Abstract
The interleukin (IL)–22R1 chain of the heterodimeric IL-22 receptor is not expressed on normal leukocytes, but this receptor is expressed on T cells from anaplastic lymphoma kinase–positive (ALK+) anaplastic large cell lymphoma (ALCL) patients. To investigate the consequences of aberrant expression of this receptor on lymphocytes, we generated transgenic mice that express IL-22R1 on lymphocytes. The health of these animals progressively deteriorated at 8 to 12 weeks of age, as they displayed respiratory distress, rough coat and sluggish movement, and subsequent lethality due to multiorgan inflammation. The IL-22R1 transgenic animals developed neutrophilia that correlated with increased levels of circulating IL-17 and granulocyte colony-stimulating factor. In addition, these mice had increased serum IL-22 levels, suggesting that T cells expressing IL-22R1 generate IL-22 in a positive autoregulatory loop. As a result of the mouse model findings, we analyzed circulating cytokine levels in ALK+ALCL patients and detected elevated levels of IL-22, IL-17, and IL-8 in untreated patient samples. Importantly, IL-22 and IL-17 were undetectable in all patients who were in complete remission after chemotherapy. This study documents a previously unknown role of IL-22R1 in inflammation and identifies the involvement of IL-22R1/IL-22 in ALK+ALCL.
Introduction
Interleukin-22 (IL-22) signals through a heterodimeric receptor complex composed of the IL-22R1 and IL-10R2 chains.1,2 Unlike the ubiquitously expressed IL-10R2 chain, the IL-22R1 chain is normally restricted to nonimmune cells such as epithelial cells and hepatocytes. IL-22 first binds to its high-affinity receptor, IL-22R1, and then recruits the low-affinity receptor chain, IL-10R2.3 IL-10R2 is constitutively expressed on virtually all cells types at high levels and is an essential component of the receptor complexes for IL-22, IL-10, IL-26, IL-28, and IL-29. When IL-22 is bound to its receptor complex, it activates multiple signaling pathways, including the signal transducer and activator of transcription 3 (STAT3) and mitogen-activated protein kinase.4 Upon activation, STAT3 translocates from the cytosol to the nucleus, where it regulates expression of genes involved in cell proliferation and survival.
Systemic anaplastic large cell lymphoma (ALCL) is a peripheral T-cell lymphoma that can present with either a T- or null-cell phenotype. It is divided into anaplastic lymphoma kinase-positive (ALK+) and negative types, with the former caused by an 80-kDa chimeric fusion protein resulting from a t(2;5) involving nucleoplasmin (NPM) and ALK genes. ALK+ALCL is characterized by the expression of IL-22R1 on the lymphoma cells and occurs predominantly in pediatric and young adult patients, where 83% of patients harbor the ALK translocation, compared with 31% of older adults.5,6 ALK+ALCL patients commonly present with clinical symptoms consistent with an inflammatory syndrome, such as high fever, lymphadenopathy, and neutrophilia. These patients often have extranodal involvement of the skin, bone, bone marrow, lung, liver, and soft tissues.
ALCL demonstrates an activated T-cell phenotype with expression of human leukocyte antigen-DR, CD25, CD30, CD45, and epithelial membrane antigen. One report suggests that aberrant expression of IL-22R1 on T cells is linked to the NPM-ALK fusion event in ALK+ALCL.5 The oncogenic role of STAT3 in ALK+ALCL has been extensively studied, and it is hypothesized that STAT3 activation inhibits apoptosis in these cells. Although STAT3 activation is normally mediated by Janus tyrosine kinases (JAKs), ALK itself mediates tyrosine phosphorylation in ALK+ALCL cells and has been shown to activate STAT3.7-9 In ALK+ALCL cell lines, JAK3 is phosphorylated, although the specific mechanism by which this occurs is unknown. Previous studies have identified IL-9 and IL-21 as important cytokines that activate the JAK3/STAT3 pathway and are aberrantly expressed in ALK+ALCL.9-12
Since the identification of ALCL, much effort has been made to understand the pathogenesis of this lymphoma. The NPM-ALK fusion protein, which encodes a constitutively active tyrosine kinase, has previously been shown to play a central role in this lymphoma. Mouse models of ALCL have been developed to understand the role of NPM-ALK in tumorigenesis. However, most of these transgenic (tg) models do not recapitulate the human ALK+ALCL disease phenotype. For instance, these tg mice usually form plasmacytomas, in contrast to the T cell–derived lymphomas that are typically found in ALK+ALCL.9,13 Moreover, none of the models address the systemic inflammation observed in ALK+ALCL patients.
To understand the biological significance of IL-22R1 expression in ALCL, we developed tg mice that express functional IL-22 receptors on lymphocytes. These animals do not develop lymphoma, which indicates that IL-22R1 expression alone may not be sufficient to cause lymphoma. However, these tg animals develop systemic inflammation that leads to premature death. Furthermore, these tg mice show autocrine amplification of IL-22, and the inflammation observed in these mice correlates with the up-regulation of serum IL-17 and granulocyte colony-stimulating factor (G-CSF). Based on our findings from the IL-22R1 tg mice, we assayed serum from ALK+ALCL patients and found elevated levels of IL-22 and IL-17 before therapy that returned to baseline in all patients who achieved complete remission. This study shows that IL-22R1 expression on lymphocytes can amplify circulating IL-22 in vivo, thus identifying this cytokine as a potentially important player in ALK+ALCL.
Methods
Gene construct and transgenic mice
The IL-22R114 cDNA (obtained from Takashi Suda, Kanazawa University, Japan) was inserted into a CD2 promoter vector (kindly provided by Paul Love, National Institutes of Health [NIH]). This expression vector was used to create a transgene in C57BL/6 mice. The founders were probed for expression of the IL-22R1 transgene by Southern blotting. Animal care was provided in accordance with the procedures outlined in the “Guide for Care and Use of Laboratory Animals” (NIH, Bethesda, MD, 1996). All animal studies were approved by the Animal Care and Use Committee at the National Cancer Institute-Frederick, NIH.
Western blot analysis
Western blot analysis was performed as previously described. Sixty micrograms of the thymus, spleen, and lymph node protein lysates were subjected to sodium dodecyl sulfate (SDS)–polyacrylamide (Invitrogen) gel electrophoresis and transferred to Immobilon-P membranes (Millipore). The membranes were blocked at room temperature in 5% milk/Tris-buffered saline Tween 20 solution. The membranes were then probed for pSTAT3 (Tyr705) and STAT3 (Cell Signaling).
ELISA and CBA analysis
Mouse serum IL-22 or human IL-2Rα was assayed by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (R&D Systems). IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-17, interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), monocyte chemotactic protein-1, G-CSF, granulocyte-macrophage colony-stimulating factor (GM-CSF), and keratinocyte chemoattractant (KC) were measured in mouse sera by cytometric bead array (CBA) flex array according to the manufacturer's instruction (BD Biosciences) on a BD FACS sort cytometer. IL-6, IL-8, IL-17, IL-22, IFN-γ, and TNF-α were measured in human ALCL patient sera or serum from severe combined immunodeficiency/nonobese diabetic (SCID/NOD) mice challenged with Karpas299 with FlowCytomix Simplex kits (eBioscience) according to the manufacturer's instructions. All clinical research samples were obtained on an institutional review board-approved protocol and patients provided informed consent.
Flow cytometry
Flow cytometry was performed on single-cell suspensions from spleen, thymus, lymph nodes, liver, bone marrow, bronchoalveolar lavage fluid (BALF), and peripheral blood lymphocytes (PBL). Antibodies used for lineage analysis were T cells: CD3 (17A2; eBioscience), CD4 (RM4-5; Pharmingen), CD8 (53-6.7; Biolegend), CD25 (PC61; Pharmingen), CD44 (IM7; Biolegend), CD62L (MEL-14; Biolegend); natural killer (NK) and NK-T cells: NK1.1 (PK136; Biolegend), NKp46 (29A1.4; eBioscience), CD1d tetramer (a gift from Jeff Subleski, National Cancer Institute-Frederick, NIH); myeloid population: CD11c (N418; eBioscience), Gr1 (Ly6C&G, RB6-8C5; eBioscience), CD11b (Mac1, M1/70; Pharmingen), F4/80 (BM8; eBioscience); B cells: CD19 (1D3; Pharmingen), B220 (RA3-6B2; Pharmingen), immunoglobulin M (R6-60.2; Pharmingen), immunoglobulin D (11-26; eBioscience), CD43 (1B11; Biolegend), and IL-22R1 (antibody kindly provided by Genentech USA) with their respective isotype controls. Antibodies used for human cell lines were pSTAT3 (4/P-STAT3; BD Biosciences) and IL-22R1 (305405; R&D Systems).
Stimulation of splenocytes and intracellular staining for IL-17 and IL-22
A quantity of 1.0 × 106/mL splenocytes from wild-type (wt) and IL-22R1 tg mice were activated by 10 ng/mL PMA (phorbol 12-myristate 13-acetate) and 1μM/mL ionomycin (Sigma-Aldrich) for 5 hours in the presence of a protein transport inhibitor containing monensin (BD Biosciences; Golgi-Stop) for 3 hours in Iscove modified Dulbecco medium (Sigma-Aldrich) supplemented with 5% heat-inactivated fetal bovine serum, penicillin (100 U/mL), streptomycin (100 μg/mL), l-glutamine, and 5 × 10−3M mercaptoethanol. The cells were stained with CD4 (RM4-5; Pharmingen) antibody, later fixed in BD Biosciences fix/perm buffer, and then stained with IL-17A (TC11-18H10; Pharmingen) and IL-22 (1H8PWSR; eBioscience) antibody. To measure in vivo IL-17 and IL-22 production, 0.25 mg of Brefeldin A (Sigma-Aldrich) was injected in mice intravenously and after 6 hours, intracellular cytokine staining was carried out on single-cell suspensions of lung.
Bone marrow transplantation
Bone marrow cells from IL-22R1 tg mice were transplanted into C57BL/6 recipient mice. The recipient syngeneic mice were irradiated with 900 rad of whole-body irradiation before transplantation. A quantity of 4.0 × 106 bone marrow cells were injected intravenously into the lateral tail vein of recipient mice. Animals were monitored daily for signs of morbidity and sacrificed between 8 to 12 weeks posttransplantation for analysis or subsequent serial transplantation.
Bone marrow cells from IL-22R1 mice were transplanted into 10 athymic nudes (Foxn1nu) and 10 C57BL/6 mice. The athymic nudes (Foxn1nu) and C57BL/6 mice were irradiated with 300 and 900 rad of whole-body irradiation before transplantation. A quantity of 4.0 × 106 bone marrow cells were injected intravenously into the lateral tail vein of recipient mice. Animals were monitored daily for survival. Blood was sampled from these mice to monitor blood parameters.
Isolation of BALF from mice
A tubing adaptor was inserted into the trachea of mice, and the lungs were washed with 3 mL of phosphate-buffered saline (PBS). The BALF was counted for lymphocytes and stained for B, T, and granulocyte cell-surface markers and analyzed by flow cytometry. Aliquots of cells were centrifuged onto glass slides, dried in air, and stained with Diff-quick (Dade Behring). The BALF supernatants were analyzed for IL-1α, IL-1β, IL-6, IL-4, IL-13, IL-17A, KC, IFN-γ, G-CSF, and GM-CSF using CBA flex array.
Cell lines, stimulations, and gene expression analyses
The SUDHL-115 and Karpas29916 cell lines used in this study were derived from ALK+ALCL patients (cell lines were kindly provided by Dr. Thomas Waldmann, NIH). A quantity of 1.0 × 106/mL cells were activated by 10 ng/mL PMA and 1μM/mL ionomycin for 5 hours in the presence of a Brefeldin A (eBioscience) in RPMI 1640 supplemented with 5% heat-inactivated fetal bovine serum, penicillin (100 U/mL), streptomycin (100 μg/mL), and l-glutamine. RNA was extracted from unstimulated SUDHL-1 and Karpas299 cells according to manufacturer's protocol (Norgen). The RNA was reverse transcribed to cDNA that was used to analyze expression of IL-17A (Hs00174383_m1), IL-17F (Hs00369400_m1), IL-22 (Hs01574154_m1), IL-26 (Hs00218189_m1), aryl hydrocarbon receptor (AHR; Hs00169233_m1), and RAR-related orphan receptor C (RORC; Hs01076112_m1) by real-time polymerase chain reaction.
