Abstract
Donor cell leukemia after allogeneic hematopoietic stem cell transplantation might provide a unique human model for our understanding of leukemogenesis in vivo. We hypothesized that the “2-genetic-hits model” may contribute to the “leukemization” of donor cells and first evaluated these genetic mutations that are implicated in the development of acute myeloid leukemia in a donor cell leukemia patient and donor. The patient and his donor-sister both harbored a germline mutation in CEBPA (584_589dup). Susceptible donor hematopoietic cells evolved to overt acute myeloid leukemia by developing 2 somatic CEBPA mutations (247dupC and 914_916dup) in the patient's microenvironment. These were identical to the acquired mutations identified in leukemic cells that originated from the patient during de novo acute myeloid leukemia. Our results provide the first report of multiple mutations of CEBPA contributing to the transformation of donor cells to the leukemic phenotype and provide clues to support the multiple-genetic-hits mechanism of donor cell leukemia.
Introduction
Leukemia relapse after allogeneic hematopoietic stem cell transplantation (allo-HSCT) that arises in cells of donor origin in the transplant recipient, so-called donor cell leukemia (DCL), is a rare disease entity, and only 51 cases were reported since 1971.1-4 The precise etiologic mechanisms of DCL remain unknown, and no common mechanism can be identified in most of the cases reported in the literature. Careful analysis of the mechanisms with respect to the oncogenic transformation of donor-derived cells might provide a unique human model for our understanding of leukemogenesis in vivo.
According to the “2-genetic-hits model,” cooperation between 2 classes of genetic mutations contributes to leukemogenesis.5 One group (class 1) comprises mutations in the fms-related tyrosine kinase 3 gene (FLT3) or the neuroblastoma RAS viral oncogene homolog gene (NRAS), which increase the proliferation and/or survival of hematopoietic stem/progenitor cells.6,7 The other complementation group (class 2) comprises mutations in CEBPA, the gene that encodes the CCAAT enhancer-binding proteinα (C/EBPα); the myeloid-lymphoid or mixed-lineage leukemia gene (MLL); or the nucleophosmin gene (NPM1), which cause impaired differentiation.8-10 The most common mutations, which include internal tandem duplications restricted to exons 14 and 15 and point mutation of Asp 835 within the TK domain of FLT3, codon 12/13 in exon 1 and codon 61 in exon 2 of NRAS, the entire coding region of CEBPA, partial tandem duplications that span exons 2-6 or exons 2-8 of MLL, and mutations in exon 12 of NPM1, have been described extensively in acute myeloid leukemia (AML).11-14 We hypothesized that the 2-genetic-hits model may contribute to the “leukemization” of donor cells in DCL. We screened these genetic mutations implicated in the development of common forms of AML in a DCL patient and donor.
Methods
The study was approved by the Zhejiang University ethics committee. The patient and donor gave their written informed consent in accordance with the Declaration of Helsinki.
Patient and donor
A more detailed case report is included in the supplemental Data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In brief, a 36-year-old male was diagnosed with AML. Cytogenetic evaluation demonstrated the patient had an abnormal clone 46,XY,del(9)(q11;q34) in 10 of 10 cells, which could still be observed during his lasting complete remission (CR) before transplantation. He underwent myeloablative allogeneic peripheral blood stem cell transplantation from an HLA-identical sister during his first CR. Cyclosporine was discontinued 3 months after transplantation, with no evidence of acute GVHD; however, the patient developed extensive chronic GVHD (liver) 8 months after transplantation. He received a combination of prednisolone, tacrolimus, and mycophenolate mofetil, with excellent response. The immunosuppressive treatment was eventually tapered to tacrolimus 1.5 mg/d. Thirteen months after transplantation, the patient became thrombocytopenic, and bone marrow aspiration revealed leukemia relapse. PCR amplification and detection of 14 short tandem repeat markers on chromosomes 2, 3, 4, 5, 7, 8, 11, 12, 13, 16, 18, and 21 and amelogenin gene showed complete donor chimerism. The karyotype of the patient's bone marrow cells remained identical to that of the donor: 45,XX,der(15;22)(q10;q10) in 10 of 10 cells. Molecular evaluation suggested that the patient had developed DCL after allogeneic peripheral blood stem cell transplantation from an HLA-identical sibling.
Screening for genetic mutations
A series of stained archival slides of bone marrow from the patient were available that included specimens taken at the time of original diagnosis, at CR after induction chemotherapy, during lasting CR, before transplantation, and 1, 9, and 12 months after allo-HSCT, as well as samples of mononuclear-cell–enriched bone marrow taken at the time of relapse and at CR after relapse. A buccal mucosal swab specimen obtained from the patient during remission was also available. A buccal mucosal swab specimen and samples of mononuclear-cell–enriched peripheral blood and bone marrow were also available from the donor. Mutations in the FLT3-TKD, NRAS, CEBPA, and NPM1 genes were bidirectionally sequenced directly by use of an ABI 3130 XL sequencer (Applied Biosystems). The FLT3 internal tandem duplication was verified by agarose gel electrophoresis. The partial tandem duplication of MLL was examined by extra-long PCR and agarose gel electrophoresis. The primers for PCR amplification and sequencing reactions are listed in supplemental Table 1.
Results and discussion
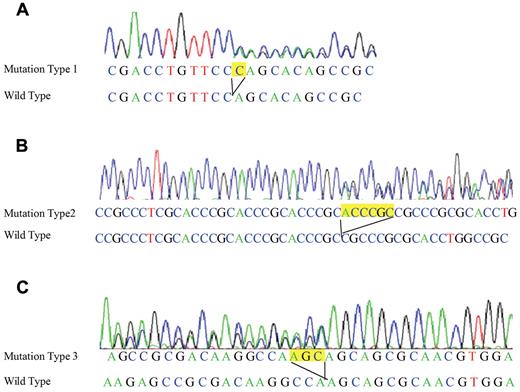
DNA obtained from the patient at the time of the original diagnosis of AML demonstrated 3 different CEBPA gene mutations (GenBank accession no. NC_000019). Mutation 1 was a duplication of a cytosine residue at nt 247 (247dupC; Figure 1A). Mutation 2 was a 6-bp duplication that included nt 584-589 (584_589dup; Figure 1B). Mutation 3 was a 3-bp duplication that included nt 914-916 (914_916dup; Figure 1C). The 584_589dup mutation was also found in DNA from all patient specimens during the lasting remission before allo-HSCT and in DNA from his buccal swab specimen (germline DNA), which indicates that this mutation was a germline mutation. In contrast, the other 2 mutations were not found in the specimens taken during remission or in the patient's buccal swab specimen, which indicates that these were somatically acquired mutations.
Sequence analyses of the N- and C-terminal CEBPA mutations in the patient and donor. (A) Leukemic cells originating from the patient at diagnosis and from the donor at DCL showed a cytosine residue duplication in the N-terminal region of the CEBPA gene (247dupC). (B) Bone marrow cells from the patient at diagnosis, remission, and DCL and his buccal mucosal specimen, as well as bone marrow cells and the buccal mucosal specimen from the donor, harbored a 6-bp germline duplication in the N-terminal region of the CEBPA gene (584_589dup). (C) Leukemic cells originating from the patient at diagnosis and from the donor at DCL showed a 3-bp duplication in the C-terminal region of the CEBPA gene (914_916dup).
Sequence analyses of the N- and C-terminal CEBPA mutations in the patient and donor. (A) Leukemic cells originating from the patient at diagnosis and from the donor at DCL showed a cytosine residue duplication in the N-terminal region of the CEBPA gene (247dupC). (B) Bone marrow cells from the patient at diagnosis, remission, and DCL and his buccal mucosal specimen, as well as bone marrow cells and the buccal mucosal specimen from the donor, harbored a 6-bp germline duplication in the N-terminal region of the CEBPA gene (584_589dup). (C) Leukemic cells originating from the patient at diagnosis and from the donor at DCL showed a 3-bp duplication in the C-terminal region of the CEBPA gene (914_916dup).
Intriguingly, the 584_589dup germline mutation was also found in DNA from peripheral blood and bone marrow cells, as well as in the buccal swab specimen from the donor. Donor-derived cells in the patient during CR at 1, 9, and 12 months after allo-HSCT only showed this single germline mutation. However, when the patient relapsed with DCL, donor-derived leukemic cells harboring this germline mutation were found to develop 2 additional somatic CEBPA mutations in the patient's microenvironment; these were identical to those observed in the patient with de novo leukemia. These 2 somatic mutations were not observed in donor-derived cells in the patient after he achieved CR after relapse. No other mutations were identified in the FLT3, NRAS, MLL, or NPM1 genes in the patient at diagnosis, remission, or relapse or in the donor.
The most important finding of the present study is that this is the first record of multiple CEBPA mutations that contributed to the transformation of donor cells to the leukemic phenotype. C/EBPα is a transcription factor that is strongly implicated in hematopoiesis, through control of the proliferation and differentiation of myeloid progenitors. It consists of N-terminal transactivation domains (TAD1 and TAD2) and a C-terminal basic and leucine zipper region necessary for specific DNA-sequence binding and homodimerization or heterodimerization, respectively (Figure 2A). The patient and his donor-sister both harbored a germline CEBPA mutation, 584_589dup, which results in an internal tandem duplication of amino acids 195-196 (His195_Pro196dup) in TAD2 of C/EBPα (Figure 2C). In vitro studies have established that the region that includes this duplication is responsible for the direct inhibition of cyclin-dependent kinase 2 and cyclin-dependent kinase 4 and causes cell proliferation arrest.15 However, the fact that the donor remained healthy indicates that this single mutation is not sufficient for leukemogenesis. The patient developed AML after acquiring 2 additional somatic mutations, 247dupC and 914_916dup. CEBPA can be translated from the ATG at nt1/aa1, which encodes the 42-kDa normal C/EBPα, and from ATG at nt358/aa120, which encodes the 30-kDa form that lacks the TAD1 domain (Figure 2A). Evidence from knockout mouse models showed that the 42-KDa C/EBPα was required for the control of myeloid progenitor proliferation, and loss of the 42-KDa C/EBPα and retention of the 30-KDa isoform resulted in the development of AML with complete penetrance.16 The N-terminal mutation, 247dupC, causes the premature termination of the 42-KDa protein at codon 106 (Gln83fsX106) and overproduction of the 30-KDa isoform, which causes loss of function of C/EBPα (Figure 2B). The C-terminal mutation, 914_916dup, causes the duplication of aa305 (Gln305dup) in the basic and leucine zipper domain, which is predicted to prevent dimerization of C/EBPα (Figure 2D).
Schematic representation of C/EBPα functional domain and mutations resulting in C/EBPα dysregulation. (A) Two transactivation domains (TAD1 and TAD2 [pink]) and the basic region and leucine zipper (bZip [blue]) are indicated. The mRNA translated from the ATG at nt1/aa1 encodes the wild-type 42-kDa C/EBPα, whereas the 30-kDa form is translated from ATG at nt358/aa120. (B) The 247dupC mutation results in a truncated N-terminal product with an altered amino acid sequence (green). The 30-kDa product that is generated from the alternative start codon lacks TAD1. (C) The 584_589dup mutation results in amino acid sequence His195_Pro196dup (orange) in TAD2. (D) The 914_916dup mutation results in amino acid sequence Gln305dup (orange) in bZip.
Schematic representation of C/EBPα functional domain and mutations resulting in C/EBPα dysregulation. (A) Two transactivation domains (TAD1 and TAD2 [pink]) and the basic region and leucine zipper (bZip [blue]) are indicated. The mRNA translated from the ATG at nt1/aa1 encodes the wild-type 42-kDa C/EBPα, whereas the 30-kDa form is translated from ATG at nt358/aa120. (B) The 247dupC mutation results in a truncated N-terminal product with an altered amino acid sequence (green). The 30-kDa product that is generated from the alternative start codon lacks TAD1. (C) The 584_589dup mutation results in amino acid sequence His195_Pro196dup (orange) in TAD2. (D) The 914_916dup mutation results in amino acid sequence Gln305dup (orange) in bZip.
The present case is also the first to provide laboratory data to support the hypothesis about a multiple-genetic-hits mechanism in DCL. The first genetic mutation involved in leukemogenesis takes place in the donor, and those susceptible donor cells can acquire second or third mutations in different genes, or recessive mutations in a single gene, in the recipient's particular microenvironment after transplantation, thus leading to overt leukemic transformation.17 Data indicating that the donor is related to the recipient in 80% of DCL cases suggest that any genetic predisposition might be shared by family members.4 Furthermore, CEBPA is the only gene known to be associated with familial AML. A germline CEBPA mutation is present in a family in which multiple individuals have AML.18 Although current evidence suggests that donors are not destined to develop leukemia, the present case raises an important question regarding the need for donor selection, notification, and tracking. The limitation of the present study is that we have no direct evidence to evaluate the contribution of the patient's microenvironment. However, leukemic cells that originated from the patient at de novo AML and from the donor at DCL after allo-HSCT exhibited the same gene mutations. This supports the theory that during DCL, the host environment in which the original malignancy developed might trigger a similar oncogenic process in donor cells, favored by the immunosuppressive status after transplantation.19
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Shanghai Genesky Bio-Tech Genetic Core Lab for their excellent technical assistance with sequencing analysis. We also thank the HLA Typing Laboratory of the Blood Center of Zhejiang Province for their assistance. We thank the patient's family for their trust.
This work was supported by Zhejiang Provincial Key Medical Discipline (Medical Tissue Engineering) and National Natural Science Foundation of China (30871098).
Authorship
Contribution: H.X., J.S., and H.H. conceived and designed the study; H.X., J.S., Y.L., and H.H. analyzed and interpreted the data; H.X. and H.H. drafted the article; J.S., Y.L., Y.T., J.H., W.X., and H.H. provided study materials or patients; X. Yu, Z.C., M.L., X. Ye, and H.H. obtained funding; and H.X., L.Z., Y.W., L.L., and K.W. performed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: He Huang, MD, PhD, Bone Marrow Transplantation Center, The First Affiliated Hospital, Zhejiang University School of Medicine, No 79 Qingchun Rd, Hangzhou, 310003, Zhejiang Province, People's Republic of China; e-mail: hehuang.zju@gmail.com.
References
Author notes
H.X. and J.S. contributed equally to this study.

![Figure 2. Schematic representation of C/EBPα functional domain and mutations resulting in C/EBPα dysregulation. (A) Two transactivation domains (TAD1 and TAD2 [pink]) and the basic region and leucine zipper (bZip [blue]) are indicated. The mRNA translated from the ATG at nt1/aa1 encodes the wild-type 42-kDa C/EBPα, whereas the 30-kDa form is translated from ATG at nt358/aa120. (B) The 247dupC mutation results in a truncated N-terminal product with an altered amino acid sequence (green). The 30-kDa product that is generated from the alternative start codon lacks TAD1. (C) The 584_589dup mutation results in amino acid sequence His195_Pro196dup (orange) in TAD2. (D) The 914_916dup mutation results in amino acid sequence Gln305dup (orange) in bZip.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/19/10.1182_blood-2010-12-326322/4/m_zh89991170610002.jpeg?Expires=1767816799&Signature=rr6cFvwFxaSagqKo1BCksDYjenBQsieIz2z7dJG1HZVZU4txNhCrWzebn~qkc495g5peR9-sXXoygKyuq7W~Ycfyd8NgeUnOp9WQp1jPhW004fi9CEJQsfZSuPMvfjLjrdXgt4PA515XYrwP3szmXYEachxa1kEDR9WkfwVe4P~l8o86uVxycLxDA1tVU-lAiPv-G2b89zEJmovRiig7gYRD4x-ksM3DW8SFeD83TTyg-BZNIuFUhNhL85vg~Wkwi8vzZd6ZuQRj6pEC3-4U6Y~ppfNRdcN86QfYRJjLA7hXVqgrNFbv4pCa5sMOYUkyzrKM9OawlI-MZ~rqijVraQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)