Abstract
Aged or damaged RBCs are effectively removed from the blood circulation by Kupffer cells in the liver, but little is known regarding the mechanism of the clearance process. Here we show that stabilin-1 and stabilin-2 in hepatic sinusoidal endothelial cells (HSECs) are critical in effectively clearing damaged RBCs in mouse liver. Damaged RBCs and phosphatidylserine (PS)–coated beads were effectively sequestered in the hepatic sinusoid regardless of the presence of Kupffer cells, suggesting a role for HSECs in PS-dependent sequestration of PS-exposed RBCs in the liver. HSECs mediate tethering of damaged RBCs in a PS-dependent manner via stabilin-1 and stabilin-2. In a sinusoid-mimicked coculture system consisting of macrophages layered over HSECs, there was significant enhancement of the phagocytic capacity of macrophages, and this was mediated by stabilin-1 and stabilin-2 in HSECs. Liver-specific knockdown of stabilin-1 and stabilin-2 inhibited the sequestration of damaged RBCs in the hepatic sinusoid and delayed the elimination of damaged cells in an in vivo animal model. Thus, the roles of stabilin-1 and stabilin-2 in hepatic sequestration of PS-exposed RBCs may represent a potential mechanism for the clearance of damaged RBCs by Kupffer cells and for the control of some pathologic conditions such as hemolytic anemia.
Introduction
The rapid removal of damaged and aged RBCs from the peripheral circulation is important for tissue homeostasis. Based on a life span of 120 days, 170 billion RBCs must be removed daily from the blood circulation by the liver and spleen. Phagocytes need to engulf 2 million RBCs per second.1 Clearance of aged and damaged RBCs is mediated by the recognition of phosphatidylserine (PS) on the cell surface.2,3 Kupffer cells in the liver are known to be responsible for engulfing damaged RBCs in a PS-dependent manner.4 The number of PS-exposed RBCs to be removed is remarkably increased in patients with several hemolytic anemia, including sickle cell disease, β-thalassemia, malaria, and chronic renal failure.5-8 Furthermore, apoptotic and senescent neutrophils are almost entirely eliminated from the circulation (clearance half-time = 6.7 hours) by Kupffer cells in the liver, but rarely by macrophages in the spleen, lung, and peripheral blood.9,10 Kupffer cells are also involved in the clearance of macromolecules and pathogens.11 The many functions of Kupffer cells suggest that an auxiliary mechanism is required for apoptotic and senescent cell clearance in the liver.
Stabilin-1 and stabilin-2 are type I transmembrane receptors with multiple functions, including cell adhesion, endocytosis, and phagocytosis. They are expressed in certain populations of macrophages as well as in sinusoidal endothelial cells in the liver, spleen, and lymph nodes.12-15 Although stabilin-1 and stabilin-2 have different ligands during endocytosis,16 they share common functions such as cell-cell adhesion.17,18 Recently, stabilin-2 was suggested as a novel member of PS-receptor along with Tim-4 and BAI1.15,19,20 More recently, we showed that stabilin-1 is also involved in apoptotic cell clearance via recognition of PS in alternatively activated macrophages.21 Although stabilin-2 and its functional homolog stabilin-1 are involved in aged and apoptotic cell clearance in macrophages via PS recognition,15,21 their role as PS receptors in sinusoidal endothelial cells remains an open question. Hepatic sinusoidal endothelial cells (HSECs) are the major cell population of the hepatic sinusoid and play several important roles in the physiology and pathology of the liver, including Ag presentation and clearance of macromolecules and apoptotic cells.22,23 Thus, we hypothesized that there is a potential mechanism for the efficient removal of aged or damaged RBCs in the liver, where sinusoidal endothelial cells play a role in facilitating clearance by Kupffer cells. In this study, we present evidence that stabilin-1 and stabilin-2 in HSECs function as receptors in the PS-specific sequestration of damaged RBCs for their effective removal from the blood circulation.
Methods
Mice, reagents, and Abs
Six- to 7-week-old male BALB/c mice were used. These mice were maintained under specific pathogen-free conditions, and the use committee established at the Medical College of Kyungpook National University and Kyungpook National University Hospital approved the protocol.
Normal goat, rabbit, and mouse IgG were obtained from Santa Cruz Biotechnology. For function blocking and immunostaining of human stabilin-1 and stabilin-2, a goat polyclonal anti–human stabilin-1 Ab (N-13) was purchased from Santa Cruz Biotechnology, whereas a mouse monoclonal anti–human stabilin-2 Ab (5G3) was generated as previously described.15 For immunostaining of mouse stabilin-1 and stabilin-2, rabbit polyclonal anti–mouse stabilin-1 (1b-R1), and anti–mouse stabilin-2 (16R-2) Abs were generated by AbFrontier. Rat monoclonal anti-CD31 Ab was obtained from BD Pharmingen. Rabbit polyclonal and rat monoclonal anti-F4/80 Abs were purchased from Santa Cruz Biotechnology and AbDSerotec, respectively. CellTracker Violet BMQC and Alexa Fluor 568– and 647–conjugated secondary antibodies were purchased from Molecular Probes. NBD-PC (1-Oleoyl-2-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl]-sn-Glycero-3-Phosphocholine) and all phospholipids were purchased from Avanti-Polar Lipids.
Cell culture
Hepatic sinusoidal endothelial cells (HSECs) were obtained from ScienCell and cultured in endothelial cell medium (ECM) supplemented with 5% FBS. L cells stably expressing empty vector (L/Mock), stabilin-1 (L/Stab-1), and stabilin-2 (L/Stab-2) were grown in DMEM supplemented with 10% FBS and 400 μg/mL G418, as previously described.15,21 P388D1, a mouse monocytic cell line, was maintained in RPMI 1640 medium containing 10% FBS. 293T cells were grown in DMEM (high glucose) containing 10% FBS.
Production of anti–mouse stabilin-1 and stabilin-2 Abs
Production of polyclonal anti-mouse stabilin-1 and stabilin-2 Abs was performed by AbFrontier. Briefly, peptide sequences CEPFDDSVLEEDFPDT-NH2 (for mouse stabilin-1) and CDPFTDSGERELENSD-NH2 (for mouse stabilin-2) were synthesized and coupled to the immunogenic carrier protein keyhole limpet hemocyanin (KLH) via an additional cysteine of the synthetic peptide by the N-γ-maleimidobutyryloxylsuccinimide (GMBS) conjugation method. To generate polyclonal anti–mouse stabilin-1 and stabilin-2 Abs, New Zealand white rabbit (typically 10-weeks-old, mostly female) was immunized by subcutaneous injection of the peptide-KLH conjugate. For the first injection, 400 μg of each immunogen was emulsified with CFA (Sigma-Aldrich). Three subcutaneous booster injections consisting of 400 μg of each immunogen in IFA (Sigma-Aldrich) were given every 3 weeks. The antisera were further purified by Protein A affinity chromatography (Amersham Pharmacia) in accordance with the manufacturer's protocols. The specificities of the polyclonal anti-stabilin-1 (1bR1) and stabilin-2 (16R-2) Abs were examined by Western blotting (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Preparation of phospholipid-coated beads
Coating of the beads with phospholipids was performed as previously described.21,24 Briefly, Nucleosil 120-5 C18 beads (5 μm; Richard Scientific) were dissolved in chloroform, and either DOPC or a mixture of PC:PS (50:50 mol%) was added before the mixture was dried under nitrogen. The beads were rehydrated with PBS and briefly sonicated before use.
Liposomal clodronate preparation and macrophage depletion
Clodronate liposomes were prepared as previously described.25 Briefly, a mixture of 8 mg of cholesterol (Sigma-Aldrich) and 86 mg of egg-phosphatidylcholine (Avanti-Polar Lipids) was dissolved in chloroform and then evaporated under nitrogen. The clodronate solution was prepared by dissolving 1.2 g of dichloromethylene diphosphonic acid (Sigma-Aldrich) in 5 mL of sterile PBS, which was then added to the prepared liposome and mixed thoroughly. The solution was sonicated and ultracentrifuged at 10 000g for 1 hour at 4°C, after which the liposome pellet was resuspended in 5 mL of PBS. After 3 washes in PBS followed by ultracentrifugation, the liposomal clodronate solution was prepared in PBS at a final concentration of 5 mg/mL and used within 48 hours. A single dose of clodronate liposomes was administered by intravenous injection at 48 hours before RBC transfusion. Forty-eight hours after injection, Kupffer cells were completely depleted in the liver.
Liver uptake of damaged RBCs in mice
The in vivo clearance of damaged RBCs or PS beads was examined according to a previously described method26 with slight modification. Six- to 7-week-old male BALB/c mice were used. Venous blood was drawn from a male BALB/c mouse using a tube containing EDTA. After 3 washes with PBS, RBCs were incubated in PBS (20% hematocrit) for 4-5 days at 37°C to induce PS exposure, followed by labeling with FITC (Sigma-Aldrich). Exposure of PS on the cell surface was detected using annexin V–FITC (supplemental Figure 2A). Clodronate-treated or control (PBS-treated) mice were injected intravenously with 200 μL of the FITC-labeled RBCs. After 15 minutes, the mice were killed and the liver was collected. Liver tissues were dehydrated and embedded in OCT compound (Sakura Finetek) in cryomolds, then cut into 6-μm sections using a rotary microtome. The sections were fixed in 4% paraformaldehyde (PFA) in PBS for 5 minutes at 4°C, then permeabilized with 0.1% Triton X-100. Nonspecific immunoglobulin binding was prevented by blocking with PBS supplemented with 2% BSA. The sections were incubated with Abs against F4/80 (for Kupffer cells) and CD31 (for sinusoidal endothelial cells). After incubation for 1 hour with Alexa Fluor 568– and 647–conjugated secondary antibodies (Invitrogen), RBCs, CD31+, and F4/80+ cells were visualized by confocal microscopy (Carl Zeiss). The number of RBCs in the liver section was counted according to a previously described method.27 For each animal, a total tissue area of approximately 1.2 mm2 (45 fields, 0.0267 mm2/field) was counted from a random sample of 5 sections.
Binding assay of damaged RBCs in HSECs
PS-exposed, damaged RBCs were prepared by incubation in PBS (20% hematocrit) for 4-5 days at 37°C as previously described.15,28 Exposure of PS on the surface of damaged RBCs was detected using an Annexin V Apoptosis detection kit (Santa Cruz Biotechnology; supplemental Figure 2B). Damaged RBCs were added to the HSECs and incubated for 1 hour to assess binding in the presence of 10μM liposomes containing PC or 50:50 molar ratios of PS:PC, PI:PC, or PE:PC (all phospholipids obtained from Avanti-Polar Lipids). After extensive washing with PBS, the binding of damaged RBCs was quantified by light microscopy. The binding index was determined as the number of bound damaged RBCs or PS beads per HSEC. At least 200 cells were scored per well, and experiments were repeated at least 3 times. In some experiments, to exclude the effect of serum factors, the binding assays were performed in serum-free medium containing 5mM CaCl2.
For lentivirus-mediated knockdown, lentiviruses harboring stabilin-1 shRNA (shStab-1), stabilin-2 shRNA (shStab-2), and scrambled shRNAs of each (shCont-1 or shCont-2) were prepared as previously described.15,21 HSECs were infected with lentivirus harboring the indicated shRNA. At 48 hours postinfection, the binding assays were performed as above.
Quantitative real-time PCR
Total RNA was extracted from HSECs using TRIzol reagent in accordance with the manufacturer's instructions (Invitrogen). cDNA produced by reverse transcription was diluted 2.5-fold, and SYBR green master mix (Roche Applied Science) was used to amplify stabilin-1, stabilin-2, CD36, Tim-1, Tim-4, BAI1, and β-actin control. Real-time PCR amplification was carried out using a LightCycler 480 (Roche Applied Science) as follows: initial denaturation at 95°C for 5 minutes; 45 cycles of amplification with denaturation at 95°C for 30 seconds, annealing at 58°C for 30 seconds and extension at 72°C for 30 seconds; 1 cycle of melting at 95°C for 5 seconds, 65°C for 1 minute, and 97°C continuously; and a final cooling step at 40°C for 30 seconds. All samples were run in triplicate. The following primers were used: Stab1 forward, 5′-CAGTTGTGGTTAGCCGTATC-3′; Stab1 reverse, 5′-AGAAGTCGTCATCAGCATCA-3′; Stab2 forward, 5′-AGCACGCCACCTGTCAGATG-3′; Stab2 reverse, 5′-AACAGTGGTATCCTGGAAGTG-3′; Cd36 forward primer, 5′-ATGCTGTATTTGAATCCGAC-3′; Cd36 reverse, 5′-TCCAAGTATGTCCTATGTTC-3′; Tim-1 forward, 5′-ACAACGAGCATTCCAACAAC-3′; Tim-1 reverse, 5′-AGCAAGAAGCACCAAGACAG-3′; Tim-4 forward, 5′-CAGAATCAGAAACTGTCCTC-3′; Tim-4 reverse, 5′-CAATGCGAAGAGCACAAATC-3′; Bai1 forward, 5′-AGTACAGCATCCACATTGAC-3′; Bai1 reverse, 5′-CGACATTCTCGTTCTTGGTG-3′. The qRT-PCR results were analyzed using the comparative cycle threshold (CT) method.
Immunofluorescence staining
HSECs were incubated with NBD-PC–labeled PS- or PC-coated beads at 37°C for 30 minutes. After extensive washing with PBS, the cells were fixed in 4% formaldehyde in PBS for 15 minutes at room temperature and then permeabilized with 0.1% Triton X-100. Nonspecific binding was minimized by incubating the cells in PBS containing 2% BSA for 1 hour. After 3 washes with PBS, the slides were incubated for 1 hour at room temperature with polyclonal anti–stabilin-1 (N-13) and monoclonal anti–stabilin-2 (5G3) Abs. The slides were washed 3 times for 5 minutes each with PBS at room temperature. Alexa Fluor 568– and Alexa Fluor 647–conjugated anti–rabbit IgG (Molecular Probes) were added, followed by incubation for 1 hour at room temperature. The slides were washed 3 times with PBS for 5 minutes each and then treated with a solution of SlowFade (Molecular Probes). The slides were viewed with a Zeiss light microscope using Axioplan2 imaging.
Phagocytosis assays in coculture system
To perform 2-color flow cytometry, PS-beads were labeled with NBD-PC (Molecular Probes), and HSEC cells were labeled with CellTracker Violet BMQC (Molecular Probes). For coculture of HSECs and P388D1 cells, HSECs were seeded on a collagen-coated plate, after which P388D1 cells were grown into a HSEC monolayer the next day. PS beads were added to the P388D1 cells or the coculture of the HSEC and P388D1 cells, after which incubation was carried out for 1 hour to assess binding in the presence or absence of anti–stabilin-1 Ab (N-13, 20 μg/mL) and/or anti–stabilin-2 Ab (5G3, 20 μg/mL). Each isotype-matched Ab (IgG1, 20 μg/mL) was used as a control. Phagocytosis of PS beads by P388D1 macrophages was quantified by flow cytometry. In experiments using lentivirus-mediated shRNA, P388D1 cells were seeded on a stabilin-1kd HSEC, stabilin-2kd HSEC, or irrelevant knockdown HSEC monolayer and then incubated with NBD-PC–labeled PS beads for 1 hour.
For coculture of P388D1 and L-cell transfectants, P388D1 cells were seeded onto an L/Mock, L/Stab-1, or L/Stab-2 monolayer and then incubated with NBD-PC–labeled PS beads for 1 hour. Phagocytosis of PS beads was quantified by 2-color FACS analysis.
Time-lapse video microscopy
HSECs and P388D1 cells were cocultured in collagen type I–coated 8-well plates (Nunc). The culture dishes were placed into a chamber on a microscope (Leica) at 37°C in 5% CO2 atmosphere. DIC (differential interference contrast) images were acquired using a 200× objective and a cooled CCD camera (Cool Snap HQ; Photometrics). Images were controlled by Metamorph image processing software (Universal Imaging Corp). Thirty minutes after the addition of PS-coated beads, images were collected every 10 seconds for 30-45 minutes.
Knockdown of mouse stabilin-1 and stabilin-2 in vivo
Target sequences for shRNA (19- or 20-nt) were designed based on the mouse stabilin-1 (GenBank accession no. NM_015136) and mouse stabilin-2 genes (GenBank accession no. NM_107564): mouse Stab-1, CAU CGC AGC UAA UGG GGU C; mouse Stab-2, UGG CAA GGA CAG CUG ACU UC; mouse Stab-1-scrambed, UAC GUG CUG ACA GGA UCC G; mouse Stab-2-scrambled, UAC GAG CGA GUA CUA CCG GU. Candidate oligonucleotides were synthesized, annealed and cloned into pSuper/basic vector (Oligoengine). pSuper/shmStab-1 and -2 were found to have significantly down-regulated stabilin-1 and -2 in stably transfected L cells, respectively (supplemental Figure 4). Each scrambled shRNA (shCont-1 and shCont-2) was used as a control for mouse stabilin-1 shRNA (shmStab-1) or stabilin-2 shRNA (shmStab-2). All constructs were verified by sequence analysis. In vivo liver-specific knockdown was performed by hyperbolic injection using a TransIT-QR Hydrodynamic Delivery Starter Kit (Mirus) in accordance with the manufacturer's instructions. Briefly, 50 μg of shRNA vector was dissolved in 2 mL of Hydrodynamic Delivery Solution (Mirus), followed by rapid injection (within 4-7 seconds) into the tail vein at a constant rate using a syringe pump (Harvard Apparatus). After 24 hours, shmStab1/2-treated or shCont1/2-treated mice were injected intravenously with 200 μL of FITC-labeled RBCs. After 15 minutes, mice were killed and the liver collected. Liver sections were stained with Abs against F4/80 (Kupffer cells) and Stab1/2 (for sinusoidal endothelial cells). Damaged RBCs, Stab1+, Stab2+, and F4/80+ cells were visualized by confocal microscopy (Carl Zeiss Inc).
Blood decay of damaged RBCs in mice
Decay of RBCs was performed as previously described.26 Briefly, damaged RBCs were labeled with FITC (Sigma-Aldrich) and resuspended in sterile 0.9% NaCl to 30% (vol/vol). Recipients were given 200 μL of FITC-labeled RBCs, and clearance of the fluorescent RBCs was measured by flow cytometry (BD Biosciences) of the 10-μL blood samples collected from the tail vein at the time points indicated. The fraction of the total number of recipient RBCs studied (200 000 per sample) that was fluorescent was determined. The data were normalized to the fraction at 1 hour or 10 minutes after injection, which is set as 100% (donor RBCs constituted 4 to 6% of the total number of RBCs at this time point).
Statistical analysis
Statistical significance was assessed by t test. P < .05 was considered statistically significant.
Results
Damaged RBCs are sequestered in the hepatic sinusoid in a PS-dependent manner regardless of the presence of Kupffer cells
Intravenous injection of dichloromethylene diphosphonate (clodronate) liposomes eliminates macrophages.29 To investigate whether or not there is an auxiliary mechanism for the removal of damaged RBCs, we transfused clodronate-treated and control mice with FITC-labeled damaged RBCs. In the control mice, damaged RBCs, but not normal RBCs, were effectively sequestered in the liver, where they were mostly colocalized with Kupffer cells (Figure 1A-B). We observed engulfment of damaged RBCs or PS beads by Kupffer cells in images captured in sequential scanning mode using a confocal microscope (supplemental Figure 3). Interestingly, even in clodronate-treated mice, damaged RBCs were found in the hepatic sinusoid to the same degree as in control mice (Figure 1A-B). Recognition of phosphatidylserine (PS) on the cell surface is an important mechanism of aged and damaged RBC clearance.2,3 Therefore, we investigated whether or not PS-coated beads behave like damaged RBCs. PS-coated beads, but not PC-coated beads, were able to bind to the hepatic sinusoid regardless of the presence of Kupffer cells, similar to what was observed with damaged RBCs (Figure 1C-D). In contrast, sequestration of phospholipid-coated beads in the spleen was independent of PS (supplemental Figure 4). Thus, these results suggest that a PS-specific recognition system was present in the liver, whereas another population of liver cells other than Kupffer cells mediated the PS-dependent sequestration of damaged RBCs for their effectively removal from the blood circulation.
Damaged RBCs are sequestered in the hepatic sinusoid in a PS-dependent manner regardless of the presence of Kupffer cells. (A) Clodronate liposome-treated or control (PBS-treated) mice were injected with FITC-labeled normal or damaged RBCs (green), after which the livers were harvested 15 minutes later. Frozen sections were stained with Abs directed against CD31 (red; a marker for endothelial cells) and F4/80 (yellow; a marker for Kupffer cells) and analyzed by confocal microscopy. Scale bars, 10 μm. (B) Microscopic quantitation of sequestration of normal or damaged RBCs in liver section. Each experiment was independently performed 3 times. Results represent mean ± SD for 6 mice per group; t test: *P < .01 versus NR. NR indicates normal RBCs; and DR, damaged RBCs. (C) Clodronate liposome-treated or control (PBS-treated) mice were injected with NBD-PC–labeled PS or PC beads (green), after which the livers were harvested 15 minutes later. Frozen sections were stained with Abs directed against CD31 (red) and F4/80 (yellow). PC or PS beads, CD31+ cells, and F4/80+ cells were visualized by confocal microscopy. Liver from control mice effectively sequestered PS-coated beads, which were colocalized with Kupffer cells. Even in clodronate-treated mice PS-coated beads were found in the sinusoid to the same degree as in control mice. Scale bar, 10 μm. (D) Clodronate liposome-treated or control (PBS-treated) mice were injected with NBD-PC–labeled PC-coated or PS-coated beads, and their sequestration in liver section was quantified by confocal microscopy. Each experiment was independently performed 3 times. Results represent mean ± SD for 6 mice per group; t test: *P < .01 versus PC. PC indicates PC-coated beads; and PS, PS-coated beads.
Damaged RBCs are sequestered in the hepatic sinusoid in a PS-dependent manner regardless of the presence of Kupffer cells. (A) Clodronate liposome-treated or control (PBS-treated) mice were injected with FITC-labeled normal or damaged RBCs (green), after which the livers were harvested 15 minutes later. Frozen sections were stained with Abs directed against CD31 (red; a marker for endothelial cells) and F4/80 (yellow; a marker for Kupffer cells) and analyzed by confocal microscopy. Scale bars, 10 μm. (B) Microscopic quantitation of sequestration of normal or damaged RBCs in liver section. Each experiment was independently performed 3 times. Results represent mean ± SD for 6 mice per group; t test: *P < .01 versus NR. NR indicates normal RBCs; and DR, damaged RBCs. (C) Clodronate liposome-treated or control (PBS-treated) mice were injected with NBD-PC–labeled PS or PC beads (green), after which the livers were harvested 15 minutes later. Frozen sections were stained with Abs directed against CD31 (red) and F4/80 (yellow). PC or PS beads, CD31+ cells, and F4/80+ cells were visualized by confocal microscopy. Liver from control mice effectively sequestered PS-coated beads, which were colocalized with Kupffer cells. Even in clodronate-treated mice PS-coated beads were found in the sinusoid to the same degree as in control mice. Scale bar, 10 μm. (D) Clodronate liposome-treated or control (PBS-treated) mice were injected with NBD-PC–labeled PC-coated or PS-coated beads, and their sequestration in liver section was quantified by confocal microscopy. Each experiment was independently performed 3 times. Results represent mean ± SD for 6 mice per group; t test: *P < .01 versus PC. PC indicates PC-coated beads; and PS, PS-coated beads.
HSECs mediate tethering of damaged RBCs in a PS-dependent manner via stabilin-1 and stabilin-2
Recently, it was reported that microvascular endothelial cells interact with PS-positive RBCs and express a functional receptor for the PS-specific interaction,30,31 indicating that PS-specific interactions occur between erythrocytes and the endothelium. Growing evidence has revealed that HSECs play an important role in the blood clearance of macromolecules and apoptotic cells.22,23,32 These findings suggest that HSECs may be directly involved in the engulfment or sequestration of PS-exposed RBCs from the blood circulation by Kupffer cells in the liver. We examined the role of HSECs in the binding and engulfment of damaged RBCs using primary HSECs. Damaged RBCs but not normal RBCs significantly bound to HSECs; however, damaged RBCs were not engulfed by the HSECs (Figure 2A-B). The binding of damaged RBCs to HSECs was specifically inhibited by PS liposomes but not other phospholipid liposomes (Figure 2C). To exclude the effect of serum factors, we performed binding assays under serum-free conditions. Damaged RBCs significantly bound to HSECs, similar to what was observed in 10% FBS medium (Figure 2D). Under serum-free conditions, treatment with PS liposomes inhibited the binding of damaged RBCs to HSECs in a time- and dose-dependent manner (Figure 2E-F). These results suggest the involvement of a PS-specific receptor. Real-time PCR analysis showed that among all PS-recognizing receptors,15,19,20,33 stabilin-1 and stabilin-2 mRNA were highly expressed in HSECs (Figure 2G), which is in agreement with previous immunostaining results that showed high expression of stabilin-2 in HSECs.12 We then examined the localization of stabilin-1 and stabilin-2 in HSECs after incubation with PS- or PC-coated beads. When HSECs were incubated with PS- or PC-coated beads, stabilin-1 and stabilin-2 in HSECs were enriched on the bound PS beads but not the PC beads (Figure 2H). We next investigated the roles of stabilin-1 and staiblilin-2 in damaged RBC binding using lentivirus-mediated knockdown, which was used previously to successfully down-regulate human stabilin-1 and stabilin-2.15,21 As shown as Figure 2I, down-regulation of stabilin-1 or stabilin-2 resulted in significant reduction of damaged RBC binding to HSECs. These results suggest that stabilin-1 and stabilin-2 in HSECs were involved in sequestering damaged cells from the blood circulation for efficient phagocytosis.
Stabilin-1 and stabilin-2 in HSECs mediate tethering of damaged RBCs in a PS-dependent manner. (A) Representative images of binding of damaged RBCs (DR) or normal RBCs (NR) in HSECs. Scale bar, 10 μm. (B) Microscopic quantification of binding or engulfment of normal or damaged RBCs in HSECs. Binding or phagocytosis index was determined based on the number of bound or engulfed RBCs per HSEC. Results represent mean ± SD from at least 3 experiments; t test: *P < .01 between DR and NR. (C) Binding of damaged RBCs by HSECs in the presence of various phospholipid liposomes (10μM). PC indicates phosphatidylcholine; PS, phosphatidylserine; PE, phosphatidylethanolamine; and PI, phosphatidylinositol; t test: *P < .01 versus untreated control. (D) HSECs were incubated with normal of damaged RBCs in serum-free medium containing 5mM CaCl2, and binding index was then determined; t test: *P < .01 between DR and NR. (E) HSECs were incubated with damaged RBCs in the presence of 3 different concentrations (0.1, 1, or 10μM) of PS liposomes. Binding assays were preformed under serum-free conditions, and the binding index of damaged RBCs was determined. (F) HSECs were preincubated with PS liposomes (10μM) during the indicated times and then incubated with damaged RBCs for 1 hour. Binding assays were preformed under serum-free conditions, and binding index of damaged RBCs was determined. (G) Expression levels of PS recognition receptors in HSECs were analyzed by quantitative real-time PCR. (H) HSECs were incubated with NBD-PC–labeled PS or PC beads (green) for 1 hour at 37°C. The cells were fixed and stained with antibodies directed against stabilin-1 (yellow) and stabilin-2 (red). The white arrowhead indicates the bound PC bead. (I) Down-regulation of stabilin-1 and stabilin-2 expression decreased damaged RBC binding in HSECs. Results represent mean ± SD from at least 3 experiments; t test: *P < .01 versus untreated control.
Stabilin-1 and stabilin-2 in HSECs mediate tethering of damaged RBCs in a PS-dependent manner. (A) Representative images of binding of damaged RBCs (DR) or normal RBCs (NR) in HSECs. Scale bar, 10 μm. (B) Microscopic quantification of binding or engulfment of normal or damaged RBCs in HSECs. Binding or phagocytosis index was determined based on the number of bound or engulfed RBCs per HSEC. Results represent mean ± SD from at least 3 experiments; t test: *P < .01 between DR and NR. (C) Binding of damaged RBCs by HSECs in the presence of various phospholipid liposomes (10μM). PC indicates phosphatidylcholine; PS, phosphatidylserine; PE, phosphatidylethanolamine; and PI, phosphatidylinositol; t test: *P < .01 versus untreated control. (D) HSECs were incubated with normal of damaged RBCs in serum-free medium containing 5mM CaCl2, and binding index was then determined; t test: *P < .01 between DR and NR. (E) HSECs were incubated with damaged RBCs in the presence of 3 different concentrations (0.1, 1, or 10μM) of PS liposomes. Binding assays were preformed under serum-free conditions, and the binding index of damaged RBCs was determined. (F) HSECs were preincubated with PS liposomes (10μM) during the indicated times and then incubated with damaged RBCs for 1 hour. Binding assays were preformed under serum-free conditions, and binding index of damaged RBCs was determined. (G) Expression levels of PS recognition receptors in HSECs were analyzed by quantitative real-time PCR. (H) HSECs were incubated with NBD-PC–labeled PS or PC beads (green) for 1 hour at 37°C. The cells were fixed and stained with antibodies directed against stabilin-1 (yellow) and stabilin-2 (red). The white arrowhead indicates the bound PC bead. (I) Down-regulation of stabilin-1 and stabilin-2 expression decreased damaged RBC binding in HSECs. Results represent mean ± SD from at least 3 experiments; t test: *P < .01 versus untreated control.
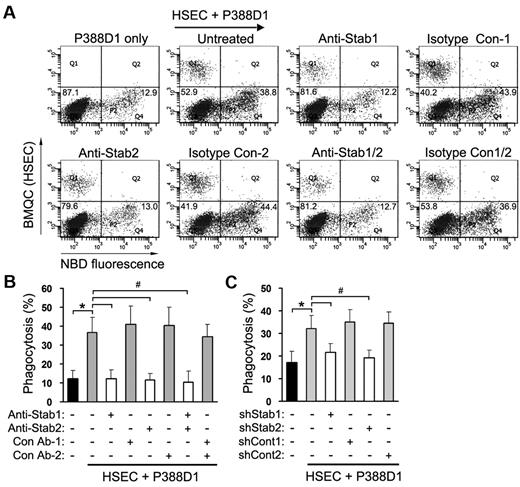
Stabilin-1 and stabilin-2 in HSECs significantly enhance PS-bead engulfment by macrophages
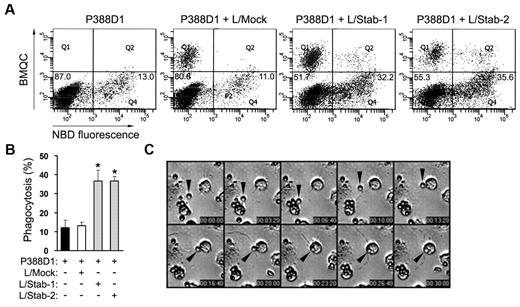
To investigate whether or not HSECs enhance the phagocytic activity of macrophages under sinusoid-mimicked conditions, we generated a coculture system consisting of the macrophage line P388D1 layered over HSECs and then examined the phagocytic activity of P388D1 cells using PS-coated beads. Although P388D1 cells expressed stabilin-2 mRNA,15 the proteins of stabilin-1 and stabilin-2 were not observed (supplemental Figure 5). We used this macrophage-like cell line instead of stabilin-deficient Kupffer cells for coculture experiments. The percentage of phagocytosis in P388D1 alone was ∼ 12.3% ± 4.2%, whereas the phagocytic activity of P388D1 cells was increased to 36.5% ± 8.2% in the presence of HSECs (Figure 3A-B). We next investigated whether or not stabilin-1 and -2 in HSECs are involved in the clearance of PS beads in our coculture system. Treatment with stabilin-1 or stabilin-2 Ab significantly decreased the engulfment of PS beads by macrophages (Figure 3A-B). Knockdown of stabilin-1 or stabilin-2 in HSECs also resulted in a significant decrease in PS-bead phagocytosis (Figure 3C). We also cocultured P388D1 cells with L cells transfected with empty vector (L/Mock), human stabilin-1 (L/Stab-1), or human stabilin-2 (L/Stab-2) and then examined the phagocytic activity of P388D1 cells. In agreement with findings of the coculture with HSECs, the percentages of phagocytosis were increased to 36.6% ± 5.7% and 36.6% ± 2.3% when P388D1 cells were cocultured with L/Stab-1 or L/Stab-2 cells, respectively (Figure 4A-B). These data suggest that stabilin-1 and stabilin-2 in HSECs were involved in the enhancement of phagocytosis by macrophages in a PS-dependent manner. We also analyzed the interaction between HSECs and P388D1 cells during the engulfment process using time-lapse video microscopy. In some case, we found that a PS bead was captured by HSECs and then presented to the adjacent macrophage for engulfment (Figure 4C and supplemental Video 1), suggesting that stabilin-1 and stabilin-2 in HSECs may act as receptors for capturing damaged cells and presenting them to macrophages for efficient clearance.
Stabilin-1 and stabilin-2 in HSECs significantly enhance PS-bead engulfment by macrophages. (A-B) Blockade of stabilin-1 and stabilin-2 abolishes the HSEC-mediated enhancement of PS-bead engulfment in coculture. P388D1 cells were seeded on the BMQC-labeled HSEC monolayer, and the next day, NBD-PC–labeled PS beads were added to the coculture of P388D1 and HSEC cells in the presence or absence of anti-stabilin-1 and/or anti-stabilin-2 Abs. After incubation for 1 hour, engulfment of PS beads by P388D1 cells (Q4 regions) was quantified by FACS analysis. A representative result is shown in panel A. Data represent mean ± SD from at least 3 experiments in panel B; t test: *P < .01 versus P388D1 only; #P < .01 versus untreated control. (C) Phagocytosis assays were performed in a coculture of P388D1 and stabilin-1 or stabilin-2 shRNA-treated HSECs. Data represent mean ± SD from at least 3 experiments; t test: *P < .01 versus P388D1 only; #P < .01 versus untreated control.
Stabilin-1 and stabilin-2 in HSECs significantly enhance PS-bead engulfment by macrophages. (A-B) Blockade of stabilin-1 and stabilin-2 abolishes the HSEC-mediated enhancement of PS-bead engulfment in coculture. P388D1 cells were seeded on the BMQC-labeled HSEC monolayer, and the next day, NBD-PC–labeled PS beads were added to the coculture of P388D1 and HSEC cells in the presence or absence of anti-stabilin-1 and/or anti-stabilin-2 Abs. After incubation for 1 hour, engulfment of PS beads by P388D1 cells (Q4 regions) was quantified by FACS analysis. A representative result is shown in panel A. Data represent mean ± SD from at least 3 experiments in panel B; t test: *P < .01 versus P388D1 only; #P < .01 versus untreated control. (C) Phagocytosis assays were performed in a coculture of P388D1 and stabilin-1 or stabilin-2 shRNA-treated HSECs. Data represent mean ± SD from at least 3 experiments; t test: *P < .01 versus P388D1 only; #P < .01 versus untreated control.
Stabilin-1– or stabilin-2–expressing cells significantly enhance PS-bead engulfment by macrophages. (A) Phagocytosis assays were performed in a coculture of P388D1 and L cells expressing empty vector, stabilin-1, or stabilin-2. P388D1 cells were seeded on BMQC-labeled L-cell transfectants, and the next day, NBD-PC–labeled PS-coated beads were added to the coculture of P388D1 and L-cell transfectants. After incubation for 1 hour, engulfment of damaged RBCs by P388D1 cells (Q4 regions) was quantified by FACS analysis. Percentages of phagocytosis for P388D1 alone and in coculture with L/Mock cells were 12.2% ± 3.9% and 13.0% ± 2.0%, respectively, whereas in the presence of L/Stab-1 or L/Stab-2 cells phagocytic activity of P388D1 cells was increased by 36.6% ± 5.7% and 36.6% ± 2.3%, respectively. A representative result is shown. (B) Phagocytosis assays were performed in a coculture of P388D1 and L cells expressing empty vector, stabilin-1, or stabilin-2. Data represent mean ± SD from at least 3 experiments; t test: *P < .01 versus L/Mock. (C) DIC images from supplemental Video 1 show the engulfment of PS bead (arrowheads) by P388D1 cells in coculture. The gray dotted lines indicate the boundary of HSECs.
Stabilin-1– or stabilin-2–expressing cells significantly enhance PS-bead engulfment by macrophages. (A) Phagocytosis assays were performed in a coculture of P388D1 and L cells expressing empty vector, stabilin-1, or stabilin-2. P388D1 cells were seeded on BMQC-labeled L-cell transfectants, and the next day, NBD-PC–labeled PS-coated beads were added to the coculture of P388D1 and L-cell transfectants. After incubation for 1 hour, engulfment of damaged RBCs by P388D1 cells (Q4 regions) was quantified by FACS analysis. Percentages of phagocytosis for P388D1 alone and in coculture with L/Mock cells were 12.2% ± 3.9% and 13.0% ± 2.0%, respectively, whereas in the presence of L/Stab-1 or L/Stab-2 cells phagocytic activity of P388D1 cells was increased by 36.6% ± 5.7% and 36.6% ± 2.3%, respectively. A representative result is shown. (B) Phagocytosis assays were performed in a coculture of P388D1 and L cells expressing empty vector, stabilin-1, or stabilin-2. Data represent mean ± SD from at least 3 experiments; t test: *P < .01 versus L/Mock. (C) DIC images from supplemental Video 1 show the engulfment of PS bead (arrowheads) by P388D1 cells in coculture. The gray dotted lines indicate the boundary of HSECs.
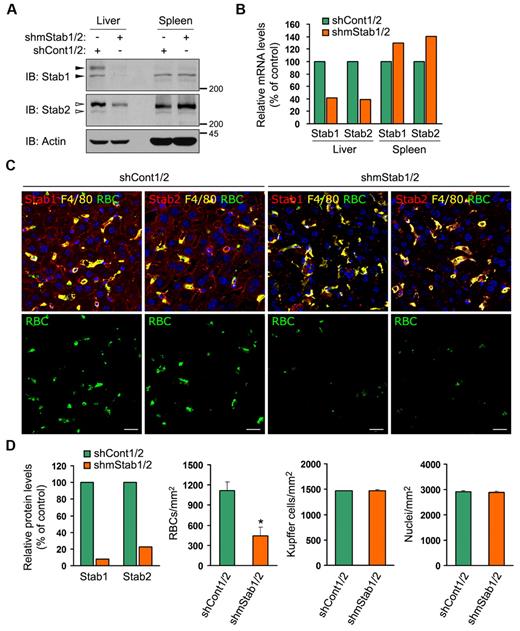
Stabilin-1 and stabilin-2 are necessary for the hepatic clearance of damaged RBCs
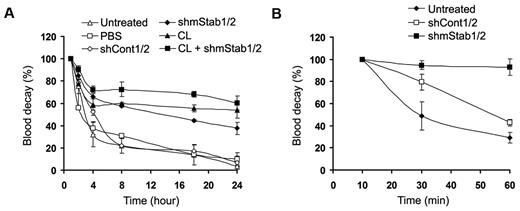
To verify the potential involvement of stabilin-1 and stabilin-2 in capturing damaged RBCs in vivo, we generated constructs expressing short hairpin RNAs (shRNA) against mouse stabilin-1 and stabilin-2 (supplemental Figure 6) and performed in vivo liver-specific knockdown via hydrodynamic-based transfection.34,35 When stabilin-1 and stabilin-2 shRNA vectors (shmStab1/2) were transfused into mice, the mRNA and protein expression of stabilin-1 and stabilin-2 were significantly suppressed in the liver but not the spleen (Figure 5A-B). Down-regulation of stabilin-1 and stabilin-2 expression significantly decreased the sequestration of damaged RBCs in the hepatic sinusoid by ∼ 40%, compared with that in shCont1/2-treated control mice (Figure 5C bottom panels and D). The numbers of Kupffer cells and nuclei were comparable in the liver sections from shStab1/2- or shCont1/2-treated mice (Figure 5D). To further confirm the roles of stabilin-1 and stabilin-2 in damaged RBC removal, we examined the decay of transfused damaged RBCs from the blood circulation. In untreated mice, damaged RBCs, but not normal RBCs, were effectively removed from the blood circulation (supplemental Figure 7). They were rapidly eliminated within 4 hours after injection and mostly removed at 24 hours (Figure 6A). Treatment with stabilin shRNA vectors (shmStab1/2) significantly delayed the decay of damaged RBCs regardless of the presence of Kupffer cells (Figure 6A closed diamonds). Treatment of control shRNA vectors (shCont1/2) had no effect (Figure 6A open diamonds). We also investigated the clearance of damaged RBCs from the blood circulation at an early time point. Damaged RBCs were rapidly eliminated from the blood of untreated or shCont1/2-treated mice within 1 hour, whereas in shmStab1/2-treated mice, damaged RBCs were not removed within 1 hour (Figure 6B). However, transfused damaged RBCs in shmStab1/2-treated mice were slowly removed from 8 hours after injection, and 24 hours of blood decay was less effective than in clodronate-treated mice (Figure 6A). One possible explanation is the compensatory enhancement of damaged RBC elimination in the spleen. We found that sequestration of damaged RBCs was increased in the spleen of shmStab1/2-treated mice (supplemental Figure 8).
Stabilin-1 and stabilin-2 in HSECs are necessary for the sequestration of damaged RBCs. (A-B) Verification of liver-specific knockdown using hydrodynamic injection. Liver and spleen were harvested from stabilin shRNAs (shmStab1/2)– or control shRNAs (shCont1/2)–treated mice. Expression of stabilin-1 and stabilin-2 were analyzed by Western blotting (A) and quantitative real-time PCR (B). A representative result is shown. The closed arrowheads indicate stabilin-1 proteins and the open arrowheads indicate stabilin-2 proteins. (C) FITC-labeled damaged RBCs were injected into stabilin shRNAs (shmStab1/2)– or control shRNAs (shCont1/2)–treated mice, after which the livers were harvested 15 minutes later. Frozen sections were stained with Abs against stabilin-1 or stabilin-2 (red) and F4/80 (yellow) and then analyzed by confocal microscopy (top panels). Damaged RBCs are shown in green (bottom panels). Scale bar, 10 μm. (D) Quantitation of stabilin-1 and stabilin-2 protein in the liver of shCont1/2- or shmStab1/2-treated mice (left panel). Immunoblot intensities for Stabilin/Actin were quantitated by densitometry and expressed in arbitrary units. The intensity for shCont1/2-treated controls was set to 100%. A representative result is shown. Microscopic quantitations of damaged RBCs, Kupffer cells, and nuclei in the liver section (right 3 panels). Each experiment was independently performed 3 times. Results represent mean ± SD for 6 mice per group; t test: *P < .01 versus shCont1/2.
Stabilin-1 and stabilin-2 in HSECs are necessary for the sequestration of damaged RBCs. (A-B) Verification of liver-specific knockdown using hydrodynamic injection. Liver and spleen were harvested from stabilin shRNAs (shmStab1/2)– or control shRNAs (shCont1/2)–treated mice. Expression of stabilin-1 and stabilin-2 were analyzed by Western blotting (A) and quantitative real-time PCR (B). A representative result is shown. The closed arrowheads indicate stabilin-1 proteins and the open arrowheads indicate stabilin-2 proteins. (C) FITC-labeled damaged RBCs were injected into stabilin shRNAs (shmStab1/2)– or control shRNAs (shCont1/2)–treated mice, after which the livers were harvested 15 minutes later. Frozen sections were stained with Abs against stabilin-1 or stabilin-2 (red) and F4/80 (yellow) and then analyzed by confocal microscopy (top panels). Damaged RBCs are shown in green (bottom panels). Scale bar, 10 μm. (D) Quantitation of stabilin-1 and stabilin-2 protein in the liver of shCont1/2- or shmStab1/2-treated mice (left panel). Immunoblot intensities for Stabilin/Actin were quantitated by densitometry and expressed in arbitrary units. The intensity for shCont1/2-treated controls was set to 100%. A representative result is shown. Microscopic quantitations of damaged RBCs, Kupffer cells, and nuclei in the liver section (right 3 panels). Each experiment was independently performed 3 times. Results represent mean ± SD for 6 mice per group; t test: *P < .01 versus shCont1/2.
Stabilin-1 and stabilin-2 in HSECs play important roles in blood decay of damaged RBCs in vivo. (A) FITC-labeled damaged RBCs were injected into clodronate liposomes (CL)–, stabilin shRNAs (shmStab1/2)–, or control shRNAs (shCont1/2)–treated mice. At the indicated times, 5 μL of venous blood was sampled from the tail vein and analyzed by flow cytometry for the fraction of fluorescent RBCs. Each experiment was independently performed 3 times. Data were normalized to the level at 1 hour after injection and represent mean ± SD for 6 mice per group. (B) FITC-labeled damaged RBCs were injected into stabilin shRNAs (shmStab1/2)– or control shRNAs (shCont1/2)–treated mice. At the indicated times, 5 μL of venous blood was sampled from the tail vein and analyzed by flow cytometry for the fraction of fluorescent RBCs. Each experiment was independently performed 3 times. Data were normalized to the level at 10 minutes after injection and represent mean ± SD for 6 mice per group.
Stabilin-1 and stabilin-2 in HSECs play important roles in blood decay of damaged RBCs in vivo. (A) FITC-labeled damaged RBCs were injected into clodronate liposomes (CL)–, stabilin shRNAs (shmStab1/2)–, or control shRNAs (shCont1/2)–treated mice. At the indicated times, 5 μL of venous blood was sampled from the tail vein and analyzed by flow cytometry for the fraction of fluorescent RBCs. Each experiment was independently performed 3 times. Data were normalized to the level at 1 hour after injection and represent mean ± SD for 6 mice per group. (B) FITC-labeled damaged RBCs were injected into stabilin shRNAs (shmStab1/2)– or control shRNAs (shCont1/2)–treated mice. At the indicated times, 5 μL of venous blood was sampled from the tail vein and analyzed by flow cytometry for the fraction of fluorescent RBCs. Each experiment was independently performed 3 times. Data were normalized to the level at 10 minutes after injection and represent mean ± SD for 6 mice per group.
Discussion
Clearance of aged and damaged cells from the liver and spleen has been well established. However, much remains to be determined regarding the molecular mechanism, including specific receptors and ligands. Although several studies have shown that the recognition of PS on the cell surface is important for clearance of aged and damaged RBCs,2,3 an in vivo inhibition study suggested that clearance of damaged RBCs by macrophages is likely dependent on scavenger receptors rather than specific PS receptors.4 However, several studies have shown that the clearance of damaged RBCs and neutrophils is PS dependent in the liver but not in the spleen,4,10 implying that an auxiliary mechanism is involved in PS-specific recognition in the liver. Previously, it was reported that P-selectin mediates sequestration of PMNs in the hepatic sinusoid, followed by phagocytosis of PMNs by Kupffer cells.10 However, this does not satisfactorily explain PS-specific sequestration of damaged RBCs in the hepatic sinusoid. We concentrated on the possibility that PS receptors are involved in hepatic sequestration of damaged RBCs. Among recently indentified PS receptors, stabilin-1 and stabiln-2 are highly expressed in HSECs.12 In this study, we provide evidence that stabilin-1 and stabilin-2 in HSECs sequestered damaged RBCs in the hepatic sinusoid and enhanced phagocytic capacity of macrophages. Furthermore, the results from real-time video microscopy suggest that stabilin-1 and stabilin-2 in HSECs captured damaged cells in a PS-dependent manner and presented them to Kupffer cells, thus establishing cellular cooperation between HSECs and Kupffer cells for the efficient clearance of PS-exposed cells. Thus, the role of stabilin-1 and stabilin-2 in the capture of aged and damaged RBCs might be an important mechanism for the clearance of aged and damaged cells in the liver.
Previously, we demonstrated that stabilin-1 and stabilin-2 mediate engulfment of aged and apoptotic cells in macrophages.15,21 Especially, stabilin-2 mediates both tethering (recognition PS) and tickling (internalization of tethered corpses and activation of downstream signaling pathways) functions.15 However, stabilin-2 in HSECs failed to mediate engulfment of damaged RBCs. One possible explanation is that HSECs may be deficient in a signal required for the tickling process. Although stabilin-1 and stabilin-2 in HSECs enhance the phagocytic activity of macrophages, blockade of either stabilin-1 or stabilin-2 inhibited phagocytosis of damaged RBCs to the same degree as seen with P388D1 alone, suggesting a possibility that the 2 proteins are functionally linked in HSECs. Immunostaining in the present study revealed that stabilin-1 and stabilin-2 shared similar cellular localization in HSECs. Furthermore, we also showed that stabilin-2 was associated with stabilin-1 using coimmunoprecipitation assays (S.-Y.P. and I.-S.K., unpublished data). Previous study suggests that a multimeric form of stabilin-2 may be required to mediate effective clearance of hyaluronan molecules from the blood circulation.36 Thus, it is possible that stabilin-1 and stabilin-2 form a multimer for effective recognition of PS on damaged RBCs.
Although we showed that stabilin-1 and stabilin-2 in HSECs sequestered damaged RBCs in a PS-dependent manner for effective phagocytosis, the roles of stabilin-1 and stabilin-2 in splenic sinusoidal endothelial cells remain unclear.12 A recent study showed that PS liposomes efficiently inhibited the uptake of oxRBCs in the liver, whereas uptake in the spleen was not influenced by PS liposomes,4 indicating that the clearance of damaged RBCs in the spleen is independent of PS. In agreement with this finding, we showed that sequestration of phospholipid-coated beads in the spleen was independent of PS (supplemental Figure 2). The mechanism of aged and damaged cell clearance in the spleen was different from that in the liver since damaged and senescent RBCs became mechanically trapped upstream of narrow interendothelial slits of the open, slow red pulp microcirculation in the spleen.37,38 Thus, sinusoidal endothelial cells may play a minor role in PS-exposed cell clearance in the spleen, whereas the roles of stabilin-1 and stabilin-2 in spleen remain to be investigated.
In patients with several hemolytic anemia such as sickle cell disease, β-thalassemia, malaria, and chronic renal failure, PS-exposed erythrocytes, which play an important role in pathophysiology, are remarkably increased.5-8 Considering that PS is an important signal for phagocytic clearance, our PS-specific sequestration mechanism based on stabilin-1 and stabilin-2 may represent a potential mechanism for the control of pathologic conditions such as hemolytic anemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Julia Kzhyshkowska for the expression vector for mouse stabilin-1.
This work was supported by grant no. RTI04-01-01 from the Regional Technology Innovation Program of the Ministry of Knowledge Economy; by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (2010-0029206); by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (R11-2008-044-03 001-0); by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0021626); and by the Brain Korea 21 Project.
Authorship
Contribution: S-.J.L. designed and performed research; S-.Y.P., M-.Y.J. and S.M.B performed research; I-.S.K. designed and supervised research; and S-.Y.P. and I-.S.K. analyzed and interpreted data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: In-San Kim, Department of Biochemistry and Cell Biology, Cell and Matrix Research Institute, Kyungpook National University School of Medicine, 101 Dongin 2-ga, Junggu, Daegu 700-422, Republic of Korea; e-mail: iskim@knu.ac.kr.
References
Author notes
S.-J.L. and S.-Y.P. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal