualized with a video-triggered epifluorescence illumination using 20× water-immersion objectives (Carl Zeiss). Microvascular network images were analyzed off-line by playback of videotape. Velocity of labeled RBCs and free-floating leukocytes were analyzed in venules with an internal diameter of 18-25μM using Image Pro-Plus 5.0 imaging software (Media Cybernetics). Velocity of red blood cells (VRBC) was measured along the microvessel center line and converted to bulk (mean) velocity (cells plus plasma) using the empirical correction factor of 1.6.18 An average of 5 to 8 RBC velocity measurements taken along the centerline of the vessel was used to define the mean centerline VRBC. The wall shear rate was calculated using the following formula: 8 × Vmean/D, where D is the venular diameter, and Vmean is estimated as VRBC/1.6.19 In experiments studying leukocyte adhesion, the velocity of free-flowing leukocytes was used to calculate hemodynamic parameters.20 RBC adhesion events (> 1 second) were quantified as events per minute in a 100-μm length of the same vessel by frame-by-frame analysis of video replay.21 Rolling leukocyte flux was determined by counting the number of cells passing a fixed point per minute. Leukocyte adhesion was quantified by counting, for each vessel, the number of adherent cells (stationary for > 30 seconds) in a 100-μm length.22 Hemodynamic parameters and leukocyte behavior were evaluated in 18-21 venules of 4-5 mice in each group. Mortality rates associated with experimental procedures were defined as the percentage of the number of mice that were treated with either saline, ARC5690, or ARC5694 but did not survive the experiments, to the total number of mice in a group. Only mice that survived the entire experimental procedure were included in the analysis and those that died in the middle of experiments due to surgical complications were not counted.
Statistical analysis
All data were expressed as means ± SE. The data were analyzed by Student t test or Mann-Whitney rank sum test using a computer-based software package (SigmaStat 3.0). A Fisher exact test was used for comparing mortality rates between groups. P values less than .05 were considered significant.
Results
Characterization of the binding specificity of anti–mouse P-selectin aptamer
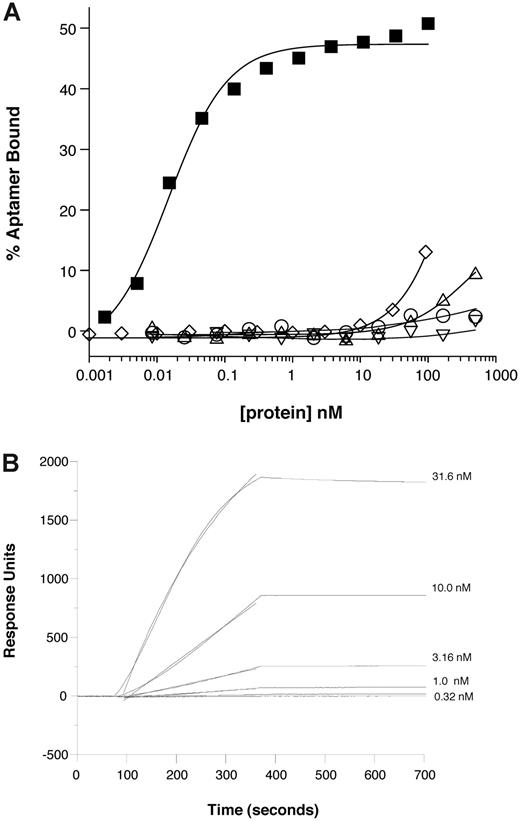
We initially evaluated the binding specificity of the anti–mouse P-selectin aptamer ARC5690 to mouse P-selectin by nitrocellulose filtration and surface plasmon resonance assays. The oligonucleotide core of ARC5690 bound to recombinant mouse P-selectin with a KD of approximately 15pM in vitro, as measured by nitrocellulose filtration assays, in which limiting concentrations of radiolabeled aptamer oligonucleotide were titrated with increasing concentrations of protein ligand (Figure 3A). These results indicate that the aptamer ARC5690 is highly specific for mouse P-selectin because little or no binding to mouse E-selectin or to human P-selectin is observed at concentrations ranging up to 500nM, and only slight binding to mouse L-selectin is observed at this concentration (Figure 3A). Although binding of the scrambled aptamer ARC5694 to mouse P-selectin is apparent at 100nM, ARC5694 interacts with mouse P-selectin with approximately 104 weaker affinity than its parent ARC5690 (Figure 3A). The surface plasmon resonance assay was performed using immobilized aptamer and increasing concentrations of mouse P-selectin in the flow (Figure 3B). The aptamer:mouse P-selectin complex dissociated with a very slow off-rate such that a firm estimate cannot be determined but appears to be < 1 × 10−4/s (t1/2 > 2 hours). These results demonstrate that ARC5690 binds mouse P-selectin with high specificity.
Anti–P-selectin aptamer binds to murine P-selectin with high specificity. (A) Nitrocellulose filtration assays were performed with 5′-32P-radiolabeled ARC5690 (lacking 5′-PEG) in the presence of increasing concentrations of mouse P-selectin (■), E-selectin (○), L-selectin (▵), or human P-selectin (▿). The aptamer binds to mouse P-selectin with a dissociation constant (KD) of approximately 15pM, and to other proteins with KD > 500nM (the highest protein concentration tested). Radiolabeled scrambled aptamer binds to mouse P-selectin (◇) with KD > 100nM (the highest protein concentration tested). (B) Surface plasmon resonance assay was performed with 5′-biotin–labeled ARC5690 (lacking 5′-PEG) immobilized to a streptavidin-derivatized microfluidics chip. Different concentrations of recombinant murine P-selectin (31.6, 10.0, 3.16, 1.00, and 0.32nM) were injected over the aptamer-coated surface. Surface plasmon resonance data were used to estimate a bimolecular association rate constant (kA) of 2 × 105M−1s−1 and dissociation rate constant (kD) of < 1 × 10−4/s (t1/2 > 2 hours) corresponding to a KD estimate of < 0.5nM.
Anti–P-selectin aptamer binds to murine P-selectin with high specificity. (A) Nitrocellulose filtration assays were performed with 5′-32P-radiolabeled ARC5690 (lacking 5′-PEG) in the presence of increasing concentrations of mouse P-selectin (■), E-selectin (○), L-selectin (▵), or human P-selectin (▿). The aptamer binds to mouse P-selectin with a dissociation constant (KD) of approximately 15pM, and to other proteins with KD > 500nM (the highest protein concentration tested). Radiolabeled scrambled aptamer binds to mouse P-selectin (◇) with KD > 100nM (the highest protein concentration tested). (B) Surface plasmon resonance assay was performed with 5′-biotin–labeled ARC5690 (lacking 5′-PEG) immobilized to a streptavidin-derivatized microfluidics chip. Different concentrations of recombinant murine P-selectin (31.6, 10.0, 3.16, 1.00, and 0.32nM) were injected over the aptamer-coated surface. Surface plasmon resonance data were used to estimate a bimolecular association rate constant (kA) of 2 × 105M−1s−1 and dissociation rate constant (kD) of < 1 × 10−4/s (t1/2 > 2 hours) corresponding to a KD estimate of < 0.5nM.
Anti–mouse P-selectin aptamer reduces sickle RBC adhesion and increases microvascular flow velocities
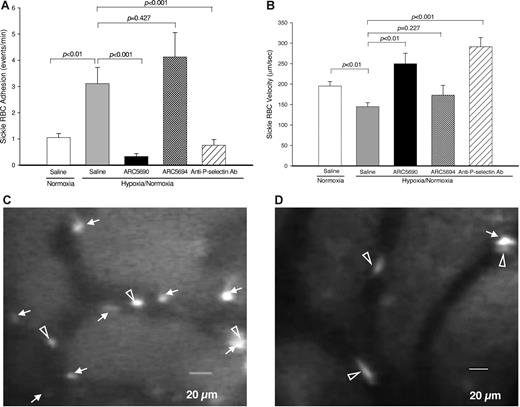
To investigate the antiadhesive properties of anti–P-selectin aptamer, we first examined the adhesion of sickle RBCs to endothelial cells in mouse skull bone marrow microvessels. Specifically we measured (1) sickle RBC adhesion, (2) RBC velocity, (3) vessel diameter, and (4) calculated wall shear rate; the results of the first 2 are shown in Figure 4A-B). We measured these parameters for sickle RBC adhesion under a hypoxic condition, under which sickle RBCs are assumed to adhere to endothelial cells in SCD patients.23 To improve the survival of SCD mice under hypoxic conditions, we modified the protocol by Kaul and Hebbel22 as shown in Figure 2. SCD mice were injected intraperitoneally with the anti–P-selectin aptamer ARC5690 or control scrambled aptamer ARC5694 at a dose of 20 mg/kg bw, or 0.9% saline (10 μL/g bw), and subjected to the hypoxic stress protocol. A total of 18-21 vessels from 4-5 mice per group were evaluated. The hypoxia/normoxia stress protocol used in this study caused a significant increase in sickle RBC adhesion (Figure 4A columns 1-2, P < .01) and decreased RBC velocity (Figure 4B columns 1-2; P < .01), compared with normoxic conditions, which indicates that a hypoxic stress at 12% O2 of as short a duration as 1 hour substantially affected sickle RBC adhesion and RBC velocity. However, administration of anti–P-selectin aptamer ARC5690 before the hypoxia/normoxia stress significantly decreased the number of adherent sickle RBC cells by approximately 90%, from 3.1 ± 0.62 events/min to 0.33 ± 0.11 events/min (Figure 4A columns 2-3; P < .001); representative video images demonstrate a significant reduction in sickle RBC adhesion by ARC5690 (Figure 4D) compared with saline (Figure 4C). The levels of sickle RBC adhesion in aptamer-injected SCD mice were even lower than those of SCD mice under normoxia (Figure 4A column 1 vs column 3; P = .019). In contrast, the scrambled aptamer ARC5694 had no inhibitory effects on sickle RBC adhesion (P = .427). Next, we compared the antiadhesive activity of anti–P-selectin aptamer with that of anti–P-selectin antibody.22 The inhibitory effect of anti–P-selectin aptamer on sickle RBC adhesion was even stronger than that of anti–P-selectin antibody that exhibited 75% inhibition of sickle RBC adhesion (Figure 4A column 3 vs column 5). Injection of ARC5690 before the hypoxia/normoxia stress also increased sickle RBC velocities by 72% (P < .01) compared with saline-treated mice (Figure 4B column 2 vs column 3), while the scrambled aptamer ARC5694 had no effects on the sickle RBC velocities. The increases in sickle RBC velocities after the administration of ARC5690 were comparable with those seen with anti–P-selectin antibody (Figure 4B columns 3 and 5; P = .099). The anti–P-selectin aptamer ARC5690 and anti–P-selectin antibody did not change vessel diameters (20.2 ± 0.95 for saline, 20.9 ± 0.53 for ARC5690, and 21.7 ± 0.56 for anti–P-selectin antibody; all groups had P values of higher than .05 compared with those injected with saline); these data are shown in supplemental Figure 4. The calculated wall shear rates, which have regulatory effects on cell-cell interactions,24 were increased accordingly (57.1 ± 2.13 for saline and 95.3 ± 9.72 for ARC5690, P < .01; and 108.8 ± 9.00 for anti–P-selectin antibody, P < .01 compared with those injected with saline), as shown in supplemental Figure 4.
Anti–P-selectin aptamer ARC5690 reduces sickle RBC adhesion and increases microvascular flow velocities. (A) ARC5690 inhibits sickle RBC adhesion. Adhesion of labeled RBCs was defined as the number of events (adhesion time more than 1.0 seconds/min in a 100-μm length of vessels by frame–by–frame analysis of video replay. Treatment with ARC5690 before hypoxia/normoxia stress reduced the number of adherent RBCs by 90% compared with saline-treated mice (P < .001). No difference in sickle-RBC adhesion was observed in the ARC5694-treated group compared with saline group (P = .427). (B) ARC5690 improves sickle RBC velocities. Sickle RBC velocities were determined off-line by frame–by–frame analysis of video-recorded microscopic images using Image Pro-Plus 5.0 software. Five to 8 RBC velocity measurements were performed along the centerline of the vessel and used to define the mean centerline VRBC. Mean VRBC (Vmean) was calculated using a conversion factor of 1.6 (VRBC/Vmean = 1.6). Compared with saline-treated mice, RBC velocities were increased in ARC5690 treated group (P < .01), but not in ARC5694-treated mice (P = .227). (C-D) Frame-captured images from videotaped intravital microscopy of bone marrow venules in SCD mice injected with saline (C) and ARC5690 (D). Saline-treated mice show more adherent sickle RBCs (white arrows). Pretreatment with ARC5690 reduced the number of adherent sickle RBCs. Open arrowheads identify RBCs moving slowly in saline-treated mice (C) and rapidly in the aptamer-treated mice (D). Values were mean ± SE obtained from 4 to 5 mice in each group. The number of venules in experimental groups ranged from 18 to 21. P values for statistical analyses are shown on top of the figures.
Anti–P-selectin aptamer ARC5690 reduces sickle RBC adhesion and increases microvascular flow velocities. (A) ARC5690 inhibits sickle RBC adhesion. Adhesion of labeled RBCs was defined as the number of events (adhesion time more than 1.0 seconds/min in a 100-μm length of vessels by frame–by–frame analysis of video replay. Treatment with ARC5690 before hypoxia/normoxia stress reduced the number of adherent RBCs by 90% compared with saline-treated mice (P < .001). No difference in sickle-RBC adhesion was observed in the ARC5694-treated group compared with saline group (P = .427). (B) ARC5690 improves sickle RBC velocities. Sickle RBC velocities were determined off-line by frame–by–frame analysis of video-recorded microscopic images using Image Pro-Plus 5.0 software. Five to 8 RBC velocity measurements were performed along the centerline of the vessel and used to define the mean centerline VRBC. Mean VRBC (Vmean) was calculated using a conversion factor of 1.6 (VRBC/Vmean = 1.6). Compared with saline-treated mice, RBC velocities were increased in ARC5690 treated group (P < .01), but not in ARC5694-treated mice (P = .227). (C-D) Frame-captured images from videotaped intravital microscopy of bone marrow venules in SCD mice injected with saline (C) and ARC5690 (D). Saline-treated mice show more adherent sickle RBCs (white arrows). Pretreatment with ARC5690 reduced the number of adherent sickle RBCs. Open arrowheads identify RBCs moving slowly in saline-treated mice (C) and rapidly in the aptamer-treated mice (D). Values were mean ± SE obtained from 4 to 5 mice in each group. The number of venules in experimental groups ranged from 18 to 21. P values for statistical analyses are shown on top of the figures.
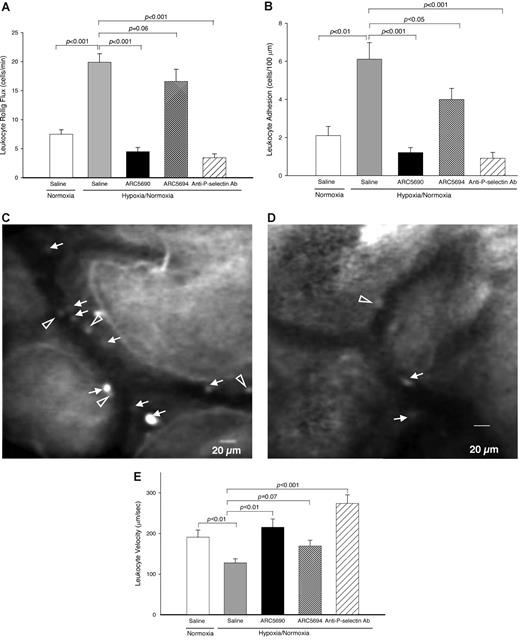
Anti–mouse P-selectin aptamer reduces leukocyte rolling flux and leukocyte adhesion to endothelial cells
Next, we investigated the effects of anti–P-selectin aptamer on leukocyte flow dynamics in SCD mice (Figure 5). Compared with normoxic conditions, the hypoxia/normoxia stress used in this study significantly increased leukocyte rolling flux (Figure 5A columns 1-2; P < .001), which is an initial step for leukocyte adhesion, as well as the adhesion of leukocytes to the endothelium (Figure 5B columns 1-2; P < .01) in SCD mice. These results are consistent with those of a transgenic SCD mouse study by Kaul and Hebbel.22 Pretreatment of SCD mice with the anti–P-selectin aptamer ARC5690 inhibited hypoxia/normoxia stress-induced leukocyte rolling flux to endothelial cells by more than 75% (Figure 5A column 2 vs column 3; P < .001), whereas no reduction in the leukocyte rolling flux was seen with scrambled aptamer (Figure 5A column 2 vs column 4; P = .06). The levels of inhibition of ARC5690 on the leukocyte rolling flux were similar to those seen with anti–P-selectin antibody (Figure 5A column 3 vs column 5; 4.5 ± 0.72 for ARC5690 and 3.5 ± 0.67 for anti–P-selectin antibody, P = .35).
Anti–P-selectin aptamer ARC5690 decreases leukocyte rolling flux and leukocyte adhesion. (A) ARC5690 decreases leukocyte rolling flux. Leukocyte rolling flux was determined by the number of cells rolling through a fixed point per minute (cells/min). Compared with saline-treated mice, pretreatment of SCD mice with ARC5690 (P < .001), but not with ARC5694 (P = .06), decreased leukocyte rolling flux under hypoxia/normoxia stress. (B) ARC5690 decreases leukocyte adhesion to endothelial cells. Leukocyte adhesion was quantified by counting the number of adherent cells (stationary for > 30 seconds) in a 100-μm length of the vessel. Pretreatment with ARC5690 before hypoxia/normoxia stress reduced the number of adherent leukocytes compared with saline-treated mice (P < .001). Scrambled aptamer ARC5694 showed some inhibition of leukocyte adhesion (P < .05; see “Discussion”). (C-D) Frame-captured images from videotaped intravital microscopy of bone marrow venules in SCD mice injected with saline (C) and ARC5690 (D) after infusion of PE rat anti–mouse CD45. Saline-treated mice show a higher number of adherent (white arrows) and rolling (open arrowheads) leukocytes. (E) ARC5690 improves velocities of free-flowing leukocytes. Velocities of free-flowing leukocytes were determined using Image Pro-Plus 5.0 software. Five to 8 velocity measurements were performed along the centerline of the vessel and used to calculate hemodynamic parameters. Velocity of free-flowing leukocytes increased after treatment with ARC5690 but not with the scrambled aptamer ARC5694 compared with saline treated mice. Values were mean ± SE obtained from 4 to 5 mice in each group. The number of venules in experimental groups ranged from 18 to 21. P values for statistical analyses are shown on top of the figures.
Anti–P-selectin aptamer ARC5690 decreases leukocyte rolling flux and leukocyte adhesion. (A) ARC5690 decreases leukocyte rolling flux. Leukocyte rolling flux was determined by the number of cells rolling through a fixed point per minute (cells/min). Compared with saline-treated mice, pretreatment of SCD mice with ARC5690 (P < .001), but not with ARC5694 (P = .06), decreased leukocyte rolling flux under hypoxia/normoxia stress. (B) ARC5690 decreases leukocyte adhesion to endothelial cells. Leukocyte adhesion was quantified by counting the number of adherent cells (stationary for > 30 seconds) in a 100-μm length of the vessel. Pretreatment with ARC5690 before hypoxia/normoxia stress reduced the number of adherent leukocytes compared with saline-treated mice (P < .001). Scrambled aptamer ARC5694 showed some inhibition of leukocyte adhesion (P < .05; see “Discussion”). (C-D) Frame-captured images from videotaped intravital microscopy of bone marrow venules in SCD mice injected with saline (C) and ARC5690 (D) after infusion of PE rat anti–mouse CD45. Saline-treated mice show a higher number of adherent (white arrows) and rolling (open arrowheads) leukocytes. (E) ARC5690 improves velocities of free-flowing leukocytes. Velocities of free-flowing leukocytes were determined using Image Pro-Plus 5.0 software. Five to 8 velocity measurements were performed along the centerline of the vessel and used to calculate hemodynamic parameters. Velocity of free-flowing leukocytes increased after treatment with ARC5690 but not with the scrambled aptamer ARC5694 compared with saline treated mice. Values were mean ± SE obtained from 4 to 5 mice in each group. The number of venules in experimental groups ranged from 18 to 21. P values for statistical analyses are shown on top of the figures.
The decrease in leukocyte rolling flux after treatment with anti–P-selectin aptamer ARC5690 was accompanied by the reduction in the number of adherent leukocytes to endothelial cells (Figure 5B column 2 vs column 3; P < .001). The effect of ARC5690 on leukocyte adhesion was again comparable with those of anti–P-selectin antibody (Figure 5B column 3 vs column 5; 1.2 ± 0.26 for ARC5690 and 0.9 ± 0.31 for anti–P-selectin antibody, P = .44); representative video images demonstrate leukocyte adhesion in SCD mice after the injection of saline (Figure 5C) and anti–P-selectin aptamer ARC5690 (Figure 5D). It is of note that the scrambled aptamer ARC5694 inhibited leukocyte adhesion by 35% (Figure 5B column 2 vs column 4; P < .05).
Next, we examined the effects of anti–P-selectin aptamer on velocities of free-flowing leukocytes. Although the hypoxia/normoxia stress decreased the velocities of free-flowing leukocytes (Figure 5E, column 1 vs column 2; P < .01), ARC5690 significantly increased the velocities to the levels in SCD mice under normoxia. In addition, the injection of anti–P-selectin antibody increased leukocyte velocities (Figure 5E column 2 vs column 5; P < .001). Since neither anti–P-selectin aptamer nor anti–P-selectin antibody changed the vessel diameter (20.9 ± 1.28 for saline, 20.6 ± 0.64 for ARC5690, and 21.5 ± 0.65 for anti–P-selectin antibody; all groups P > .05 compared with saline), the calculated wall shear rates increased accordingly (50.0 ± 5.34 for saline, 84.4 ± 8.54 for ARC5690, and 101.8 ± 6.97 for anti–P-selectin antibody; ARC5690 vs saline P < .01; anti–P-selectin antibody vs saline P < .001). The figures for vessel diameter and wall shear rates are shown in supplemental Figure 5. No significant changes in cell velocities (Figure 5E, P = .07) and calculated wall shear rates (P = .105) were observed after administration of scrambled aptamer (supplemental Figure 5).
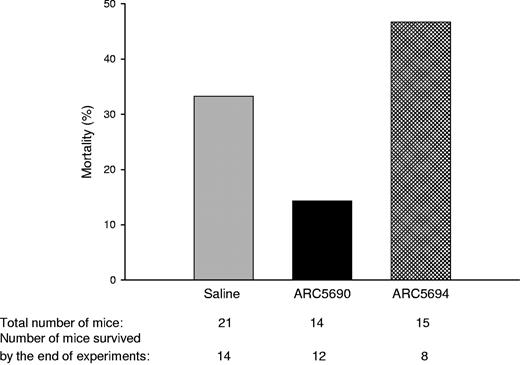
Finally, we investigated whether anti–P-selectin aptamer improved the mortality rate of SCD mice which was associated with experimental procedures including surgical operations for intravital microscopy observation. SCD mice treated with anti–P-selectin aptamer ARC5690 under the hypoxia/normoxia stress exhibited a lower mortality rate than those injected with saline or ARC5694 (Figure 6).
ARC5690 decreases mortality in SCD mice associated with experimental procedures. Mortality rates were defined as the percentage of the number of mice pretreated with either saline, ARC5690, or ARC5694 that did not survive through experiments, to the total number of mice in a group. The numbers of the total mice and those surviving hypoxia/normoxia stress are shown at the bottom of the figure.
ARC5690 decreases mortality in SCD mice associated with experimental procedures. Mortality rates were defined as the percentage of the number of mice pretreated with either saline, ARC5690, or ARC5694 that did not survive through experiments, to the total number of mice in a group. The numbers of the total mice and those surviving hypoxia/normoxia stress are shown at the bottom of the figure.
Discussion
Although anti–cell adhesion agents could provide a therapeutic option for SCD patients,25-27 no such therapeutics are currently available for the treatment. In this study, aiming at the development of a novel anti–cell adhesion agent, we have generated an anti–mouse P-selectin aptamer and investigated its anti–cell adhesion activities using SCD model mice under hypoxia/normoxia.22 We have found that the anti–mouse P-selectin aptamer efficiently inhibits the adhesion of both sickle RBCs and leukocytes to the vascular endothelium and improves the flow velocities of both cells in the microcirculation under hypoxia without affecting venule diameter. Because P-selectin–mediated cell adhesion is believed to impair the microvascular flow in SCD,3,8 the inhibition of blood cell–endothelial cell interactions by anti–P-selectin aptamer likely contributes to the increase in microvascular flow velocities. Importantly, compared with SCD mice injected with saline, those injected with anti–P-selectin aptamer demonstrated a lower mortality rate associated with the experimental procedures including hypoxia/reperfusion injury and surgery for intravital microscopy. The low mortality rate is presumably relevant to the decrease in the adhesion of both RBCs and leukocytes to endothelial cells and resultant improvements of microvascular flow. Together, these results demonstrate that P-selectin is an important molecule involved in the adhesion of both sickle RBCs and leukocytes to endothelial cells, and more importantly, anti–P-selectin aptamer has a potential as a novel anti–cell adhesion agent for the treatment of SCD.
Vigorous investigations have been performed to clarify the mechanisms underlying hypoxia-induced sickle RBC adhesion to endothelial cells, but most studies used in vitro settings or heterologous systems involving injection of human sickle RBCs into the rat.21,23,28 To gain insight into the mechanisms using knockout-transgenic SCD mice, we modified the hypoxia/reoxygenation protocol from a study by Kaul and Hebbel22 to improve their survival under hypoxia. Our intravital microscopy studies demonstrated that the adhesion of sickle RBCs in the microvasculature significantly increases in SCD model mice even under mild hypoxia of 12% O2.
Given that several molecular interactions are likely involved in the adhesion of sickle RBCs,29 it is of particular interest that anti–P-selectin aptamer suppressed sickle RBC adhesion to endothelial cells under hypoxia by approximately 90%, suggesting that P-selectin plays a major role in sickle RBC adhesion. However, the legitimate ligand for P-selectin on the cell surface of sickle RBCs remains unclear. In addition to its canonical ligand, P-selectin glycoprotein 1,30 P-selectin is able to interact with several carbohydrate-based ligands, all of which share an anionic character, including sialylated, fucosylated glycoproteins31 and several sulfated glycosaminoglycans (eg, heparin).32 Inhibition of sickle RBC adhesion to endothelial cells by the soluble tetrasaccharide sialyl Lewisx that binds to all selectins suggested that Lewis RBC antigens can be a potential ligand for P-selectin on RBCs.3 However, the affinity of this antigen to P-selectin is rather weak and RBCs do not synthesize these antigens.31 Nonetheless, our in vivo studies using SCD mice clearly demonstrate a critical role for P-selectin in sickle RBC adhesion to endothelial cells, which is in agreement with a study by Embury et al.8 More intriguingly, molecules other than P-selectin, which include fibronectin,33 von Willebrand factor,34 CD36 binding thrombospondin,35 α4β1-integrin,36 vascular intercellular adhesion molecule-1,23 and CD4437 have been shown to be involved in sickle RBC adhesion. Given our in vivo data showing a major role of P-selectin in sickle RBC adhesion, these studies may suggest that stable adherence of sickle RBCs to endothelial cells requires the interactions of multiple, not single, molecules between sickle RBCs and vascular endothelial cells, and that the interruption of a single molecular interaction by an anti–cell adhesion compound like anti–P-selectin aptamer may suffice to dissociate sickle RBCs from endothelial cells. However, the efficiency to induce sickle RBC dissociation may vary depending upon the molecular interaction inhibited by a compound.
The roles of leukocytes in developing vaso-occlusion are increasingly acknowledged.4 Recently, Frenette and colleagues demonstrated that human immunoglobulin reduces adherent leukocyte numbers and inhibits the adhesive interactions between sickle RBCs and leukocytes in SCD mice.26,38 There are substantial differences between their results and those of the present study. First, their SCD mice were treated with tumor necrosis factor-α (TNF-α). Although TNF-α is a critical cytokine that promotes inflammatory reactions in SCD and induces E-selectin expression,2 the plasma levels of TNF-α vary substantially among SCD patients.39 In contrast, we studied the effects of anti–P-selectin aptamer on adhesive interactions under a hypoxia/normoxia stress, which is a more physiologic event that precipitates crises in SCD.22 Second, although human immunoglobulin increased the number of rolling leukocytes in SCD mice,38 the anti–P-selectin aptamer inhibited leukocyte rolling flux, an initial step that precedes firm leukocyte adhesion, as well as leukocyte adhesion to endothelial cells. Anti–P-selectin aptamers may have more global effects on leukocyte adhesion as a consequence of inhibiting leukocyte recruitment to the inflamed endothelium at an early stage.40 Third, there are continued concerns with the toxicity of immunoglobulin infused intravenously.41 In contrast, aptamers have demonstrated little toxicity in preclinical studies.42 These advantages of anti–P-selectin aptamer over human immunoglobulin may encourage performing clinical trials for SCD patients under crisis.
It is of note that the scrambled aptamer ARC5694 inhibited leukocyte adhesion by 35%. The cause of this mild effect observed with the srambled aptamer may be related to the mechanisms underlying the selectin/ligand interaction. As stated above, P-selectin is capable of interacting with mutiple carbohydrate-based ligands such as sialylated, fucosylated glycoproteins30,31 and sulfated glycosaminoglycans. These molecules share an anionic character and inhibit leukocyte adhesion.32,43 Thus, the polyanionic phosphoribosyl backbone of ARC5694 may exhibit some inhibitory activity against P-selectin, when used at high concentrations. Alternatively, ARC5694 may have nonspecific affinities to other molecules involved in leukocyte adhesion.44
Potential anti-adhesive compounds that are currently investigated include human immunoglobulin,26 unfractionated heparin,25 and αVβ3 antagonists.27 Although all these potential therapeutics including anti–P-selectin aptamer require injectable administration, the features of anti–P-selectin aptamer include low toxicity and lack of immungenicity.10 Although aptamers have shorter half-lives in peripheral blood, it is possible to extend their half-lives by modifying their chemical structures.10 Currently several aptamers directed at inhibiting a variety of hematologic and cardiovascular targets are being investigated. They include anti–von Willebrand factor aptamer ARC1779, which has completed a phase I clinical study with healthy volunteers and is now in a phase II study in patients with von Willebrand factor-related platelet function disorders.15,45,46 REG1, anti–factor IX aptamer, was evaluated in both phase I and phase II studies of cardiopulmonary bypass surgery.47,48 NU172, a direct thrombin inhibitor, has completed a phase I study.13
In conclusion, we have shown using SCD model mice that anti–P-selectin aptamer efficiently inhibits the adhesion of sickle RBCs and leukocytes to endothelial cells, demonstrating an important role for P-selectin in cell adhesion. To our knowledge, this is the first in vivo demonstration of cell adhesion inhibition by an anti–P-selectin aptamer in knockout-transgenic SCD model mice. Together with other anti–cell adhesion compounds, aptamers that block the function of adhesion molecules such as P-selectin with high specificity may represent a novel class of therapeutics for SCD.
Note added in proof: After submitting this manuscript, we learned that a study by Chang et al was accepted by this journal. These authors showed that GMI-1070, a new pan-selectin antagonist, inhibits vaso-occlusions in mice that were transplanted with bone marrow from sickle cell mice (DOI 10.1182/blood-2009-12-260513).49
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the following employees of Archemix Corporation for contributions of in vitro data: Michael Schwartz and Kathleen McGinness for the nitrocellulose filtration data and Yuxun Wang and Shuhao Zhu for the Biacore data. Pharmacokinetic data were contributed by Renta Hutabarat of Archemix Corporation. We thank Nadine Odo for editing the manuscript.
This study was supported by research funding from Archemix Corporation to D.R.G. and C.A.H. T.I. was supported in part by a Southeastern Clinical and Translational Research Institute grant and a grant (P20 MD003383) from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: D.R.G. designed and conducted the research, analyzed the data, and wrote the manuscript; J.B.P. and S.D.Y. performed research; J.C.K. and R.G.S. developed aptamers and participated in designing the research and writing the manuscript; T.I. analyzed the data and wrote the manuscript; and C.A.H. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: J.C.K. and R.G.S. are employees of Archemix Corporation. The remaining authors declare no competing financial interests.
Correspondence: C. Alvin Head, Department of Anesthesiology and Perioperative Medicine, Medical College of Georgia, 1120 15th St, BIW 2144, Augusta, GA 30912-2700; e-mail: ahead@mcg.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal