Abstract
Mechanisms governing stress-induced hematopoietic progenitor cell mobilization are not fully deciphered. We report that during granulocyte colony-stimulating factor–induced mobilization c-Met expression and signaling are up-regulated on immature bone marrow progenitors. Interestingly, stromal cell–derived factor 1/CXC chemokine receptor-4 signaling induced hepatocyte growth factor production and c-Met activation. We found that c-Met inhibition reduced mobilization of both immature progenitors and the more primitive Sca-1+/c-Kit+/Lin− cells and interfered with their enhanced chemotactic migration to stromal cell–derived factor 1. c-Met activation resulted in cellular accumulation of reactive oxygen species by mammalian target of rapamycin inhibition of Forkhead Box, subclass O3a. Blockage of mammalian target of rapamycin inhibition or reactive oxygen species signaling impaired c-Met–mediated mobilization. Our data show dynamic c-Met expression and function in the bone marrow and show that enhanced c-Met signaling is crucial to facilitate stress-induced mobilization of progenitor cells as part of host defense and repair mechanisms.
Introduction
During steady state conditions, adhesive interactions between the bone marrow (BM) stromal cells and primitive hematopoietic cells mostly result in stem cell retention, in a noncycling and nonmotile mode. However, low levels of progenitor cells are continuously released from the BM to the blood circulation as part of homeostasis. This process is dramatically amplified during emergency situations because of damage and cell death, as part of host defense and repair, in response to stress signals, including cytokines such as granulocyte colony-stimulating factor (G-CSF). Repetitive G-CSF stimulations are commonly used in the clinic, mimicking emergency situations to harvest stem and progenitor cells from the circulation for transplantation protocols.1,2 The BM reservoir of immature and maturing leukocytes is dynamic, replenishing the blood with new cells on demand. These dynamic changes are achieved through a complex interplay between the immune and nervous systems, the bones and the BM microenvironment, involving cytokines, chemokines, proteolytic enzymes, and adhesion molecules.3 In particular, oscillations in BM levels of stromal cell–derived factor 1 (SDF-1; transiently increased and subsequently degraded) and CXC chemokine receptor-4 (CXCR4) activation play a crucial role in promoting progenitor cell egress.4,5
The cytokine hepatocyte growth factor (HGF) and its receptor c-Met control complex biologic programs known as “invasive growth” and tumor spreading.6 Reactive oxygen species (ROS) are constantly generated during intracellular metabolism and in response to cytokines. Although excess ROS can cause oxidative damage to DNA, moderate levels have important roles in cell signaling, regulating different physiologic and pathologic cellular processes, including cell-cycle progression, migration, and invasion.7 Finally, redox signaling has emerged as an important regulator of hematopoietic stem cell (HSC) self-renewal and lifespan.8,9
The Forkhead Box, class O (FOXO) family of Forkhead transcription factors is a regulator of oxidative stress.10 Loss of FOXO function in HSCs results in increased ROS levels, defective maintenance of quiescence, and reduced long-term repopulating ability.11,12 FOXOs are a direct substrate of the protein kinase Akt, a mammalian target of rapamycin inhibition (mTOR) target,13 which inactivates them by phosphorylation.14
In this study, we demonstrate that c-Met expression levels on immature and maturing leukocytes in the BM reservoir are dynamic and dramatically increased when urgent requirements for enhanced leukocyte production and recruitment emerge. Moreover, full c-Met activation requires the integrity of SDF-1/CXCR4 signaling pathways. We also show that c-Met activation generates a mTOR/FOXO3a/ROS signaling cascade, which induces quiescent and nonmotile BM hematopoietic progenitor cells to acquire migrating features that are needed for their recruitment as part of host defense and repair mechanisms.
Methods
Mice
Mobilization experiments with C57BL/6 mice (Harlan) were approved by the Animal Care and Use Committee of Weizmann Institute. G-CSF−/− mice (mixed background) were kindly provided by Prof G. J. Lieschke (Cancer and Hematology Division, The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia). Mice were mobilized with a daily subcutaneous injection of G-CSF (Filgrastim [Amgen]; 300 μg/kg in 250 μL of 0.9% NaCl, 5% fetal calf serum), pH 4.55) for 3 and 5 consecutive days. Control mice received injections of phosphate-buffered saline (PBS). c-Met signaling was blocked by intraperitoneal injections of polyclonal neutralizing anti–c-Met antibodies (Abs; R&D Systems) (10 μg in 400 μL of PBS) on days 4 and 5 of G-CSF treatment. These Abs inhibited c-Met binding to HGF and induced down-regulation of the receptor in vivo (data not shown). Nonspecific goat immunoglobulin G (R&D Systems) were used as control Ab. The c-Met inhibitor PHA-665752 (a kind gift from Dr James Christensen and Pfizer) was dissolved in 7.5 μL of dimethyl sulfoxide (DMSO) and 400 μL of PBS and delivered as 7.5 mg/kg twice a day intraperitoneally for 5 consecutive days together with G-CSF. DMSO and PBS were delivered as control vehicle. CXCR4 signaling was blocked by intraperitoneal injection of CXCR4 neutralizing Abs (10 μg) on the last 2 days of G-CSF injections.4 Mice were also mobilized by 2 daily intraperitoneal injections of 4 mg of HGF (R&D Systems). This protocol was chosen as optimal for progenitor cell mobilization in most mice. Mice requiring additional HGF stimulations for mobilization were excluded. The antioxidant N-acetyl-L-cysteine (NAC; Sigma-Aldrich) was administered subcutaneously at 50 mg/kg for 2 days alone or together with G-CSF or HGF. Rapamycin (Rapamune/Sirolimus; Wyeth) was delivered at 480 μg/kg subcutaneously for 2 days. Peripheral blood (PB) from mice asphyxiated with CO2 was collected by cardiac aspiration in heparinized tubes. White blood cell counts in the circulation were determined by hematocytometer after red blood cell lysis. Unless otherwise indicated, mice were killed 3-5 hours after the last injection.
Colony-forming assay
PB mononuclear cells were isolated by Ficoll separation. Total BM cells (1.5 × 104 cells/mL) or PB mononuclear cells (2 × 105 cells/mL) separated by Ficoll were seeded in semisolid cultures as previously described.4 Colonies were scored 7 days later, according to morphologic criteria.
Lentivirus vector preparation and in vitro cell transduction to silence MET
BM cells obtained from mice treated for 5 days with G-CSF were transduced with concentrated lentiviral vectors expressing a c-Met short hairpin RNA (shRNA) or a point-mutated nonfunctional control shRNA.15 Vector stocks were produced as described previously.16 The concentration of viral p24 antigen was assessed with the HIV-1 p24 core profile enzyme-linked immunoabsorbent assay (ELISA) kit (NEN Life Science Products) according to the manufacturer's instructions. Cells were transduced overnight in 24-well plates (5 × 105 cells/well in 0.5 mL of medium) with the use of 1 mg/mL of p24 equivalents in the presence of 8 mg/mL Polybrene (Sigma-Aldrich) and the following mouse cytokines: stem cell factor (50 ng/mL), thrombopoietin (10 ng/mL), interleukin-6 (10 ng/mL), and fms-like tyrosine kinase-3 ligand (50 ng/mL). Cells transduction was evaluated through green fluorescent protein (GFP) positivity (GFP coding sequence is present in the shRNA-containing vectors).
Migration assays
Migration assays were performed in Transwell chambers (Costar). The filter upper side was coated with 15 mg/cm2 reconstituted Matrigel basement membrane (Collaborative Research). Viable cells (105) previously transduced with c-Met or control shRNAs vectors were seeded in the upper chambers in RPMI 10% fetal bovine serum (FBS), and the lower wells were filled with RPMI 10% fetal bovine serum with SDF-1 (50 ng/mL). After 3 hours, media from the upper and lower wells were collected, and the number of GFP+ transduced cells were counted by fluorescence-activated cell sorting (FACS) and seeded for colony assays. Migration was calculated as the percentage of GFP+ migrating cells (of total GFP+ cells). The number of migrated progenitors was calculated as normalizing the number of green colonies derived from 105 BM cells that were initially loaded to migrate, with the number of colonies scored in the migrating fraction.
BM cells from mobilized mice were pretreated with c-Met inhibitor (PHA) 250nM for 40 minutes and then loaded (105 cells/well) on the upper Transwell chamber. The lower chambers contained RPMI 10% FBS with SDF-1 and PHA. After 3 hours, cells from the upper and lower chambers were collected and counted by FACS and seeded for colony assays. BM cells from control mice or mice receiving G-CSF alone or in combination with NAC were subjected to Transwell migration (105 cells/well). The lower chamber contained RPMI 10% FBS and SDF-1 (50 ng/mL).
Flow cytometry
Expression of c-Met on BM cells was detected by polyclonal rabbit anti–c-Met (R&D Systems) and subsequently with biotinylated rabbit anti–goat Ab (Dako), which was followed by streptavidin fluorescein isothiocyanate (FITC)–labeled Ab (Jackson ImmunoResearch Laboratories). Expression of c-Met on Sca-1+/c-Kit+ was detected with an anti–c-Met phycoerythrin (PE; eBioscience). Levels of Sca-1+/c-Kit+/Lin− (SKL) were determined by triple staining with FITC-conjugated Ab, indicating a lineage-positive phenotype (CD4, natural killer [NK], CD8 from eBioscience; B220, CD11b, GR-1 from BD) together with anti–Sca-1 PE and anti–c-kit allophycocyanin (eBioscience). Labeled cells (1 × 106) were analyzed with the use of FACSCalibur (BD Biosciences) with CellQuest 3.3. Intracellular expression of HGF in BM cells was detected by membrane staining for Gr-1 and CD115 and then fixatation/permeabilization by the Cyoperm/Cytofix kit according to the manufacturer's instructions (BD Biosciences). Intracellular staining with anti-HGF Ab (R&D Systems) and subsequently with biotinylated rabbit anti–goat Ab (Dako) was then followed by streptavidin-PE–labeled Ab (Jackson ImmunoResearch Laboratories). HGF expression was analyzed on Gr-1high and CD115− cells, which defines the polymorphonuclear (PMN) cell population. ROS generation was detected by incubating BM cells with 2μM hydroethidine (Molecular Probes) for 10 minutes at 37°C. Cells were then washed in PBS and stained for SKL markers. The fluorescence of oxidized hydroethidine was determined by excitation at 488 nm and emission at wavelengths 580 ± 21 nm with the use of the BD LSR II Flow Cytometer (BD Biosciences) and analyzed by FACSDiva 6.1.3 (BD Biosciences). FOXO3a levels were determined in BM and PB Lin−/c-Kit+ progenitor cells with the use of Ab, which detects the inactive form of FOXO3a (phospho Thr32 FOXO3a; Santa Cruz Biotechnology). P-FOXO3a staining was performed on fixed/permeabilized cells with the use of the BrdU (5′-bromo-2′-deoxyuridine) flow kit according to the manufacturer's instructions (BD Biosciences). Phospho–c-Met staining was performed with the use of a phospho-specific Ab (Millipore) followed by a secondary Delight 649 Ab on cells, previously fixed/permeabilized with the BrdU flow kit (BD Biosciences). CD34 staining was performed with a CD34-FITC (eBioscience) Ab. Phospho–c-Met and CD34/SKL cells were determined by FACS CyAN (Dako) and analyzed by FlowJo 8.6 (TreeStar). FACS analysis was performed on BM and blood cells collected 3-5 hours after the last injection.
Real-time reverse-transcription polymerase chain reaction
Total RNA was isolated from BM cells (5 × 106 cells per sample) with the use of Tri-reagent (Molecular Research) according to the manufacturer's protocol. Two micrograms of each RNA sample was reverse-transcribed with Moloney murine leukemia virus reverse-transcriptase (Promega) and oligodT. Real-time polymerase chain reaction (PCR) was performed with an ABI 7000 machine (Applied Biosystems) with the SYBR Green PCR Master Mix (Applied Biosystems) and the following primers: HGF sense, 5′-CCCATGAACACAGCTTTTTGC-3′; HGF antisense, 5′-TTCCCCTCGAGGATTTCGA-3′; FOXO3a sense, 5′-CGGCTCACTTTGTCCCAGAT-3′; FOXO3a antisense, 5′-GCCGGATGGAGTTCTTCCA-3′; FOXO1 sense, 5′-GCGGGCTGGAAGAATTCAAT-3′; FOXO1 antisense, 5′-TTCCTTCATTCTGCACTCGAATAA-3′; FOXO4 sense, 5′-AGGCGCTCCCATCCCTAA-3′; FOXO4 antisense, 5′-TCCAGATCCTGAGGCATTCTG-3′; Catalase sense, 5′-CAGGATCCTTCAGGTGAGTCTGT-3′; and Catalase antisense, 5′-CCATATTGTTTCCATCCTTTATCCA-3′.
Raw data from reactions that used HGF, FOXO3a, FOXO1, FOXO4, or catalase primers were normalized to the corresponding data from reactions that used hypoxantine phosphoribosyltransferase primers.
The expression of mMET was analyzed with the use of the Taqman gene-specific probes from Applied Biosystems (Mm00434924_m1, NM_008591) on the total population of BM cells, of which ∼ 50% were positive for GFP expression or on sorted GFP population. In addition, mMET expression was analyzed on sorted GFP+ BM cells.
HGF ELISA
To detect HGF protein levels, ELISA Costar strips were coated with 100 μL of goat anti–mouse HGF Ab AF2207 (R&D Systems) at 0.5 μg/mL diluted in PBS and incubated overnight at 4°C. The strips were washed 3 times with wash buffer (0.05% Tween 20 in PBS at pH 7.4) and incubated for 1 hour at room temperature with 300 μL of blocking buffer (1% bovine serum albumin, 5% sucrose, and 0.05% NaN3 in PBS). After washes, 100 μL of samples undiluted and diluted at 1/2 and 1/10 in a sample diluent (0.1% bovine serum albumin, 0.05% Tween 20 in Tris [tris(hydroxymethyl)aminomethane]–buffered saline at pH 7.3) were loaded, and strips were incubated for 2 hours at room temperature. After washes, 100 μL of biotinylated goat anti–mouse HGF detection Ab BAF2207 (R&D Systems) at 0.4 μg/mL was added, and strips were incubated for 2 hours at room temperature. After washes, 100 μL of 1 μg/mL horseradish peroxidase–streptavidin (Sigma-Aldrich) was added, and strips were incubated for 30 minutes at room temperature. After washes, 100 μL of TMB (3,3′,5,5′-tetramethylbenzidine) substrate solution (Chemicon) was added, and incubation continued for 20-30 minutes. The reaction was stopped by adding 50 μL of 1M H2SO4 solution. Optical density was determined with a microplate reader set at 450 nm. Readings at 570 nm were then subtracted from the results. Recombinant mouse HGF (R&D Systems) was used to generate a linear standard curve.
SDF-1 ELISA
Total protein content in BM fluids was quantified by Bradford assay, and equal protein amounts were assayed for SDF-1 according to previously published protocols.4 SDF-1 was quantified on BM samples of mice collected after 3-5 hours from the last injection.
c-Met immunoprecipitation
BM cells were lysated with ice-cold RIPA buffer supplemented with fresh 1% protease inhibitors cocktail (Sigma-Aldrich P8340) and 0.2mM per-vanadate as phosphatase inhibitor. Immunoprecipitation was performed according to standard protocols with the use of anti–c-Met Abs B-2 (Santa Cruz Biotechnology). Purified proteins were resolved by 8% sodium dodecylsulfate–polyacrylamide gel electrophoresis under reducing conditions, transferred onto Hybond C nitrocellulose membranes (Whatman GmbH), and immunoblotted with antiphosphotyrosine clone 4G10 (Upstate Biotech) or anti–c-Met (R&D Systems). Final detection was performed with an enhanced chemiluminescence system (Pierce Chemical).
Gelatin zymography
Bones were flushed in PBS, and supernatants were kept on ice. Protein concentration was measured by Bradford protein assay (Bio-Rad Laboratories Inc) Five micrograms of BM proteins were loaded on 10% sodium dodecylsulfate–polyacrylamide gel electrophoresis gels containing 1 mg/mL gelatin. Gels were rinsed for 30 minutes in 2.5% Triton X-100, washed with double-distilled H2O, and incubated at 37°C for 16 hours with developing buffer consisting of 50mM Tris (pH 8), 5mM CaCl2, 200mM NaCl, and 0.02% Brij (Sigma-Aldrich). Gels were stained with 0.25% Coomassie blue for 3 hours and destained with 5% acetic acid and 10% methanol.
Statistical analysis
Significance levels of data were determined by t test for the differences in mean values.
Results
c-Met is up-regulated and activated by G-CSF by SDF-1 signaling
We first investigated the potential roles of HGF and c-Met in a murine model of G-CSF–induced mobilization. Our data show that HGF is accumulated in the blood after 5 consecutive G-CSF injections or even after a single one (which induces progenitor cell release17 ; Figure 1A). Moreover, after 5 consecutive injections of G-CSF we observed an increased HGF production in BM leukocytes (Figure 1B). Because the most relevant source of HGF is myeloid PMN cells,18 we examined also HGF production in these cells. Figure 1C shows that HGF levels are increased in PMN cells after 3 days of G-CSF stimulation, peaking at day 5 of G-CSF treatment (Figure 1C), correlating with the degree of SKL cell mobilization (Figure 1D). Note, exogenous administration of G-CSF to G-CSF−/− mice, which lack granulocytes,19 failed to mobilize SKL cells (Figure 1D) and to promote HGF elevation in the plasma (Figure 1E). We then examined the effect of G-CSF administration on c-Met expression at the cell membrane. Compared with controls, mice injected with G-CSF showed significantly higher c-Met levels on the surface of BM cells, PMN cells, immature c-Kit+/Lin− cells, and primitive CD34−/SKL cells (Figure 1F-G). Importantly, G-CSF was able to promote c-Met activation in circulating primitive SKL cells. Although a very low percentage of circulating SKL cells expressed phosphorylated c-Met in control mice, we found a significant increase in the percentage of this population that express phosphorylated c-Met on the administration of G-CSF (Figure 1K-L).
HGF and c-Met are up-regulated by G-CSF in a CXCR4/SDF-1–dependent manner. (A) Plasma HGF levels, evaluated by ELISA, in mice that received 1 injection of G-CSF and were killed 24 hours afterward or in mice receiving 5 consecutive injections of G-CSF. (B) Mice were given G-CSF for 5 consecutive days. HGF intracellular levels were determined in BM leukocytes by FACS analysis. (C) Intracellular HGF levels in BM PMN cells of control mice or mice receiving 3 or 5 consecutive injections of G-CSF were determined by FACS analysis. (D) G-CSF knockout (KO) mice or wild-type (WT) counterparts were injected with PBS or with 3 or 5 consecutive daily injections of G-CSF. The levels of circulating SKL cells were determined. (E) Plasma HGF levels in G-CSF KO and WT mice receiving PBS or 5 consecutive injections of G-CSF were determined by ELISA. (F) Cell-surface c-Met levels were quantified by FACS analysis. Representative c-Met expression on total BM cells of control (b) or 5 days of G-CSF in treated mice (c), together with control secondary staining (a) is shown. (G) A summary of 4 independent experiments showing membrane c-Met expression on BM cells, PMN cells, BM c-kit+/Lin− cells, or SKL/CD34− cells. (H-I) BM cells were left untreated or treated in vitro with SDF-1. c-Met activation (H) and intracellular HGF expression (I) were measured. (J) Circulating SKL cells in control mice or mice receiving 3 or 5 injections of G-CSF alone, or together with anti CXCR4 neutralizing Abs on the last 2 days of G-CSF treatment. (K) Percentage of phospho–c-Met on SKL cells in the PB of control mice, mice treated with 5 injections of G-CSF alone, or with anti-CXCR4 neutralizing Abs on days 4 and 5 of G-CSF treatment. (L) Representative FACS analysis showing the percentage of phospho–c-Met expression on circulating SKL cells. Percentages indicate the frequency of phospho c-Met–positive cells. (M) HGF levels in BM PMN cells from control mice or mice receiving 3 injections of G-CSF alone, or with anti-CXCR4 neutralizing Abs on days 2 and 3 of G-CSF treatment. Indicated values are presented as mean ± SE in ≥ 3 independent experiments, 6 mice in each group. *P < .05; **P < .001. SSC indicates side scatter.
HGF and c-Met are up-regulated by G-CSF in a CXCR4/SDF-1–dependent manner. (A) Plasma HGF levels, evaluated by ELISA, in mice that received 1 injection of G-CSF and were killed 24 hours afterward or in mice receiving 5 consecutive injections of G-CSF. (B) Mice were given G-CSF for 5 consecutive days. HGF intracellular levels were determined in BM leukocytes by FACS analysis. (C) Intracellular HGF levels in BM PMN cells of control mice or mice receiving 3 or 5 consecutive injections of G-CSF were determined by FACS analysis. (D) G-CSF knockout (KO) mice or wild-type (WT) counterparts were injected with PBS or with 3 or 5 consecutive daily injections of G-CSF. The levels of circulating SKL cells were determined. (E) Plasma HGF levels in G-CSF KO and WT mice receiving PBS or 5 consecutive injections of G-CSF were determined by ELISA. (F) Cell-surface c-Met levels were quantified by FACS analysis. Representative c-Met expression on total BM cells of control (b) or 5 days of G-CSF in treated mice (c), together with control secondary staining (a) is shown. (G) A summary of 4 independent experiments showing membrane c-Met expression on BM cells, PMN cells, BM c-kit+/Lin− cells, or SKL/CD34− cells. (H-I) BM cells were left untreated or treated in vitro with SDF-1. c-Met activation (H) and intracellular HGF expression (I) were measured. (J) Circulating SKL cells in control mice or mice receiving 3 or 5 injections of G-CSF alone, or together with anti CXCR4 neutralizing Abs on the last 2 days of G-CSF treatment. (K) Percentage of phospho–c-Met on SKL cells in the PB of control mice, mice treated with 5 injections of G-CSF alone, or with anti-CXCR4 neutralizing Abs on days 4 and 5 of G-CSF treatment. (L) Representative FACS analysis showing the percentage of phospho–c-Met expression on circulating SKL cells. Percentages indicate the frequency of phospho c-Met–positive cells. (M) HGF levels in BM PMN cells from control mice or mice receiving 3 injections of G-CSF alone, or with anti-CXCR4 neutralizing Abs on days 2 and 3 of G-CSF treatment. Indicated values are presented as mean ± SE in ≥ 3 independent experiments, 6 mice in each group. *P < .05; **P < .001. SSC indicates side scatter.
We next investigated the mechanisms underlying G-CSF–induced c-Met activation, focusing on SDF-1/CXCR4 signaling because this pathway is involved in egress from the BM.1,2,4,20 Figure 1H shows that c-Met activation is indeed strongly increased in BM cells after a short incubation with SDF-1 in vitro. Interestingly, we found that SDF-1 stimulation in vitro caused a significant increase in HGF expression on BM leukocytes and, in particular, on BM PMN cells (Figure 1I). We then evaluated the effect of CXCR4 neutralization on c-Met activation. CXCR4 neutralization impaired SKL cell mobilization by G-CSF (Figure 1J), in line with results previously obtained by our group with the use of neutralizing Abs4 or by another group that used CXCR4−/− hematopoietic chimeras.21 Consistently, CXCR4 neutralization in vivo reduced the percentage of circulating SKL cells expressing phospho–c-Met (Figure 1K-L). CXCR4 neutralization also abrogated the G-CSF–induced increase in HGF production observed on BM PMN cells after 3 days of G-CSF stimulations (Figure 1M). These results indicate that G-CSF promotes the expression of functional c-Met through SDF-1/CXCR4–mediated regulation of HGF production on BM PMN cells.
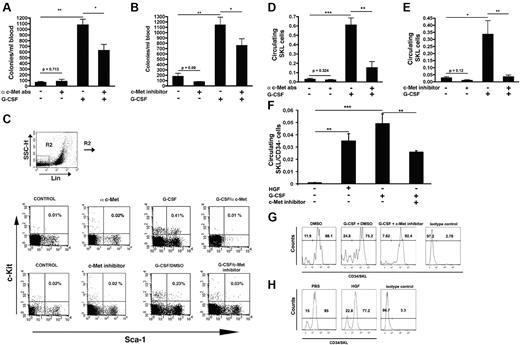
c-Met neutralization reduces G-CSF–induced mobilization of immature progenitor cells
To determine the role of c-Met in mobilization we blocked its signaling during G-CSF treatment. A significant decrease in the number of circulating progenitors was observed in mice coinjected with G-CSF and c-Met neutralizing Abs on days 4 and 5 of G-CSF treatment, compared with animals receiving G-CSF alone (Figure 2A). This phenomenon was accompanied by a marked decrease in the number of circulating SKL cells (Figure 2C-E). Administration of immunoglobulin G control Ab did not affect mobilization (data not shown).
c-Met inhibition impairs progenitor cell mobilization. (A) Mice were injected with anti–c-Met Abs, 5 consecutive injections of G-CSF or anti–c-Met on days 4 and 5 of G-CSF administration. The frequency of circulating progenitor cells was analyzed. (B) Numbers of circulating progenitors in mice injected with vehicle, PHA, 5 consecutive doses of G-CSF with vehicle, or c-Met inhibitor. (C) A representative FACS analysis showing, in the upper lane, the percentage of circulating SKL cells in control mice or mice receiving c-Met Abs, or G-CSF alone or together with c-Met Abs. The lower lane depicts the percentage of SKL in the blood of mice injected with vehicle or c-Met inhibitor or G-CSF with vehicle or c-Met inhibitor. (D-E) A summary of ≥ 3 independent experiments showing the percentage of circulating SKL cells (D) in mice that were given anti–c-Met Abs, or G-CSF alone or G-CSF in combination with anti c-met Abs or (E) in mice that were injected with vehicle or with c-Met inhibitor, or G-CSF together with vehicle or c-Met inhibitor. (F) Percentage of circulating SKL/CD34− cells in mice that were injected with vehicle, HGF, G-CSF together with vehicle, or c-Met inhibitor. In all the experiments, mobilization was evaluated after 3-5 hours after the last injection. (G) Representative FACS plot showing the percentage of CD34− cells within the SKL population in the circulation of mice treated with DMSO, G-CSF and vehicle, or G-CSF and c-Met inhibitor. (H) Representative FACS plot showing the percentage of CD34− cells within the SKL population in the circulation of mice treated with PBS or HGF. In all the experiments, mobilization was evaluated after 3-5 hours from the last injection. Indicated values are presented as mean ± SE in ≥ 3 independent experiments, 6 mice in each group. *P < .05; **P < .001.
c-Met inhibition impairs progenitor cell mobilization. (A) Mice were injected with anti–c-Met Abs, 5 consecutive injections of G-CSF or anti–c-Met on days 4 and 5 of G-CSF administration. The frequency of circulating progenitor cells was analyzed. (B) Numbers of circulating progenitors in mice injected with vehicle, PHA, 5 consecutive doses of G-CSF with vehicle, or c-Met inhibitor. (C) A representative FACS analysis showing, in the upper lane, the percentage of circulating SKL cells in control mice or mice receiving c-Met Abs, or G-CSF alone or together with c-Met Abs. The lower lane depicts the percentage of SKL in the blood of mice injected with vehicle or c-Met inhibitor or G-CSF with vehicle or c-Met inhibitor. (D-E) A summary of ≥ 3 independent experiments showing the percentage of circulating SKL cells (D) in mice that were given anti–c-Met Abs, or G-CSF alone or G-CSF in combination with anti c-met Abs or (E) in mice that were injected with vehicle or with c-Met inhibitor, or G-CSF together with vehicle or c-Met inhibitor. (F) Percentage of circulating SKL/CD34− cells in mice that were injected with vehicle, HGF, G-CSF together with vehicle, or c-Met inhibitor. In all the experiments, mobilization was evaluated after 3-5 hours after the last injection. (G) Representative FACS plot showing the percentage of CD34− cells within the SKL population in the circulation of mice treated with DMSO, G-CSF and vehicle, or G-CSF and c-Met inhibitor. (H) Representative FACS plot showing the percentage of CD34− cells within the SKL population in the circulation of mice treated with PBS or HGF. In all the experiments, mobilization was evaluated after 3-5 hours from the last injection. Indicated values are presented as mean ± SE in ≥ 3 independent experiments, 6 mice in each group. *P < .05; **P < .001.
Next, we inhibited c-Met activation with the use of the c-Met–specific chemical inhibitor PHA-665752.22 When coinjected with G-CSF, this inhibitor significantly reduced progenitor numbers (Figure 2B), primitive SKL cells (Figure 2C,E), and stem cell containing CD34−/SKL cells (Figure 2F-G). Consistently, mice injected with HGF mobilized primitive CD34−/SKL cells (Figure 2E-F). Both the administration of c-Met neutralizing Abs or c-Met inhibitor alone in steady state did not significantly influence the egress of progenitors (Figure 2A-B) and SKL cells (Figure 2C-E). These treatments did not decrease the number of BM progenitor cells or BM SKL cells, did not affect BM cell viability, or alter the BM composition (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Mice injected with c-Met neutralizing Abs or with c-Met inhibitor presented similar frequencies of BM progenitor cells or SKL cells compared with their counterparts receiving G-CSF alone (supplemental Table 2).
Altogether, these results show that c-Met signaling is required for optimal mobilization of stem and progenitor cells during stress-induced conditions.
c-Met signaling regulates SDF-1–induced chemotaxis of progenitor cells
We next investigated whether c-Met was able to directly influence the motility of progenitor cells. For this purpose we inhibited c-Met expression in BM-derived cells by shRNA technology15 (supplemental Figure 1). As shown in Figure 3A, c-Met silencing on leukocytes obtained from the BM of G-CSF–treated mice reduced their ability to migrate toward a gradient of SDF-1, thereby indicating functional interactions between c-Met and SDF-1 pathways in BM leukocytes. c-Met silencing also impaired the migration of progenitor cells (Figure 3A). Similar results were obtained when BM cells from G-CSF–treated mice were incubated with c-Met inhibitor and then subjected to SDF-1–induced migration (Figure 3B). Of notice, c-Met silencing or inhibition did not affect spontaneous migration of BM leukocytes or progenitor cells (Figure 3C-D). These results indicate involvement of c-Met in regulating SDF-1–induced migration.
c-Met promotes chemotaxis toward a SDF-1 gradient. (A,C) BM cells from G-CSF–treated mice were transduced with lentiviral vectors expressing a c-Met shRNA or a nonfunctional control shRNA and subsequently subjected to a chemotaxis assay through Matrigel-coated filters. (A) SDF-1–induced migration or (C) spontaneous migration of BM leukocytes or progenitor cells are shown. (B,D) BM cells taken from mice injected with G-CSF; cells were preincubated with c-Met inhibitor and then subjected to a chemotaxis assay through Matrigel-coated filters. (B) SDF-1–induced migration or (D) spontaneous migration of BM leukocytes or progenitor cells are shown. (E) MMP-9 activity in the BM of mice receiving c-Met Abs, DMSO, or 5 injections of G-CSF with or without anti–c-Met Abs on days 4 and 5 of G-CSF treatment or mice that received 5 injections of G-CSF with vehicle or c-Met inhibitor. Gelatin zymography has been performed on BM supernatants. The data are expressed as mean ± SE in 3 independent experiments *P < .05, **P < .001.
c-Met promotes chemotaxis toward a SDF-1 gradient. (A,C) BM cells from G-CSF–treated mice were transduced with lentiviral vectors expressing a c-Met shRNA or a nonfunctional control shRNA and subsequently subjected to a chemotaxis assay through Matrigel-coated filters. (A) SDF-1–induced migration or (C) spontaneous migration of BM leukocytes or progenitor cells are shown. (B,D) BM cells taken from mice injected with G-CSF; cells were preincubated with c-Met inhibitor and then subjected to a chemotaxis assay through Matrigel-coated filters. (B) SDF-1–induced migration or (D) spontaneous migration of BM leukocytes or progenitor cells are shown. (E) MMP-9 activity in the BM of mice receiving c-Met Abs, DMSO, or 5 injections of G-CSF with or without anti–c-Met Abs on days 4 and 5 of G-CSF treatment or mice that received 5 injections of G-CSF with vehicle or c-Met inhibitor. Gelatin zymography has been performed on BM supernatants. The data are expressed as mean ± SE in 3 independent experiments *P < .05, **P < .001.
We further confirmed the role of c-Met in leukocyte motility by analyzing its effect on the activity of matrix metalloproteinase 9 (MMP-9), a protease involved in enhanced, stress-induced progenitor cell trafficking.23 Figure 3E shows that, although G-CSF strongly activated MMP-9, the administration of c-Met neutralizing Abs or inhibitor significantly reduced it, indicating its role in MMP-9 activation. Delivery of anti–c-Met Abs or DMSO in steady state did not alter MMP-9 levels (Figure 3E).
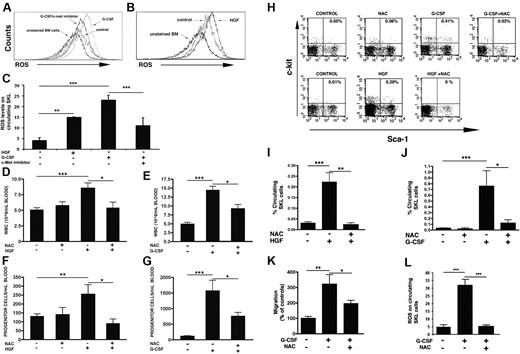
c-Met regulates ROS production in BM progenitors
Next, we examined the mechanisms underlying c-Met–induced progenitor cell recruitment, focusing on ROS. c-Met silencing in a mouse cell line decreased the production of ROS.24 A role for ROS in the regulation of HSC-niche interactions9,25 and repopulation8,11 have been shown.
G-CSF induced a strong increase in ROS production in BM SKL cells, whereas c-Met inhibition substantially impaired it (Figure 4A). Moreover, PHA-665752 inhibited G-CSF–induced production of ROS in circulating SKL cells (Figure 4C). Consistently, HGF-induced mobilization was accompanied by an increase in ROS production in BM (Figure 4B) and circulating SKL cells (Figure 4C), indicating a c-Met–dependent generation of ROS.
c-Met regulates progenitor cell egress by ROS generation. (A) A representative FACS plot showing ROS levels on BM SKL cells of mice receiving vehicle or G-CSF with vehicle or c-Met inhibitor. (B) A representative FACS analysis of ROS levels on BM SKL of control mice or mice injected for 2 consecutive days with HGF. (C) ROS levels in circulating SKL cells in mice receiving 2 injections of HGF or 5 consecutive injections of G-CSF together with DMSO or c-Met inhibitor. (D) Circulating leukocytes in mice receiving 2 consecutive injections of NAC, HGF alone, or together with NAC or (E) in mice injected for 5 days with G-CSF alone or NAC on days 4 and 5 of G-CSF treatment. (F-G) Colony-forming progenitor cells in the blood of mice that were given NAC, HGF, HGF together with NAC (F) or G-CSF alone or in combination with NAC (G). (H) A representative FACS analysis of circulating SKL cells in mice injected with NAC, G-CSF alone or with NAC, or HGF alone or in association to NAC. (I) A summary of circulating SKL in mice receiving HGF alone or in combination with NAC. (J) A summary of SKL in the circulation of mice receiving NAC, G-CSF, or G-CSF and NAC. (K) SDF-1 induced migration of BM cells derived from control mice or mice treated with G-CSF alone or in combination with NAC. (L) ROS levels in circulating SKL cells in mice receiving 5 daily injections of G-CSF alone or in combination with 2 consecutive injections of NAC. Indicated values represent the mean ± SE in ≥ 3 independent experiments, 6 mice in each group. **P < .001; *P < .05.
c-Met regulates progenitor cell egress by ROS generation. (A) A representative FACS plot showing ROS levels on BM SKL cells of mice receiving vehicle or G-CSF with vehicle or c-Met inhibitor. (B) A representative FACS analysis of ROS levels on BM SKL of control mice or mice injected for 2 consecutive days with HGF. (C) ROS levels in circulating SKL cells in mice receiving 2 injections of HGF or 5 consecutive injections of G-CSF together with DMSO or c-Met inhibitor. (D) Circulating leukocytes in mice receiving 2 consecutive injections of NAC, HGF alone, or together with NAC or (E) in mice injected for 5 days with G-CSF alone or NAC on days 4 and 5 of G-CSF treatment. (F-G) Colony-forming progenitor cells in the blood of mice that were given NAC, HGF, HGF together with NAC (F) or G-CSF alone or in combination with NAC (G). (H) A representative FACS analysis of circulating SKL cells in mice injected with NAC, G-CSF alone or with NAC, or HGF alone or in association to NAC. (I) A summary of circulating SKL in mice receiving HGF alone or in combination with NAC. (J) A summary of SKL in the circulation of mice receiving NAC, G-CSF, or G-CSF and NAC. (K) SDF-1 induced migration of BM cells derived from control mice or mice treated with G-CSF alone or in combination with NAC. (L) ROS levels in circulating SKL cells in mice receiving 5 daily injections of G-CSF alone or in combination with 2 consecutive injections of NAC. Indicated values represent the mean ± SE in ≥ 3 independent experiments, 6 mice in each group. **P < .001; *P < .05.
We next administered to mice the ROS inhibitor NAC,11 which blocked ROS generation in circulating SKL cells (Figure 4L). NAC administration reduced HGF-induced mobilization of maturing leukocytes (Figure 4D), progenitors (Figure 4F), and SKL cells (Figure 4H-I). NAC decreased G-CSF–induced mobilization of mature cells (Figure 4E), caused a 3-fold reduction in the number of circulating progenitors (Figure 4G), and strongly prevented mobilization of SKL cells (Figure 4H,J). Of note, NAC delivery in steady state did not affect the egress of maturing leukocytes (Figure 4D), progenitors (Figure 4F), and primitive SKL cells (Figure 4H,J). Moreover, it did not alter the BM composition or the number of BM progenitor and SKL cells, and it did not induce apoptosis (supplemental Table 1).
NAC treatment impaired the enhanced, stress-induced ability of BM leukocytes to migrate toward an SDF-1 gradient in vitro (Figure 4K), indicating a role for ROS in promoting cell motility during stress situations.
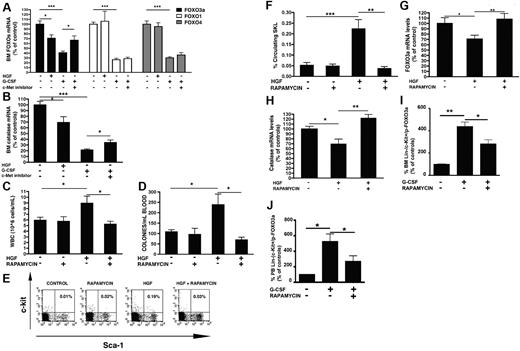
ROS are generated by c-Met via mTOR-mediated inhibition of FOXO3a
We subsequently investigated how c-Met activation leads to ROS generation in the BM. As shown in Figure 5A, the expression of FOXO3a, a transcriptional factor that mediates resistance to oxidative stress,11,12 was down-regulated in the BM after HGF and G-CSF administration. G-CSF–induced decrease of FOXO3a was prevented after treatment with c-Met inhibitor (Figure 5A). Although G-CSF was able to down-regulate also FOXO1 and FOXO4, 2 other members of the FOXO family with a role in the hematopoietic system,11 c-Met inhibition failed to revert this down-regulation (Figure 5A). In accordance, no decrease in FOXO1 or FOXO4 was observed after HGF delivery, therefore indicating a selective regulation of FOXO3a by c-Met signaling in the BM (Figure 5A). Of note, the transcription of catalase, a main FOXO3a target,12 was decreased after HGF or G-CSF treatment but not when c-Met inhibitor was injected together with G-CSF (Figure 5B).
ROS are produced by c-Met by mTOR-mediated inhibition of FOXO3a. (A) Mice were injected with HGF or with G-CSF with vehicle or with PHA, and the BM transcriptional levels of FOXO3a, FOXO1, and FOXO4 mRNA were determined. (B) Catalase mRNA levels in the BM of mice receiving HGF or G-CSF or G-CSF and c-Met inhibitor. (C-H) Mice were injected with rapamycin or HGF alone or in combination with rapamycin. The levels of circulating leukocytes (C), progenitors (D), and primitive SKL cells (E-F) were evaluated. (G) BM FOXO3a and (H) catalase transcriptional levels in the BM of control mice or mice injected with HGF with or without rapamycin. Values represent the mean ± SE of ≥ 3 independent experiments, 6 mice in each group. (I-J) Percentage of Lin−/c-kit+/phosphor-Thr32-FOXO3a+ cells in the BM (I) and peripheral blood (J) after treatment with G-CSF alone or in combination with rapamycin. ** P < .001; * P < .05.
ROS are produced by c-Met by mTOR-mediated inhibition of FOXO3a. (A) Mice were injected with HGF or with G-CSF with vehicle or with PHA, and the BM transcriptional levels of FOXO3a, FOXO1, and FOXO4 mRNA were determined. (B) Catalase mRNA levels in the BM of mice receiving HGF or G-CSF or G-CSF and c-Met inhibitor. (C-H) Mice were injected with rapamycin or HGF alone or in combination with rapamycin. The levels of circulating leukocytes (C), progenitors (D), and primitive SKL cells (E-F) were evaluated. (G) BM FOXO3a and (H) catalase transcriptional levels in the BM of control mice or mice injected with HGF with or without rapamycin. Values represent the mean ± SE of ≥ 3 independent experiments, 6 mice in each group. (I-J) Percentage of Lin−/c-kit+/phosphor-Thr32-FOXO3a+ cells in the BM (I) and peripheral blood (J) after treatment with G-CSF alone or in combination with rapamycin. ** P < .001; * P < .05.
To elaborate the signaling cascade, we blocked mTOR signaling by delivering its inhibitor rapamycin.26 HGF-induced egress of maturing leukocytes (Figure 5C), immature progenitor cells (Figure 5D), and primitive SKL cells (Figure 5E-F) was strongly reduced after rapamycin administration. On the contrary, administration of rapamycin in steady state did not alter the egress of mature leukocytes, progenitor cells, or SKL cells (Figure 5C-F). Similarly, the numbers of BM progenitor cells or SKL cells were not affected by rapamycin treatment (supplemental Table 1). Rapamycin treatment also impaired the HGF-induced decrease in the expression of FOXO3a (Figure 5G) and its target gene catalase in the BM (Figure 5H). In addition, rapamycin delivery inhibited G-CSF–induced progenitor and SKL cell mobilization.26 We detected changes in the levels of the inactive phosphorylated FOXO3a levels: rapamycin treatment caused inhibition of the G-CSF–induced increase in the number of BM and PB Lin−/c-kit+/p-FOXO3a+ progenitor cells (Figure 5I and 5J, respectively). All together these findings indicate that c-Met activation induces mTOR signaling, down-regulation of FOXO3a levels, and ultimately increased ROS production.
Discussion
In this study we show an essential role for c-Met in optimal hematopoietic progenitor cell mobilization by regulating their enhanced motility by signaling through the mTOR, FOXO3a, and ROS cascade. Although the involvement of c-Met in cancer progression is well established, not much is known about c-Met functions in normal adult hematopoiesis. Most studies were limited to the analysis of the synergistic effects exerted in vitro by HGF on hematopoietic cell proliferation, in combination with other growth factors.27-30 With the use of functional, in vivo mobilization models, we describe a new role for c-Met and its ligand HGF in the regulation of stress induced, accelerated immature and maturing leukocyte recruitment as part of host defense and repair mechanisms. We first show that both the ligand and its receptor are modulated by stress conditions, such as G-CSF–repeated stimulations. Confirming previous studies in mobilized healthy donors,18,31 we observed an increase in HGF serum levels in mice receiving G-CSF. Our data also indicate that G-CSF induced an increase in HGF production both in BM leukocytes and PMN cells, the main source for HGF in the hematopoietic compartment.32
In addition to modulating HGF levels, we show that c-Met expression and activation status are dynamic and are inducible after stress situations such as G-CSF administration. Interestingly, the main regulator of c-Met transcription, hypoxia inducible factor-1 α, was up-regulated by G-CSF stimulations, correlating progenitor mobilization with an expansion of hypoxic murine BM areas.33 Thus, the increase in c-Met after G-CSF treatment may be mediated by hypoxia inducible factor-1 α, which also regulates expression of other genes involved in stem cell trafficking such as SDF-1/CXCR4 and vascular endothelial growth factor.34,35 Moreover, primitive, quiescent BM HSCs from G-CSF–treated mice have reduced long-term repopulation ability.36 We show regulation of c-Met activation by SDF-1/CXCR4 signaling. This axis has been extensively reported to be involved in stem cell mobilization. Multiple SDF-1 injections are able to induce osteoclast activation and progenitor cell mobilization37 ; furthermore, a single dose of SDF-1 is already able to induce progenitor cell release into the circulation.17 Moreover, neutralizing anti-CXCR4 Abs block progenitor cell mobilization (Petit et al4 ; Figure 1). In support, chimeric mice reconstituted with CXCR4−/− hematopoietic cells do not mobilize in response to G-CSF.21 Our results indicate that G-CSF–induced c-Met activation requires functional CXCR4 signaling because neutralizing CXCR4 Abs that prevent SKL mobilization (Petit et al4 ; Figure 1J) also inhibit c-Met activation. Moreover, SDF-1 was able to strongly activate c-Met by up-regulating HGF production in BM leukocytes and PMN cells. The need for CXCR4/SDF-1 signaling for optimal c-Met activation, together with the observation that c-Met is needed for enhanced leukocyte migration to a gradient of SDF-1, suggests mutual interactions between the c-Met and CXCR4 cascades. Interestingly, repeated HGF stimulations were accompanied by a decrease in BM SDF-1 levels, which is mandatory for an optimal progenitor mobilization4,5 (supplemental Figure 2). Furthermore, HGF was shown to up-regulate CXCR4 expression on immature human CD34+ progenitor cells,37 thus suggesting that the interactions between the CXCR4 and c-Met signaling pathways are reciprocal.
Our results show that the strong up-regulation of c-Met expression and signaling is instrumental to face the accelerated demand for stress-induced leukocyte production and recruitment during G-CSF–induced mobilization. With the use of different approaches to interfere with c-Met signaling, we showed that c-Met has an essential role in progenitor cell recruitment, because the absence of functional c-Met signaling strongly impairs the egress of immature colony-forming progenitor cells and primitive SKL cells. Moreover, direct inhibition of c-Met expression and activity hampered the enhanced ability of BM-derived cells to migrate toward a gradient of SDF-1. These results indicate a role for c-Met in regulating progenitor cell motility. Furthermore, c-Met regulates also MMP-9 secretion. In addition, a recent in vitro study showed that HGF signaling by c-Met can increase transendothelial migration of human CD34+ progenitor cells by up-regulating the expression and activity of MT1-MMP and MMP-9.31 Protease activation was shown to be important for progenitor cell egress: MMP-9 knockout mice showed impaired HSC motility23 ; moreover, our previous study has shown the important role of MT1-MMP in G-CSF–induced mobilization of human and murine progenitor cells.26 Combining these studies together we can conclude that the HGF/c-Met signaling axis is also involved in activation of MMPs to promote leukocyte recruitment under stress conditions.
Our findings provide evidence for cell autonomous effects exerted by c-Met signaling on the enhanced migration of G-CSF–stimulated leukocytes. However, c-Met may regulate progenitor mobilization at multiple levels, not only through a direct effect on BM progenitor cells but also through indirect effects on the BM microenvironment. HGF was reported as an important regulator of the BM microenvironment, controlling osteoclast/osteoblast bone remodeling interactions, which were also shown to promote stem and progenitor cell mobilization.20,38 Of note, HGF is also a potent angiogenic factor,39 and it stimulates production of vascular endothelial growth factor, an important regulator of vascular permeability and leukocyte rolling to the vascular endothelium.40 Therefore, c-Met might promote progenitor cell mobilization by targeting also the BM vascular niche, an essential location for cell egress. In addition, although motility and proliferation of progenitor cells are regulated by similar factors, c-Met inhibition affected predominantly progenitor cell motility without altering BM progenitor or SKL frequencies. However, it has to be considered that this study focuses on G-CSF–induced mobilization after 5 consecutive G-CSF stimulations. At this time point the cells have already undergone cell proliferation (which occurs at the second and third day of G-CSF treatment),41 and the predominant phenomena are cell motility and egress. Therefore, although our results indicate that at day 5 of G-CSF stimulation, the frequency of BM SKL is not affected by c-Met inhibition, a role for c-Met in cell proliferation during G-CSF–induced mobilization cannot be ruled out.
The analysis of the molecular mechanisms underlying the c-Met role in progenitor cell mobilization pointed out the crucial role of a new, unexpected ROS signaling pathway. We describe a cascade in which c-Met activates the mTOR pathway, which down-regulates FOXO3a expression, thereby leading to ROS production (Figure 6). Notably, blockage of mTOR by rapamycin treatment reduced HGF-induced progenitor cell recruitment, reverted HGF-mediated down-regulation of FOXO3a and of its target gene catalase, in line with a previous study showing that rapamycin targets the activity of mTOR complex 2 (mTORC2).42 Our data suggest a new role for FOXO3a reduction, which allow increased ROS levels as part of progenitor cell mobilization. In support of our results, a recent finding shows that mice lacking FOXO3a develop neutrophilia.43 Of note, rapamycin delivery impaired G-CSF–mediated mobilization,26 and the phosphatidyl inositol-3 kinase pathway has been described in other systems as an essential player of the c-Met signaling cascade.44,45 Moreover, ablation of phosphatase and tensin homologue, a negative regulator of the phosphatidyl inositol-3 kinase /Akt/mTOR pathway, was shown to be associated with reduced BM retention of stem cells.46,47 Notably, the delivery of the ROS scavenger NAC inhibited G-CSF– and HGF-induced mobilization of mature leukocytes and primitive cells. In support of these results, genetic deficiency of tuberous sclerosis 1, a negative regulator of mTOR, is associated with higher ROS levels.48 Furthermore, HSCs from AKT1/2−/− mice, which are stuck in G0 and have low ROS levels, show reduced long-term repopulation capacities,49 suggesting that balanced ROS levels are required for both mobilization and repopulation.
Scheme of the proposed model. G-CSF administration causes a transient up-regulation of SDF-1,4 (1) which activates CXCR4 signaling (2) crucial for promoting stem cell egress.2,4,20,21 CXCR4/SDF-1 activation leads to an up-regulation of HGF in BM PMN cells. (3) HGF subsequently binds to c-Met and thus promotes its activation. (4) c-Met in turn induces mTOR signaling, (5) which represses FOXO3a. (6) Subsequently, FOXO3a inhibition causes increased ROS production (7); this signaling cascade ultimately promotes hematopoietic stem and progenitor cell egress out of the BM reservoir (8).
Scheme of the proposed model. G-CSF administration causes a transient up-regulation of SDF-1,4 (1) which activates CXCR4 signaling (2) crucial for promoting stem cell egress.2,4,20,21 CXCR4/SDF-1 activation leads to an up-regulation of HGF in BM PMN cells. (3) HGF subsequently binds to c-Met and thus promotes its activation. (4) c-Met in turn induces mTOR signaling, (5) which represses FOXO3a. (6) Subsequently, FOXO3a inhibition causes increased ROS production (7); this signaling cascade ultimately promotes hematopoietic stem and progenitor cell egress out of the BM reservoir (8).
In conclusion, we have shown the dynamic nature of the BM reservoir that induces a dramatic up-regulation of c-Met expression and activity, in a CXCR4/SDF-1–dependent manner, to face the rapid and increased demand for leukocytes during stress-induced mobilization. Moreover, we describe a mTOR/FOXO3a/ROS signaling cascade that promotes enhanced progenitor cell recruitment and suggests in vitro NAC treatment to restore long-term repopulation of clinically mobilized stem cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof Stephen J. Weiss, Prof Paolo Maria Comoglio, and Prof Irwin Bernstein for critically reviewing the manuscript; Loya Abel and Kfir Lapid for excellent technical assistance; Dr James Christensen and Pfizer for kindly providing the c-Met inhibitor PHA-665752; and Prof Graham Lieschke for providing G-CSF−/− mice.
This work was supported by The Sergio Lombroso Foundation fellowship (M.T.), the Minerva Foundation fellowship (E.C.B.), and the AIRC fellowship to (S.C.). This work was partially supported by grants from the Israeli Science Foundation and Minerva Foundation (T.L.), AIRC grant (S.G. and W.P.), and a grant from Regione Piemonte (S.G., S.C., and W.P.).
Authorship
Contribution: M.T. designed the research, designed and performed experiments, collected and analyzed the data, and wrote the manuscript; K.G. performed in vivo experiments and real-time PCR experiments; S.C. and S.G. designed, performed, and analyzed c-Met silencing experiments; A.S. designed, performed, and analyzed immuno-precipitation studies; Y.V. helped with in vivo rapamycin inhibition experiments; S.S. helped with in vivo experiments; A.K. designed, performed, and analyzed ELISA; L.C. and L.G. helped with c-Met silencing experiments; E.L. helped with sorting experiments; E.C.B. helped with colony assays; E.S., T.I., O.K., and I.P. helped with designing experiments; J.C. provided the c-Met inhibitor; A.T., M.A., and W.P. reviewed and provided advice on manuscript preparation; and T.L. supervised the study, including experiment design, and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tsvee Lapidot, Immunology Department, The Weizmann Institute of Science, Rehovot 76100, Israel; e-mail: tsvee.lapidot@weizmann.ac.il.