Tumorigenicity of Karpas299 cells in SCID/NOD mice
SCID/NOD was challenged with Karpas299 as previously described.17,18 Two groups of 10 SCID/NOD mice received intravenous injections of 1 × 107 Karpas299. Group 1 received 200 μL of PBS on days 7, 14, and 21 after injection of Karpas299. Group 2 received intravenous injections of 100 μg of HeFi-1 (α-CD30 antibody: NCI Frederick) on days 7, 14, and 21. Mice were monitored for survival or euthanized when the animals weight decreased by more than 20% of their initial body weight. Serum was collected to assay for human IL-22 and IL-2Rα as markers of tumor burden.
Statistical analysis
Data were analyzed with the Student t test to determine statistical significance. P values of less than .05 were considered statistically significant.
Results
IL-22R1 expression on lymphocytes induces spontaneous multiorgan inflammation and premature death
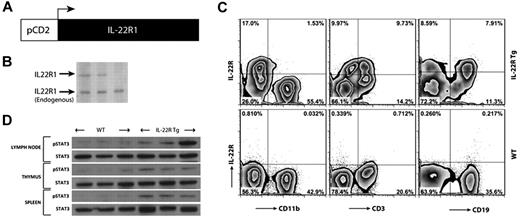
While cell-surface expression of IL-10R2 is seen on lymphocytes, IL-22R1 is not normally expressed on these cells.1 To define the potential functional consequences of IL-22R1 expression on lymphocytes, a tg mouse expressing this receptor driven by a CD2 promoter was developed. Nineteen founder mice were positive for the IL-22R1 transgene as determined by Southern blot, and 9 mice expressed IL-22R1 on lymphocytes (Figure 1A-B). The relative levels of expression of the IL-22R1 chain varied depending on the copy number of the transgene (data not shown). When analyzed by flow cytometry, IL-22R1 cell-surface expression was detected on CD3+ and CD19+ populations as expected, while the CD11b+ population was negative (Figure 1C). IL-22R1 and IL-10R2 can assemble a functional heterodimeric complex and activate STAT3, the major pathway by which IL-22 signals, and the transgene-positive founders had elevated levels of activated STAT3 in the thymus, lymph nodes, and spleen (Figure 1D). These observations support the conclusion that forced expression of the IL-22R1 chain on lymphocytes forms a heterodimeric complex with IL-10R2 resulting in activation of STAT3 signaling. The tg mice positive for IL-22R1 expression and phospho-STAT3 (Tyr705) were selected for further functional analysis.
Transgenic mice express functional IL-22R1 on lymphocytes. (A) A schematic diagram of the IL-22R1 transgene driven by a mouse CD2 promoter. (B) Southern blot analysis of IL-22R1 transgene in founders on a C57BL/6 background. (C) Zebra plots of flow cytometric analysis for IL-22R1 expression on myeloid (CD11b+), T (CD3+), and B (CD19+) cells in peripheral blood leukocytes. (D) Western blot analysis of STAT3 phosphorylation (Tyr705) in the thymus, lymph nodes, and spleen from tg founders and nontransgenic littermates.
Transgenic mice express functional IL-22R1 on lymphocytes. (A) A schematic diagram of the IL-22R1 transgene driven by a mouse CD2 promoter. (B) Southern blot analysis of IL-22R1 transgene in founders on a C57BL/6 background. (C) Zebra plots of flow cytometric analysis for IL-22R1 expression on myeloid (CD11b+), T (CD3+), and B (CD19+) cells in peripheral blood leukocytes. (D) Western blot analysis of STAT3 phosphorylation (Tyr705) in the thymus, lymph nodes, and spleen from tg founders and nontransgenic littermates.
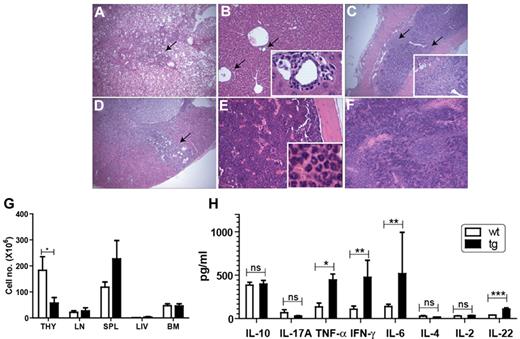
Transgenic IL-22R1 founders were healthy and developed normally for the first 8 to 12 weeks of age. However, by that time, all of the tg founders developed rough coats with a humped appearance, sluggish movement, and respiratory distress, and their overall health progressively worsened, leading to death. Histological analysis showed massive inflammation in multiple organs. Lung, liver, colon, kidney, bone marrow, and spleen were affected by lymphocytic and neutrophilic infiltrates (Figure 2A-F) as well as the gall bladder, duodenum, and pancreas (data not shown). Severe lymphoid depletion was seen in the thymus and lymph nodes in addition to acute thymic atrophy. Systemic inflammation leading to respiratory distress was found to be the primary cause of death for the IL-22R1 founders. Blood analysis showed leukocytosis caused mainly by neutrophilia (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The tg founders had lower cellularity in the thymus compared with the wt animals, whereas lymph nodes, spleen, bone marrow, and liver showed no differences in cellularity (Figure 2G).
The IL-22R1 transgenic founders show spontaneous multiorgan inflammation. Various organs of IL-22R1 tg mice were stained (hematoxylin and eosin) and examined under the microscope. Inflammation was observed in lung (×20; A), liver (×20; B), colon (×20; C), and kidney (×20; D). Hyperplasia with granulocytic infiltrates was observed in bone marrow (×20; E) and extramedullary hematopoiesis was seen in spleen (×20; F). Arrows show leukocytic infiltrates, and insets show enlargement of the portion of the slide. (G) Total cellularity of the thymus, lymph nodes, bone marrow, spleen, and liver from IL-22R1 tg mice (n = 3) and littermate controls (n = 3). (H) Expression of proinflammatory cytokines is elevated in IL-22R1 tg mice. Cytometric bead array to assay the concentrations of an inflammatory panel of cytokines in the serum of IL-22R1 (n = 7) tg founders and nontransgenic littermates (n = 9). IL-22 production in the serum of IL-22R1 tg and wt mice measured by ELISA (n = 6). The data are represented as the mean ± SEM. *P < .05, **P < .01, ***P < .001; ns, not significant. Images were acquired with an Olympus BX40 light microscope using Image Proplus 6 software.
The IL-22R1 transgenic founders show spontaneous multiorgan inflammation. Various organs of IL-22R1 tg mice were stained (hematoxylin and eosin) and examined under the microscope. Inflammation was observed in lung (×20; A), liver (×20; B), colon (×20; C), and kidney (×20; D). Hyperplasia with granulocytic infiltrates was observed in bone marrow (×20; E) and extramedullary hematopoiesis was seen in spleen (×20; F). Arrows show leukocytic infiltrates, and insets show enlargement of the portion of the slide. (G) Total cellularity of the thymus, lymph nodes, bone marrow, spleen, and liver from IL-22R1 tg mice (n = 3) and littermate controls (n = 3). (H) Expression of proinflammatory cytokines is elevated in IL-22R1 tg mice. Cytometric bead array to assay the concentrations of an inflammatory panel of cytokines in the serum of IL-22R1 (n = 7) tg founders and nontransgenic littermates (n = 9). IL-22 production in the serum of IL-22R1 tg and wt mice measured by ELISA (n = 6). The data are represented as the mean ± SEM. *P < .05, **P < .01, ***P < .001; ns, not significant. Images were acquired with an Olympus BX40 light microscope using Image Proplus 6 software.
To test whether the systemic inflammation seen in the IL-22R1 tg founders correlated with altered expression of proinflammatory cytokines, we measured the levels of various T-helper (Th)1 and Th17 cytokines in the serum. The levels of TNF-α, IFN-γ, IL-22, and IL-6 were significantly increased in the IL-22R1 tg founders compared with the wt controls (Figure 2H). The expression of IL-22 suggests generation of an autocrine loop of IL-22 signaling16 leading to chronic STAT3 phosphorylation. In addition, the elevated levels of Th1 and Th17 cytokines in the serum of IL-22R1 founders are characteristic of a cytokine profile associated with systemic inflammation.
Transplantation of bone marrow cells from IL-22R1 founders into wt recipients transfers the inflammatory phenotype
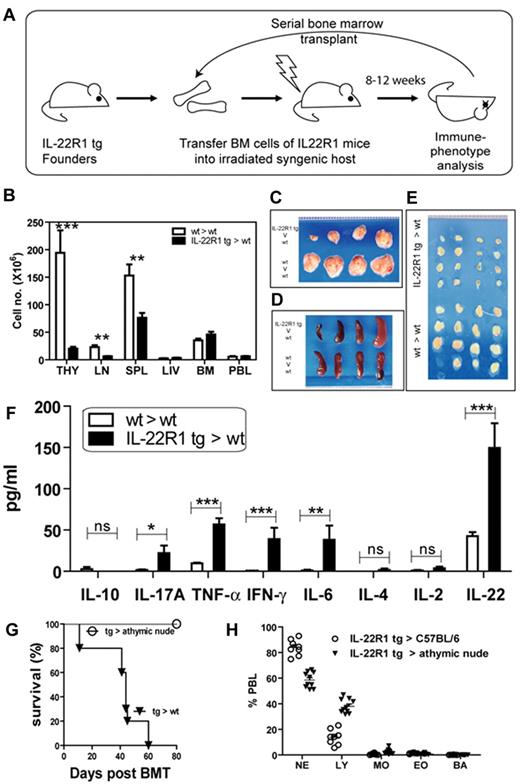
To examine whether the hematopoietic cells were sufficient to induce inflammation as seen in the IL-22R1 tg founders, we transferred 4 × 106 cells from the bone marrow of the IL-22R1 tg founders into lethally irradiated C57BL/6 syngeneic hosts (Figure 3A). As was the case in the founders, the health of these recipients deteriorated within 8 to 12 weeks after transfer, and they developed systemic inflammation leading to premature death. These animals progressed to severe multiorgan inflammation associated with lymphocyte and neutrophil infiltration. Blood analysis showed leukocytosis caused mainly by neutrophilia (supplemental Table 2). The gross structure and total cellularity of the thymus and lymph nodes were severely reduced in IL-22R1 bone marrow recipient mice, while there was a reduction in the total cellularity in spleen with no change in the morphology (Figure 3B-E). We detected elevated levels of Th1 and Th17 cytokines in the serum of the IL-22R1 tg founder bone marrow recipients (Figure 3F). Gene arrays also confirmed an increase in expression of IFN-γ, IL-1β, IL-6, and IL-22 (supplemental Figure 1). The inflammatory phenotype observed in these chimeras was similar to that seen in IL-22R1 tg animals. Even after 4 passages of bone marrow serial transplants, we continued to observe a phenotype similar to that seen in the IL-22R1 tg founders. As the founder animals would not breed, and these bone marrow recipients mimicked the disease seen in the founders, we used these chimeras to further characterize the phenotype resulting from IL-22R1 expression on lymphocytes.
Transplantation of IL-22R1 tg hematopoietic cells into wt recipients results in T cell–mediated spontaneous multiorgan inflammation. (A) Schematic diagram showing serial bone marrow transplantation in tg (IL-22R1→wt) and wt (wt→wt) recipients. (B) Total cellularity of hematopoietic organs in wt (wt→wt) and tg (IL-22R1→wt) recipients. Gross morphology of the thymus (C), spleen (D), and lymph nodes (E). Images were acquired using a digital SLR camera (Sony DSLR-A330) with a Macro 2.8/50 lens. (F) Analysis of the concentrations of inflammatory cytokines (by CBA) and IL-22 (by ELISA) in the serum of IL-22R1 tg (IL-22R1→wt) and wt (wt→wt) recipients (n = 7). The data are represented as the mean ± SEM. *P < .05, **P < 0.01, ***P < .001. (G) Kaplan-Meier survival plot of wt and Foxn1nu recipients receiving bone marrow from IL-22R1 tg mice. (H) Percentage of neutrophils (NE), lymphocytes (LY), monocytes (MO), eosinophils (EO), and basophils (BA) in peripheral blood lymphocytes from athymic nude mice compared with wt controls receiving IL-22R1 tg bone marrow.
Transplantation of IL-22R1 tg hematopoietic cells into wt recipients results in T cell–mediated spontaneous multiorgan inflammation. (A) Schematic diagram showing serial bone marrow transplantation in tg (IL-22R1→wt) and wt (wt→wt) recipients. (B) Total cellularity of hematopoietic organs in wt (wt→wt) and tg (IL-22R1→wt) recipients. Gross morphology of the thymus (C), spleen (D), and lymph nodes (E). Images were acquired using a digital SLR camera (Sony DSLR-A330) with a Macro 2.8/50 lens. (F) Analysis of the concentrations of inflammatory cytokines (by CBA) and IL-22 (by ELISA) in the serum of IL-22R1 tg (IL-22R1→wt) and wt (wt→wt) recipients (n = 7). The data are represented as the mean ± SEM. *P < .05, **P < 0.01, ***P < .001. (G) Kaplan-Meier survival plot of wt and Foxn1nu recipients receiving bone marrow from IL-22R1 tg mice. (H) Percentage of neutrophils (NE), lymphocytes (LY), monocytes (MO), eosinophils (EO), and basophils (BA) in peripheral blood lymphocytes from athymic nude mice compared with wt controls receiving IL-22R1 tg bone marrow.
Because ALK+ALCL is a T cell–derived lymphoma, we investigated whether the phenotype seen in IL-22R1 tg mice was T cell driven. IL-22R1 tg bone marrow was transferred to C57BL/6 and athymic nude mice, which cannot develop T cells due to the absence of a thymus. C57BL/6 recipients developed spontaneous inflammation and succumbed within 60 days posttransplantation, while the athymic nude mice did not develop inflammation and continued to survive 80 days posttransplantation (Figure 3G). Blood parameters showed that the C57BL/6 recipients exhibited neutrophilia, whereas the athymic mice exhibited normal neutrophil counts (Figure 3G-H).
IL-22R1 bone marrow recipient mice show thymic involution
The total cellularity of the thymus was drastically reduced in the sick IL-22R1 bone marrow recipients (Figure 3B). We analyzed the T-cell profiles of the thymus and lymph nodes and found a marked reduction in the percentage of double-positive (CD4+CD8+) cells with an increase in the percentages of single-positive (CD4 or CD8) and double-negative cells (supplemental Figure 2A). There was a gradual decrease in double-positive cells as the mice became progressively sicker (data not shown). In the lymph nodes, there was a reduction in the percentage of CD4+ cells while there was no statistically significant difference in the percentage of CD8+ cells in the IL-22R1 bone marrow recipient mice compared with the wt controls (supplemental Figure 1E). Although we have not further investigated the cause of this thymic involution in these mice, a recent report demonstrated that administration of IL-22 to wt mice induces thymic involution.19,20
IL-22R1 tg bone marrow produces a myeloproliferative disorder with high levels of neutrophils
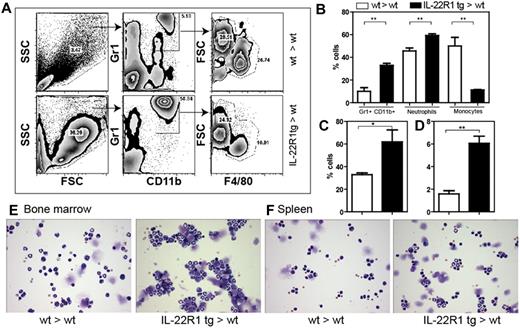
The IL-22R1 tg mice have an increased myeloid population in the blood, spleen, and bone marrow (Figure 4). Flow cytometric analysis of the myeloid population in the blood showed a higher percentage of Gr1+CD11b+ cells in IL-22R1 bone marrow recipient mice (Figure 4A-B). When the Gr1+CD11b+ population was further gated based on forward scatter and F4/80 expression to distinguish neutrophils and inflammatory monocytes, we found that the percentage of neutrophils was higher in IL-22R1 bone marrow recipient mice (Figure 4B). A similar increase in neutrophils was observed in the spleen and bone marrow (Figure 4C-D). Neutrophilia was also detected in cytospins from single-cell suspensions of spleen and bone marrow. The neutrophils in the spleen and bone marrow were mature and showed a segmented nucleus (Figure 4E-F). There was a gradual increase in the Gr1+CD11b+ population in the tg bone marrow recipients compared with the wt recipients (supplemental Figure 3). When the levels of the myeloid stimulatory cytokines, GM-CSF, G-CSF, and KC (CXCL1), were measured, high serum levels of G-CSF were detected in IL-22R1 tg founders (Figure 5E). In addition, the level of IL-17 (Figure 3F) was elevated in IL-22R1 bone marrow recipient mice. These cytokines are the major players in activating granulopoiesis, and these elevated cytokine levels are consistent with the cellular phenotype observed in the mice.
IL-22R1 tg mice exhibit neutrophilia. (A) Forward scatter/side scatter plot shows that the percentages of GR1+CD11b+ population was higher in IL-22R1 tg mice compared with the wt mice. The Gr1+CD11b+ population was further distinguished based on the FSC and F4/80 gates. (B) Percentage of neutrophils (FSChiF4/80lo) and monocytes (FSC loF4/80int) from polymorphonuclear leukocytes. (C-D) Percentage of cells that are Gr1+CD11b+ in bone marrow and spleen (n = 3), respectively. (E-F) Representative cytospins of 1 × 104 cells from bone marrow (×20; E) and spleen (×20; F) stained with Giemsa. Images were acquired with an Olympus BX40 light microscope using Image Proplus 6 software.
IL-22R1 tg mice exhibit neutrophilia. (A) Forward scatter/side scatter plot shows that the percentages of GR1+CD11b+ population was higher in IL-22R1 tg mice compared with the wt mice. The Gr1+CD11b+ population was further distinguished based on the FSC and F4/80 gates. (B) Percentage of neutrophils (FSChiF4/80lo) and monocytes (FSC loF4/80int) from polymorphonuclear leukocytes. (C-D) Percentage of cells that are Gr1+CD11b+ in bone marrow and spleen (n = 3), respectively. (E-F) Representative cytospins of 1 × 104 cells from bone marrow (×20; E) and spleen (×20; F) stained with Giemsa. Images were acquired with an Olympus BX40 light microscope using Image Proplus 6 software.
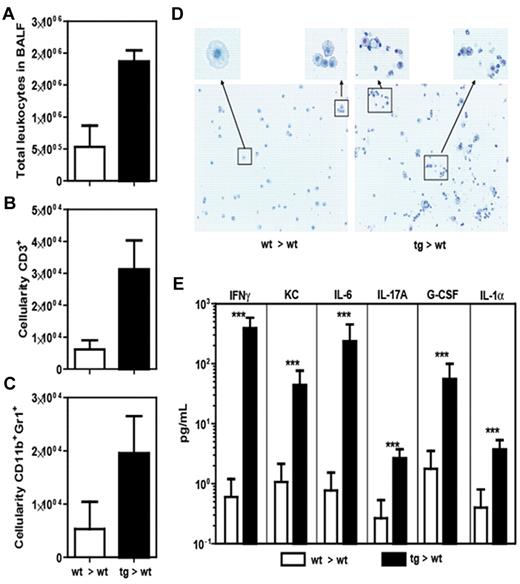
Analysis of BALF of tg and wt bone marrow recipients. Comparison of total leukocytes (A), T cells (B), and neutrophils (C) in BALF. (D) Cytospin and Giemsa staining of the BALF. (E) CBA array analysis of cytokine levels in BALF. Data from 1 experiment with 3 animals per group that is shown as mean ± SEM. ***P < .001.
Analysis of BALF of tg and wt bone marrow recipients. Comparison of total leukocytes (A), T cells (B), and neutrophils (C) in BALF. (D) Cytospin and Giemsa staining of the BALF. (E) CBA array analysis of cytokine levels in BALF. Data from 1 experiment with 3 animals per group that is shown as mean ± SEM. ***P < .001.
BALF from IL-22R1 bone marrow recipient mice has severe lymphocyte and neutrophil infiltration with high levels of inflammatory cytokines
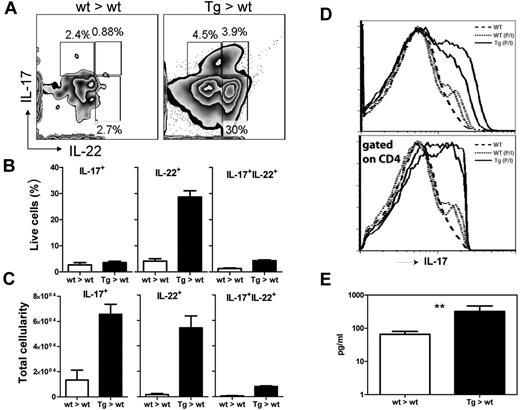
As the health of our IL-22R1 bone marrow recipient mice progressively worsened, they showed respiratory distress and necropsy revealed severe infiltration of inflammatory cells in their lungs. To investigate the cause of this respiratory distress, we analyzed the cellular infiltrates and cytokine levels in the BALF (Figure 5). As expected, there were significantly higher numbers of total leukocytes in the tg mice compared with the wt recipient controls. Based on flow cytometric analysis and analytical cytospins, we found that there was significant infiltration of lymphocytes and neutrophils in the airway (Figure 5A-D). Lymphocytic infiltrates were also confirmed by gene expression array analysis where gene transcripts for CD3 were elevated in the tg mice (supplemental Figure 4). In addition, there were elevated levels of many inflammatory cytokines in the BALF, such as IFN-γ, KC, IL-6, IL-17A, G-CSF, and IL-1α (Figure 5E). In the lungs, we observed a significant increase in the numbers of cells producing IL-22, IL-17A, and both IL-22 and IL-17A as measured by intracellular flow cytometry 6 hours after Brefeldin A intravenous injection (Figure 6A-C). Gene expression arrays confirmed these results showing an increase in proinflammatory cytokine genes including IL-18, IL-21, IL-22, and IL-27 (supplemental Figure 4).
G-CSF, IL-22, and IL-17 are elevated in IL-22R1 tg mice. (A) Zebra plot showing intracellular levels of IL-17 and IL-22 in T cells in lungs when injected with Brefeldin A for 6 hours intravenously. Graphs showing levels of IL-17 and IL-22 as percentage (B) and total cellularity (C). (D) Histogram of flow cytometric analysis of splenocytes stimulated with PMA and ionomycin for 5 hours blocked with Brefeldin A and stained with CD4 and IL-17 antibodies. (E) Cytometric bead array to assay the concentrations of G-CSF in the serum of IL-22R1 (n = 5) tg founders and nontransgenic littermates (n = 5). The data are represented as the mean ± SEM. **P < .01.
G-CSF, IL-22, and IL-17 are elevated in IL-22R1 tg mice. (A) Zebra plot showing intracellular levels of IL-17 and IL-22 in T cells in lungs when injected with Brefeldin A for 6 hours intravenously. Graphs showing levels of IL-17 and IL-22 as percentage (B) and total cellularity (C). (D) Histogram of flow cytometric analysis of splenocytes stimulated with PMA and ionomycin for 5 hours blocked with Brefeldin A and stained with CD4 and IL-17 antibodies. (E) Cytometric bead array to assay the concentrations of G-CSF in the serum of IL-22R1 (n = 5) tg founders and nontransgenic littermates (n = 5). The data are represented as the mean ± SEM. **P < .01.
ALK+ALCL-derived cell lines express IL-22R1 and Th17- associated genes
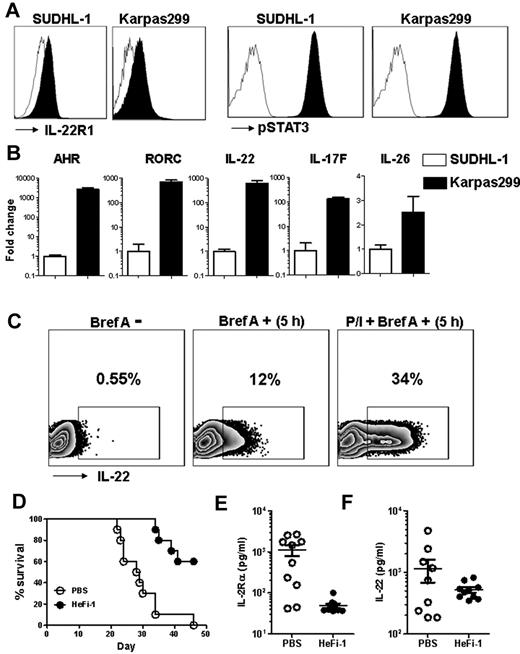
We determined that ALK+ALCL cell lines, Karpas299 and SUDHL-1, express IL-22R1 and detected constitutively activated pSTAT3 in these cells (Figure 7A). We also showed AHR, RORC, IL-22, IL-26, and IL-17F expression in both ALK+ALCL cell lines (Figure 7B). The expression of Th17 marker genes in Karpas299 was significantly higher than in SUDHL-1 cells. By flow cytometry, we detected constitutive expression of IL-22 in Karpas299 cells, and the expression level increased by 2.8-fold when activated by PMA and ionomycin (Figure 7C).
ALK+ALCL cell line characterization and leukemia model amplify IL-22, which correlates with tumor burden. (A) Histogram of flow cytometric analysis of cell lines stained for IL-22R1 and pSTAT3 (Tyr705) antibodies. (B) Relative quantification of AHR, RORC, IL-22, IL-17F, and IL-26 by real-time polymerase chain reaction. (C) Zebra-plot of flow cytometry data showing intracellular levels of IL-22 with (Bref A +) or without (Bref A −) Brefeldin A, and PMA and ionomycin stimulation with Brefeldin A (P/I+ Bref A +) for 5 hours. (D) Kaplan-Meier survival plot of the Karpas299 leukemia-bearing SCID/NOD mice injected with PBS or HeFi-1. Serum levels of sIL-2Rα (E) and IL-22 (F) in Karpas299 leukemia-bearing SCID/NOD mice at day 21 after therapy.
ALK+ALCL cell line characterization and leukemia model amplify IL-22, which correlates with tumor burden. (A) Histogram of flow cytometric analysis of cell lines stained for IL-22R1 and pSTAT3 (Tyr705) antibodies. (B) Relative quantification of AHR, RORC, IL-22, IL-17F, and IL-26 by real-time polymerase chain reaction. (C) Zebra-plot of flow cytometry data showing intracellular levels of IL-22 with (Bref A +) or without (Bref A −) Brefeldin A, and PMA and ionomycin stimulation with Brefeldin A (P/I+ Bref A +) for 5 hours. (D) Kaplan-Meier survival plot of the Karpas299 leukemia-bearing SCID/NOD mice injected with PBS or HeFi-1. Serum levels of sIL-2Rα (E) and IL-22 (F) in Karpas299 leukemia-bearing SCID/NOD mice at day 21 after therapy.
Previous studies have shown that treatment with anti-CD30 monoclonal antibody, HeFi-1, is effective in prolonging the survival of SCID/NOD mice challenged with Karpas299 cells.17,18 We used this model to determine whether IL-22 levels correlated with tumor burden and prognosis. HeFi-1 monoclonal antibody prolonged survival compared with PBS-treated mice (Figure 7D), confirming previous studies. Circulating levels of IL-2Rα, a surrogate marker of tumor burden, was significantly higher in PBS-treated mice compared with the HeFi-1–treated animals (Figure 7E). When screened for circulating levels of IL-22, PBS-treated mice had higher levels compared with the HeFi-1 treatment group (Figure 7F). In addition, early levels of IL-22 correlated with IL-2Rα levels as well as the overall prognosis of the animals.
ALK+ALCL is associated with increased serum IL-22 levels in patients
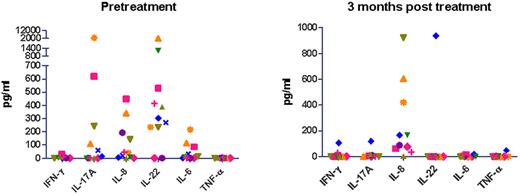
To determine whether the results observed in our IL-22R1 tg mice can serve as a model for human disease, we assayed serum cytokine levels in ALK+ALCL patients before and after chemotherapy (Figure 8). The patients received a dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) treatment but without rituximab as described for diffuse large B-cell lymphoma (DLBCL).21 We observed high levels of IL-22 protein in pretreatment serum samples from 9 of 11 ALK+ALCL patients. Some of the patients who expressed IL-22 also showed elevated levels of IL-17 (6/11), IL-6 (7/11), and IL-8 (9/11) cytokines. However, none of these patients contained measurable levels of IFN-γ or TNF-α in their serum. We next analyzed the cytokine levels in the serum of patients after treatment and observed that the levels of IL-22 were undetectable in all of the patients who were in complete remission. One patient who we assayed still had detectable serum IL-22 after treatment but subsequently had a relapse in the disease (supplemental Figure 5). We also assayed serum from DLBCL patients and found that only 50% of those patients had detectable IL-22 in their serum (10/20) compared with 82% of ALK+ALCL patients (9/11). In addition, the levels of IL-22 are significantly different (P = .0069) in that more than half of the ALK+ALCL patients have higher IL-22 than all of the DLBCL patients. IL-17 showed a similar trend, where only 15% of DLBCL patients have detectable IL-17 (3/20) compared with 55% of ALK+ALCL patients (6/11). Moreover, IL-17 and IL-22 levels are significantly higher in ALK+ALCL compared with DLBCL (supplemental Figure 6).
Th17 cytokine levels decline after chemotherapy in ALK+ALCL patients. Cytometric bead array to assay the concentrations of IFN-γ, IL-17A, IL-8, IL-22, IL-6, and TNF-α in serum of ALK+ALCL patients pre- and postchemotherapy. The patients received a dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) treatment but without rituximab.
Th17 cytokine levels decline after chemotherapy in ALK+ALCL patients. Cytometric bead array to assay the concentrations of IFN-γ, IL-17A, IL-8, IL-22, IL-6, and TNF-α in serum of ALK+ALCL patients pre- and postchemotherapy. The patients received a dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) treatment but without rituximab.
Discussion
The NPM-ALK fusion caused by chromosomal translocation is common in ALK+ALCL patients. Models of ALCL have explored the importance of NPM-ALK in tumorigenesis. Similar to other cancers, constitutively activated STAT3 is known to play a major role in survival and proliferation of ALK+ALCL cells. ALK, IL-9, IL-21, and IL-22 are known activators of STAT3 promoting survival and proliferation of ALK+ ALCL cells. In this study, our tg mice show that IL-22 plays a critical role in inflammation as a result of aberrant expression of IL-22R1 on lymphocytes. The aberrant expression of IL-22R1 may contribute to the development of the Th17 phenotype seen in ALK+ALCL. We demonstrate that ALK+ALCL cell lines expressing IL-22R1 have a Th17 phenotype that produce IL-22 and IL-17 at high levels and that ALK+ALCL patients have increased IL-22 and IL-17 in their serum.
Based on the observation that ALK+ALCL patients have IL-22R1 on their lymphoma cells, we hypothesized that expression of this receptor on lymphocytes may have an effect on ALCL pathogenesis as it signals via STAT3. The expression of IL-22R1 on lymphocytes did not, by itself, induce a lymphocytic malignancy in our animals even though the animals died after only 8 to 12 weeks. We suspect that development of T-cell lymphoma is dependent on the NPM-ALK fusion event and expression of IL-22R1 might be a consequence of preneoplastic changes. Our IL-22R1 tg mice exhibited respiratory distress, rough coat, and displayed sluggish movement with a humped appearance. Necropsy revealed that systemic multiorgan inflammation was the primary cause of their premature death. The tg mice exhibited severe neutrophilia, a hallmark of acute inflammation. These animals had high levels of Th1, Th17, and G-CSF cytokines in their serum. G-CSF is known to regulate the survival, proliferation, differentiation, and function of neutrophils and their precursors.22 IL-17 is known to induce the production of G-CSF and, thus, the increased granulopoiesis in IL-22R1 tg mice may be caused by the elevated levels of IL-17.23 In this study, we demonstrate that CD4+ T cells in IL-22R1 tg animals have already differentiated into a Th17 phenotype in vivo. This indicates the importance of this cell type in the pathogenesis of systemic inflammation in these mice. Using athymic nude (Foxn1nu) recipients, we further show that the inflammatory condition is driven by T cells as these recipients do not develop systemic inflammation when transplanted with IL-22R1 tg bone marrow. Neutrophil accumulation was also observed in the BALF of IL-22R1 tg mice, which coincides with the increased levels of IL-17, G-CSF, and KC (CXCL1). These findings correlate well with a previous study that showed that in addition to G-CSF, IL-17 also induces production of CXCL1, which is known to increase neutrophils in the airway.24
The inflammation found in our tg mice is quite similar to many of the clinical features of ALK+ALCL patients. These patients may be initially misdiagnosed as having an inflammatory disease rather than lymphoma. They frequently present with neutrophilic infiltration in multiple organs and show an increase in G-CSF levels.19,25 In our study, we found that 6 of 11 ALK+ALCL patients had increased levels of IL-17 before chemotherapy. However, IL-17 was undetectable in all samples postchemotherapy, showing that the inflammation was associated with the lymphoma. Recent studies have shown that STAT3 is critical for the development and lineage commitment of Th17 cells.19,26 In our tg model, we have shown that there is constitutive activation of STAT3 in T cells that might contribute to the increased levels of IL-17–producing T cells. We also found that ALK+ALCL cell lines express Th17-associated signature genes, including IL-17F, IL-22, IL-26, AHR, and RORC. An independent study investigated gene expression profiles in several types of T- and NK-cell lymphomas and found that Th17 genes were among the top ranked genes as molecular classifiers for ALK+ALCL.27 Although we show that the Th17 phenotype of the leukemic cells is seen in ALK+ALCL, we have not extensively tested for IL-22R1 expression and IL-22 responsiveness by other T cell–derived lymphomas. However, Th17 cytokines might also be produced by pathogenic T cells in other such malignancies.
We have documented high levels of circulating IL-22 in IL-22R1 tg founders as well as in bone marrow recipients. These data are consistent with a previous in vitro study where ALK+ALCL cell lines expressing IL-22R1 have a positive autoregulatory feedback loop that amplifies the levels of IL-22 in vitro.5 To confirm an autoregulatory loop in an in vivo model, we challenged SCID/NOD mice with Karpas299 cells and treated with PBS or HeFi-1 (α-CD30). HeFi-1–treated mice survived significantly longer than control mice. This correlated with tumor burden based on the levels of a surrogate marker of tumor burden, IL-2Rα. Strikingly, a similar difference was seen in circulating levels of IL-22 in PBS- and HeFi-1–treated animals, where higher tumor burden and lower survival rate correlated well with increased levels of IL-22. Furthermore, the animals that succumbed to the leukemia in either group had significantly higher levels of IL-2Rα and IL-22 (data not shown). In another study, a similar autoregulatory feedback loop of IL-22 was shown in a human lung cancer xenograft model.19,28 In ALK+ALCL patients, we measured high levels of IL-22 prechemotherapy, which returned to basal levels postchemotherapy. In the patients' samples, there is 100% correlation between the decrease in the level of IL-22 and development of complete remission. This is the first report to show that serum IL-22 levels are elevated in this type of lymphoma. Our findings suggest that aberrant expression of IL-22R1 leads to an increase in IL-22 in the environment. It would be of interest to evaluate the effects on survival and inflammation by neutralizing IL-22 in ALK+ALCL models such as NPM-ALK tg or SCID/NOD mice injected with Karpas299. Bard and coworkers5 have already shown that IL-22 supports proliferation of ALK+ALCL cell lines and this activity can be blocked by administering IL-22 binding protein in vitro.
There is a growing body of evidence suggesting that some nonhematopoietic cancers may use the IL-22R1/STAT3 pathway to enhance growth and survival. A recent study showed that lung cancer cell lines expressing IL-22R1 are protected from chemotherapeutic drug-induced apoptosis by IL-22 via activation of STAT3 and its downstream antiapoptotic proteins.19,28 It would be of interest to investigate the role of IL-22 in other human tumors such as colorectal adenocarcinoma, lung carcinoma, melanoma, hepatoma, and renal carcinoma, because the cell lines derived from these tumors express IL-22R1.1,28
This study reveals a previously unknown role of the IL-22/IL-22R1 pathway in inflammation, which could be important in ALK+ALCL lymphoma as these cells aberrantly express IL-22R1 and produce IL-22. Further studies are warranted to investigate the effects of IL-22R1 expression on the progression of lymphomas and systemic inflammatory conditions. Our investigation has established a new mouse model that highlights the previously underappreciated role of IL-22R1 in inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank John Wine and Kelli Czarra for conducting animal experiments; Nicole Grant, Jeff Subleski, Tim Chan, Isabelle Shuggi, and Mike Sanford for technical assistance; and Drs Doug Kuhns, John Gallin, Scott Durum, Daniel McVicar, and Pamela Schwartzberg for their advice and discussions regarding this project.
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project has been funded with federal funds from the National Cancer Institute, NIH, under contract HHSN261200800001E.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States government.
National Institutes of Health
Authorship
Contribution: R.S. and A.P.M. performed the research, analyzed the data, and wrote the paper; R.S., A.P.M., D.A.R., W.H.W., and H.A.Y. designed the experiments; R.S., A.P.M., D.A.R., K.R., M.K., H.S., and S.P. performed the experiments; D.M.K., K.D., S.K.A., and R.P.D. provided intellectual input; and L.F. made the IL-22R1 tg mice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Howard A. Young, Cancer and Inflammation Program, PO Box B, Bldg 560, Frederick, MD 21702; e-mail: YoungHow@mail.nih.gov; or Ram Savan, Cancer and Inflammation Program, PO Box B, Bldg 560, Frederick, MD 21702; e-mail: savanr@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal