Abstract
The small guanine nucleotide binding proteins of the Ras family, including in mammals the highly homologous H-ras, N-ras, and K-ras isoforms, are rapidly activated on ligation of the T-cell antigen receptor (TCR), but whether each isoform plays specific roles in T cells is largely unknown. Here, we show, with the use of mice specifically lacking H-ras or N-ras, that these isoforms are dispensable for thymocyte development and mature T-cell activation. By contrast, CD4+ T cells from Ras-deficient mice exhibited markedly decreased production of the Th1 signature cytokine IFN-γ early after TCR stimulation, concomitantly with impaired induction of the Th1-specific transcription factor T-bet. Accordingly, Ras-deficient mice failed to mount a protective Th1 response in vivo against the intracellular parasite Leishmania major, although they could be rendered resistant to infection if a Th1-biased milieu was provided during parasite challenge. Collectively, our data indicate that the TCR recruits distinct Ras isoforms for signal transduction in developing and mature T cells, thus providing a mechanism for differential signaling from the same surface receptor. Furthermore, we demonstrate for the first time that H-ras and N-ras act as critical controllers of Th1 responses, mostly by transmitting TCR signals for Th1 priming of CD4+ T cells.
Introduction
T cells differentiate in the thymus from CD4+CD8+ precursors. In the peripheral lymphoid organs, mature CD4+ T cells undergo further differentiation and become T helper effectors which mediate, among others, Th1 and Th2 immune responses controlling intracellular and extracellular pathogen infections, respectively, allergy, and autoimmunity.1,2 Both differentiation processes are initiated by signals emanating from the TCR. However, the signaling pathways differentially triggered by the TCR during thymic and peripheral differentiation of T cells are not fully understood.
The small guanine nucleotide binding proteins of the Ras family,3 encompassing in mammals the highly homologous H-ras, N-ras, and K-ras (4A and 4B splice variants) isoforms, are rapidly activated after TCR engagement.4-6 Each of the 3 mammalian Ras genes encodes a membrane-associated 21-kDa protein that acts as a molecular switch to convey extracellularly derived signals into the cell interior. Ras proteins cycle between an inactive GDP-bound state and an active GTP-bound state, because of the concerted action of guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins.7 GEFs catalyze the release of GDP, allowing binding of the more abundant GTP and inducing a conformational change in the proteins that allow them to interact with their downstream effectors. GTPase-activating proteins, however, stimulate the intrinsic GTPase activity of Ras proteins and therefore catalyze their inactivation.
The best-characterized Ras effectors are the Raf kinases, through which Ras activates the MAPK cascade, the PI3Ks, and a family of Ral GEFs.7 Although Ras proteins are ubiquitously expressed, differences in their expression in various tissues and during development,3 along with a distinct subcellular distribution and recruitment of downstream effectors,8 suggest that each Ras isoform may subsume a specialized and specific function.
Ras signaling downstream of the TCR has been implicated in several aspects of T-cell biology,9,10 including thymocyte selection11 and Th differentiation.12 However, most of these studies are based on analyses of transgenic mice expressing a dominant-negative Ras mutant protein, which, because of its mechanism of action (ie sequestration of activating GEFs13 ), cannot discriminate between contributions from different isoforms or from other members of the Ras superfamily.14 This limitation can be partly overcome by analyzing gene-targeted mice specifically lacking individual Ras isoforms. Mice lacking H-ras15 or N-ras16 develop and reproduce normally, whereas deletion of K-ras results in embryonic lethality.17 We therefore analyzed T-cell differentiation and function in mice deficient for H-ras or N-ras. Our analyses showed hitherto unidentified functions of H-ras and N-ras in regulating early IFN-γ expression and Th1 responses and provided supporting evidence for their dispensability during thymocyte development and mature T-cell activation. Thus, our results suggest that Ras isoforms, although closely related, have nevertheless evolved to control differentiation programs triggered by the TCR at distinct stages of T-cell maturation.
Methods
Mice and reagents
H-ras– and N-ras–deficient mice have been previously described.15,16 C57BL/6 mice used as wild-type (WT) controls and BALB/c mice were purchased from Harlan Interfauna Ibérica. AND TCR and OT-I TCR transgenic mice (H-2b background) have been described elsewhere.18,19 Transgenic TCRs were independently introduced onto the Ras-deficient background by breeding TCR transgenic with H-ras knockout (KO) or N-ras KO mice. F1 littermates expressing the transgenic TCR, as determined by flow cytometry in blood T cells, were bred further to obtain Ras-sufficient (+/+) and Ras KO (−/−) TCR transgenic animals. Ras genotype was determined by PCR, as previously described.15 For experiments of Leishmania major infection, Ras-deficient mice were bred on a C57BL/6 background for ≥ 6 generations. All animal experiments were done according to institutional guidelines. Antibodies were obtained from BD Biosciences and Santa Cruz Biotechnology. Soluble leishmanial antigen (SLA) was prepared as previously described.20 Lipopolysaccharide (LPS) and concanavalin A were obtained from Sigma-Aldrich. Phosphorothioate-modified CpG-oligodeoxynucleotide (ODN; 5′-TCAACGTTGA-3′ and 5′-GCTAGCGTTAGCGT-3′) were synthesized by Isogen. Cytokines were purchased from R&D Systems.
TCR stimulation
Cells were cultured for 48 hours in RPMI 1640 supplemented with 10% FCS, 2mM l-glutamine, and 50 U/mL penicillin/streptomycin, in 96-well plates (5 × 105 cells per well) coated with 1 μg of anti-CD3 in the presence or absence of 0.5 μg of soluble anti-CD28. AND TCR thymocytes were stimulated by coculture for 48 hours with I-Ek– and ICAM-1–expressing DCEK fibroblasts loaded with various amounts of moth cytochrome c 81-103 peptide (VFAGLKKANERADLIAYLKQATK). For biochemical analysis, cells were resuspended in PBS and incubated for 10 minutes at 4°C in the presence or absence of biotin-conjugated anti-CD3 (145-2C11) and anti-CD4 (RM4-5) antibodies. After removal of unbound antibody, the cells were incubated at 37°C for various periods of time with 100 μg/mL streptavidin.
Immunoblotting
After stimulation, cells were lysed in a buffer containing 1% Brij-96, 20mM Tris-HCL pH 7.8, 150mM NaCl, and a cocktail of protease and phosphatase inhibitors. Total cell lysates were resolved by SDS-PAGE, transferred to Immobilon-P (Millipore) membranes, and immunoblotted with specific antibodies. Bound antibodies were detected with an enhanced chemiluminescence system (Amersham).
Flow cytometry
Cells were stained with specific fluorochrome-conjugated or biotinylated antibodies revealed with PE–cyanine 5–streptavidin and analyzed by flow cytometry in a FACSCalibur cytometer with Cellquest software (Becton Dickinson).
Proliferation, cytokine, and NO production assays
Proliferation was determined by incorporation of [3H]-thymidine, as described.21 The methods for cytokine measurement by intracellular cytokine staining and ELISA have been described.20 Briefly, for intracellular cytokine staining, cells were cultured in the presence of Golgi transport inhibitor for the final 4 hours of culture. Cells were then surface-stained with anti–CD4-PerCP, followed by fixation and permeabilization with Cytofix/Cytoperm (BD Biosciences) according to the manufacturer's protocol. Finally, cells were stained with anti–IFN-γ–FITC and anti–IL-4–PE and analyzed by flow cytometry. For ELISA assays, 3 × 106 cells were seeded in 48-well plates at 37°C for 48 hours in the presence or absence of SLA (12 μg/mL). The release of IFN-γ and IL-4 was measured in the culture supernatant fluids with ELISA kits from Diaclone and eBioscience, respectively. IL-12 was assessed with an ELISA kit from eBioscience. IL-2 was measured by fluorescent bead assay (Bender MedSystems), following the manufacturer's instructions. NO was measured as nitrite with the use of the Greiss reaction.22
BM-derived dendritic cells and macrophages
Cells from BM were cultured for 7 days in the presence of GM-CSF (20 ng/mL; PeproTech) or M-CSF (10 ng/mL; Sigma) to generate dendritic cells (DCs) and macrophages, respectively.
Parasites and infection
The methods used for parasite growth, challenge, and quantification have been described.20 Briefly, infection was performed by intradermal inoculation in the ear of 300 metacyclic promastigotes (in 15 μL of PBS) of L major (clone V1). The evolution of the infection was monitored by measuring the diameter of the induration of the ear lesion with a metric caliper. The number of parasites was determined by limiting dilution assay.
Statistical analysis
To analyze statistical significance, we used an unpaired Student t test. P values < .05 were considered to be significant.
Results
Unimpaired thymocyte development and positive selection in mice lacking H-ras or N-ras
To evaluate the effects of the H-ras and N-ras deficiency on T-cell development, we first examined the different thymocyte subpopulations defined by expression of CD4 and CD8 in WT and Ras-deficient mice (Figure 1A; Table 1). Percentages and numbers of double-negative CD4−CD8−, double-positive CD4+CD8+, and single-positive (SP) CD4+ or CD8+ cells present in H-ras– and N-ras–deficient thymuses were comparable to those of WT mice.15,16,23 Frequencies of mature CD4+ and CD8+ SP cells in the spleen were also similar between Ras-deficient and control mice. Further, the pattern of TCR expression, measured by CD3 staining, in thymocytes and spleen CD4+ T cells of Ras-deficient mice was indistinguishable from that of WT mice (Figure 1A bottom panels).
Unaffected thymocyte development and positive selection in mice lacking H-ras or N-ras. (A) Cells from WT, H-ras–, and N-ras–deficient mice were stained for expression of CD4, CD8, and CD3 and were analyzed by flow cytometry. Numbers within dot plots indicate percentage of cells in each quadrant. Bottom panels show CD3 expression in thymocytes (left) and splenic CD4+ T cells (right) from WT (shaded curve), H-ras KO (solid line), and N-ras KO (dotted line) mice. (B) CD4 and CD8 expression in AND TCR transgenic thymocytes (gated as TCRhi) from Ras-sufficient (+/+) and deficient (−/−) littermate mice. Numbers indicate percentage of cells in the gated region. Clonotypic TCR expression in gated CD4+ SP thymocytes from Ras-sufficient (solid line) and deficient (dotted line) mice is shown in the bottom panels. (C) CD4+ SP thymocyte numbers in Ras-sufficient and -deficient and TCR transgenic mice. Results from individual mice (open circles) and the average for each population (thick bars) are shown. (D) Thymocytes were stimulated with the indicated concentration of moth cytochrome c (MCC) 81-103 peptide for 48 hours and stained for expression of CD4, CD8, and CD69. Mean CD69 expression ± SD (n = 2) in gated CD4+ SP cells is shown. (E) CD4, CD8, and clonotypic TCR expression in thymocytes from Ras-sufficient and -deficient OT-I TCR transgenic mice. CD8+ SP frequencies were (n = 4): H-ras (+/+: 13 ± 3.4; −/−: 13 ± 2.0; P = .98); N-ras (+/+: 8.7 ± 5.9; −/−: 8.9 ± 6.0; P = .96). (F) ERK activation in anti–CD3-stimulated thymocytes. Fold induction of ERK relative to unstimulated (time 0) cells is indicated. Total AKT is shown as loading control.
Unaffected thymocyte development and positive selection in mice lacking H-ras or N-ras. (A) Cells from WT, H-ras–, and N-ras–deficient mice were stained for expression of CD4, CD8, and CD3 and were analyzed by flow cytometry. Numbers within dot plots indicate percentage of cells in each quadrant. Bottom panels show CD3 expression in thymocytes (left) and splenic CD4+ T cells (right) from WT (shaded curve), H-ras KO (solid line), and N-ras KO (dotted line) mice. (B) CD4 and CD8 expression in AND TCR transgenic thymocytes (gated as TCRhi) from Ras-sufficient (+/+) and deficient (−/−) littermate mice. Numbers indicate percentage of cells in the gated region. Clonotypic TCR expression in gated CD4+ SP thymocytes from Ras-sufficient (solid line) and deficient (dotted line) mice is shown in the bottom panels. (C) CD4+ SP thymocyte numbers in Ras-sufficient and -deficient and TCR transgenic mice. Results from individual mice (open circles) and the average for each population (thick bars) are shown. (D) Thymocytes were stimulated with the indicated concentration of moth cytochrome c (MCC) 81-103 peptide for 48 hours and stained for expression of CD4, CD8, and CD69. Mean CD69 expression ± SD (n = 2) in gated CD4+ SP cells is shown. (E) CD4, CD8, and clonotypic TCR expression in thymocytes from Ras-sufficient and -deficient OT-I TCR transgenic mice. CD8+ SP frequencies were (n = 4): H-ras (+/+: 13 ± 3.4; −/−: 13 ± 2.0; P = .98); N-ras (+/+: 8.7 ± 5.9; −/−: 8.9 ± 6.0; P = .96). (F) ERK activation in anti–CD3-stimulated thymocytes. Fold induction of ERK relative to unstimulated (time 0) cells is indicated. Total AKT is shown as loading control.
Thymocyte subsets in WT and Ras-deficient mice
| . | WT . | H-ras KO . | N-ras KO . |
|---|---|---|---|
| Total number, × 106 | 83 ± 16 | 86 ± 13 | 75 ± 9 |
| CD4+CD8+, % | 84.9 ± 2.4 | 83.5 ± 2.4 (.394) | 82.4 ± 1.9 (.112) |
| CD4+ SP, % | 9.4 ± 1.1 | 10.8 ± 2.1 (0.217) | 10.6 ± 0.5 (.06) |
| CD8+ SP, % | 2.5 ± 0.6 | 2.7 ± 0.9 (.709) | 2.8 ± 0.6 (.520) |
| . | WT . | H-ras KO . | N-ras KO . |
|---|---|---|---|
| Total number, × 106 | 83 ± 16 | 86 ± 13 | 75 ± 9 |
| CD4+CD8+, % | 84.9 ± 2.4 | 83.5 ± 2.4 (.394) | 82.4 ± 1.9 (.112) |
| CD4+ SP, % | 9.4 ± 1.1 | 10.8 ± 2.1 (0.217) | 10.6 ± 0.5 (.06) |
| CD8+ SP, % | 2.5 ± 0.6 | 2.7 ± 0.9 (.709) | 2.8 ± 0.6 (.520) |
Values are mean ± SD (n = 5). P values versus WT using unpaired t test are shown in parentheses.
SP indicates single positive.
To study thymocyte development in more detail, we introduced the MHC class II–restricted cytochrome c-specific AND TCR transgene18 onto the H-ras– and N-ras–deficient background and analyzed positive selection. In Ras-deficient mice, positively selected CD4+ SP T cells expressing the AND TCR were similar to their counterparts in Ras-sufficient littermate controls with regard to cell frequencies and numbers (Figure 1B-C) and surface expression of the transgenic TCR (Figure 1B bottom panels). Because a major criterion for positive selection is responsiveness to cognate antigen, we challenged Ras-sufficient and -deficient thymocytes with antigen in vitro and assessed CD69 up-regulation in positively selected CD4+ SP cells. The pattern of CD69 up-regulation in response to antigenic peptide of H-ras– and N-ras–deficient CD4+ SP cells was comparable to that of their sufficient counterparts (Figure 1D). Moreover, positive selection of OT-I TCR19 transgenic CD8+ SP thymocytes proceeded normally in Ras-deficient mice (Figure 1E). Collectively, these data suggest that TCR-derived signals responsible for thymocyte positive selection remain mostly undisturbed in the absence of H-ras or N-ras. Further supporting this, activation of ERK kinases, which is critical for positive selection,24 was not defective but moderately augmented in thymocytes from mice lacking H-ras, in particular, or N-ras (Figure 1F). Taken together, these data indicate that loss of either H-ras or N-ras gene function does not compromise positive selection and differentiation in the thymus.
H-ras and N-ras are not required for TCR-mediated mature T-cell activation
Next, we examined the roles of H-ras and N-ras in TCR-mediated activation of peripheral T cells. For this, we stimulated splenocytes from WT and Ras-deficient mice with anti-CD3 and anti-CD28 antibodies. Up-regulation of the early activation markers CD69 and CD25 was similar in Ras-deficient and WT T cells (Figure 2A). Further, stimulation of Ras-deficient splenocytes with concanavalin A, a T-cell mitogen, or anti-CD3 plus anti-CD28 provoked similar or augmented release of IL-2, respectively, compared with WT cells (Figure 2B). In correlation, H-ras– and N-ras–deficient T cells showed enhanced proliferation in response to anti-CD3 plus anti-CD28 stimulation compared with WT counterparts (Figure 2C). Thus, H-ras and N-ras seem to be dispensable for TCR-mediated T-cell activation.
Unimpaired TCR-mediated early T-cell activation in mice lacking H-ras or N-ras. (A) Splenocytes from WT, H-ras–, and N-ras–deficient mice were stimulated without or with anti-CD3 plus anti-CD28 mAb for 48 hours and stained for CD25 and CD69 expression. Numbers indicate percentage of cells in the gated region. (B) IL-2 production by splenic T cells stimulated with anti-CD3 (solid line), concanavalin A (dotted line), or without stimulation (shaded curve), as measured after 48 hours by fluorescent bead assay. Numbers over each curve indicate IL-2 concentration (ng/mL). (C) Proliferation responses of unstimulated and anti–CD3/CD28-stimulated LN T cells. Mean [3H]-thymidine incorporation ± SD of triplicate cultures is shown (*P < .05 vs WT). Results in each panel are representative of ≥ 2 independent experiments.
Unimpaired TCR-mediated early T-cell activation in mice lacking H-ras or N-ras. (A) Splenocytes from WT, H-ras–, and N-ras–deficient mice were stimulated without or with anti-CD3 plus anti-CD28 mAb for 48 hours and stained for CD25 and CD69 expression. Numbers indicate percentage of cells in the gated region. (B) IL-2 production by splenic T cells stimulated with anti-CD3 (solid line), concanavalin A (dotted line), or without stimulation (shaded curve), as measured after 48 hours by fluorescent bead assay. Numbers over each curve indicate IL-2 concentration (ng/mL). (C) Proliferation responses of unstimulated and anti–CD3/CD28-stimulated LN T cells. Mean [3H]-thymidine incorporation ± SD of triplicate cultures is shown (*P < .05 vs WT). Results in each panel are representative of ≥ 2 independent experiments.
H-ras and N-ras deficiency impair TCR-induced IFN-γ expression in CD4+ T cells
The early induction of cytokine gene expression and subsequent Th differentiation require proximal signaling events initiated by TCR engagement,25,26 the nature of which remains poorly defined. To test whether H-ras and N-ras are implicated in early cytokine production by CD4+ T cells after TCR ligation, we stimulated splenocytes with immobilized anti-CD3 in the presence or absence of anti-CD28 and measured the expression of Th hallmark cytokines IFN-γ (Th1) and IL-4 (Th2) by intracellular cytokine staining. Anti–CD3-stimulated CD4+ T cells from WT mice showed enhanced IFN-γ expression but only marginal induction of IL-4, compared with nonstimulated cells. In addition, the IFN-γ response was strongly enhanced further by anti-CD28 antibody (Figure 3A top panels). In contrast, IFN-γ expression was consistently attenuated in stimulated CD4+ T cells from H-ras– and N-ras–deficient mice, with poor additional enhancement when anti-CD28 costimulation was provided (Figure 3A middle and bottom panels). However, this impairment in IFN-γ expression was not accompanied by a corresponding increase in the proportions of IL-4–producing cells. The addition during Th priming of IL-12 or CpG-containing oligodeoxynucleotides (CpG-ODNs), both potent inducers of IFN-γ and Th1 differentiation,25,27 failed to restore IFN-γ production to WT levels (Figure 3B). A similar defect was observed in Th1-polarized cultures of Ras-deficient T cells at later time points (data not shown). In addition, deliberate Th2 polarization, by the addition of IL-4 and anti–IFN-γ antibody, did not result in increased frequencies of IL-4–expressing cells in Ras-deficient compared with WT cells (Figure 3B).
H-ras and N-ras deficiency impair TCR-induced IFN-γ expression in CD4+ T cells. (A) Splenic T cells from mice of the indicated genotype were stimulated without or with anti-CD3 or anti-CD3 plus anti-CD28 mAb during 48 hours for analysis of intracellular IFN-γ and IL-4 expression in gated CD4+ T cells. Numbers within dot plots indicate percentage of cells in the gated regions. (B) Splenic T cells from mice of the indicated genotype were stimulated for 48 hours with anti-CD3 plus anti-CD28 in the absence or presence of either IL-12 (1 ng/mL), CpG-ODN (25 μg/mL), or IL-4 (100 ng/mL) plus anti–IFN-γ antibody (3 μg/mL) and analyzed for intracellular IFN-γ and IL-4 expression in gated CD4+ T cells by flow cytometry. Numbers within dot plots indicate percentage of cells in the gated regions.
H-ras and N-ras deficiency impair TCR-induced IFN-γ expression in CD4+ T cells. (A) Splenic T cells from mice of the indicated genotype were stimulated without or with anti-CD3 or anti-CD3 plus anti-CD28 mAb during 48 hours for analysis of intracellular IFN-γ and IL-4 expression in gated CD4+ T cells. Numbers within dot plots indicate percentage of cells in the gated regions. (B) Splenic T cells from mice of the indicated genotype were stimulated for 48 hours with anti-CD3 plus anti-CD28 in the absence or presence of either IL-12 (1 ng/mL), CpG-ODN (25 μg/mL), or IL-4 (100 ng/mL) plus anti–IFN-γ antibody (3 μg/mL) and analyzed for intracellular IFN-γ and IL-4 expression in gated CD4+ T cells by flow cytometry. Numbers within dot plots indicate percentage of cells in the gated regions.
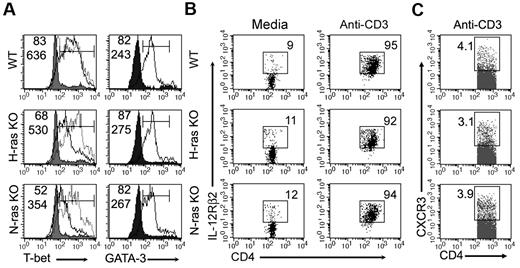
The poor capacity of H-ras– and N-ras–deficient CD4+ T cells to differentiate into IFN-γ–producing effectors correlated with impaired induction of T-bet (Figure 4A), a transcription factor that plays a major role in Th1 lineage commitment in part by activating the expression of IFN-γ.28 In contrast, induction of the Th2 transcription factor GATA-31 was not affected by the Ras deficiency (Figure 4A). Notably, IL-12Rβ225 and CXCR-3,29 also gene targets of T-bet, were normally induced in activated CD4+ T cells from Ras-deficient mice (Figure 4B-C), suggesting that expression of T-bet–dependent genes is differentially triggered by distinct levels of the transcription factor. Of interest, CpG-ODNs were capable of fully rescuing the defect in T-bet induction (Figure 4A), whereas IFN-γ expression (Figure 3B) was only partially improved by CpG-ODNs in activated Ras-deficient CD4+ T cells. The latter supports the notion that, in addition to T-bet, other H-ras– and N-ras–dependent pathways are required for optimal TCR-mediated induction of IFN-γ. To any extent, these data indicate that H-ras and N-ras play an important role in TCR-initiated signaling leading to early induction of T-bet and IFN-γ in CD4+ T cells.
T-bet and GATA-3 induction in activated CD4+ T cells from Ras-deficient mice. (A) T-bet and GATA-3 expression in splenic CD4+ T cells left unstimulated (shaded curve) or stimulated with anti-CD3/CD28 antibodies for 48 hours (solid line). T-bet expression in cells stimulated in the presence of CpG-ODN is also shown (dotted line). Top and bottom values within histograms indicate the percentage and MFI, respectively, of cells in the marked region. (B) Unstimulated or anti–CD3-stimulated (48 hours) splenocytes were stained for expression of CD4 and IL-12Rβ2. (C) CXCR3 induction in splenic CD4+ T cells stimulated with anti-CD3 antibody for 72 hours. Numbers indicate percentage of cells in the gated region. Results in each panel are representative of ≥ 2 independent experiments.
T-bet and GATA-3 induction in activated CD4+ T cells from Ras-deficient mice. (A) T-bet and GATA-3 expression in splenic CD4+ T cells left unstimulated (shaded curve) or stimulated with anti-CD3/CD28 antibodies for 48 hours (solid line). T-bet expression in cells stimulated in the presence of CpG-ODN is also shown (dotted line). Top and bottom values within histograms indicate the percentage and MFI, respectively, of cells in the marked region. (B) Unstimulated or anti–CD3-stimulated (48 hours) splenocytes were stained for expression of CD4 and IL-12Rβ2. (C) CXCR3 induction in splenic CD4+ T cells stimulated with anti-CD3 antibody for 72 hours. Numbers indicate percentage of cells in the gated region. Results in each panel are representative of ≥ 2 independent experiments.
H-ras and N-ras deficiency enhance susceptibility to L major infection because of defective development of a protective Th1 response
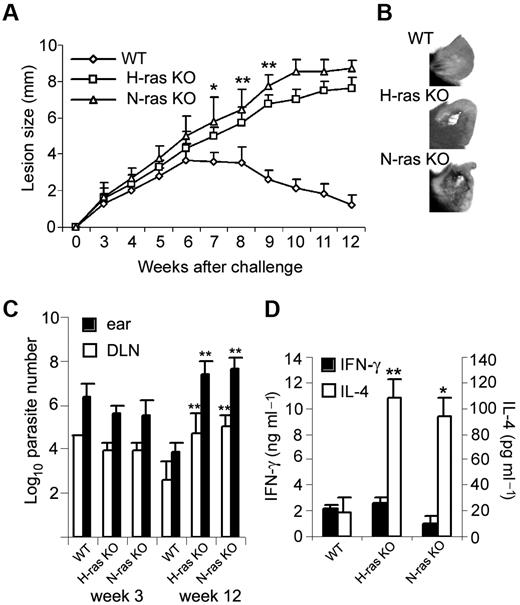
Because T-bet and IFN-γ play a major role in Th1 development,25 we next sought to determine the effect of the H-ras and N-ras deficiency on the generation of Th1 responses in vivo against the intracellular parasite L major.30 Inbred genetically resistant mouse strains such as C57BL/6 control infection by developing a curative Th1 response. Ras-deficient mice in the C57BL/6 background, in striking contrast to WT mice, developed progressively larger and nonhealing lesions (Figure 5A-B) after inoculation of a low dose of parasites into the ear dermis, which correlated with significantly enhanced parasite burden in the infection site and draining lymph node (LN) at week 12 after infection (Figure 5C). At an earlier times after infection (week 3), parasite loads were still comparable in WT and Ras-deficient mice.
H-ras and N-ras deficiency enhance susceptibility to L major infection. (A) Course of L major infection in WT, H-ras–, and N-ras–deficient mice. Mean lesion size ± SD of 4-10 mice per group are shown (*P < .001 and **P < .0005 vs WT). (B) Ear lesions with focal necrosis in infected Ras-deficient but not WT mice at week 12 after infection. (C) Parasite burden in the ear and draining LN (DLN) from infected mice at weeks 3 and 12 after infection. Results represent mean ± SD for 4 mice per group (**P < .0005 vs WT). (D) IFN-γ and IL-4 production, as determined by ELISA, by draining LN cells isolated from L major–infected mice at week 12 after infection and stimulated with SLA (12 μg/mL) for 48 hours. Values represent the mean ± SD of duplicate cultures and 4 mice in each group (*P < .001 and **P < .0005 vs WT). The data shown are representative of 2 independent experiments.
H-ras and N-ras deficiency enhance susceptibility to L major infection. (A) Course of L major infection in WT, H-ras–, and N-ras–deficient mice. Mean lesion size ± SD of 4-10 mice per group are shown (*P < .001 and **P < .0005 vs WT). (B) Ear lesions with focal necrosis in infected Ras-deficient but not WT mice at week 12 after infection. (C) Parasite burden in the ear and draining LN (DLN) from infected mice at weeks 3 and 12 after infection. Results represent mean ± SD for 4 mice per group (**P < .0005 vs WT). (D) IFN-γ and IL-4 production, as determined by ELISA, by draining LN cells isolated from L major–infected mice at week 12 after infection and stimulated with SLA (12 μg/mL) for 48 hours. Values represent the mean ± SD of duplicate cultures and 4 mice in each group (*P < .001 and **P < .0005 vs WT). The data shown are representative of 2 independent experiments.
One week after parasite challenge, frequencies of primed IFN-γ–producing CD4+ T cells were significantly reduced in Ras-deficient compared with WT mice, but without concomitant increase of IL-4–expressing cells (Table 2). In contrast, 12 weeks after challenge, the cytokine response to the parasite was biased toward a Th2-like profile dominated by IL-4 in both H-ras– and N-ras–deficient compared with WT mice (Figure 5D). Moreover, Th2-dependent IgG1 antibodies to the parasite were detected in Ras-deficient but not in WT mice (data not shown). These data clearly show that, despite their normally resistant genetic background,30 H-ras– and N-ras–deficient mice are impaired in their ability to resolve a L major infection, probably because of defective Th1 differentiation in these mice leading to gradual establishment of a disease-promoting Th2 response.
CD4+ T-cell cytokine response in WT and Ras-deficient mice early after L major challenge
| . | WT . | H-ras KO . | N-ras KO . |
|---|---|---|---|
| IFN-γ+ | 17.4 ± 1.9 | 5.5 ± 0.7 (.015*) | 5.8 ± 0.5 (.041*) |
| IL-4+ | 1.2 ± 0.9 | 1.2 ± 0.5 (.709) | 0.8 ± 0.3 (.520) |
| . | WT . | H-ras KO . | N-ras KO . |
|---|---|---|---|
| IFN-γ+ | 17.4 ± 1.9 | 5.5 ± 0.7 (.015*) | 5.8 ± 0.5 (.041*) |
| IL-4+ | 1.2 ± 0.9 | 1.2 ± 0.5 (.709) | 0.8 ± 0.3 (.520) |
Values represent the percentage (mean ± SD; 2 mice per group) of CD4+ T cells positive for the indicated cytokine, as determined by cytometric intracellular staining, in anti–CD3/CD28-stimulated (48 hours) draining lymph node cells 1 week after parasite challenge. P values versus WT using unpaired t test are shown in parentheses.
Significant difference.
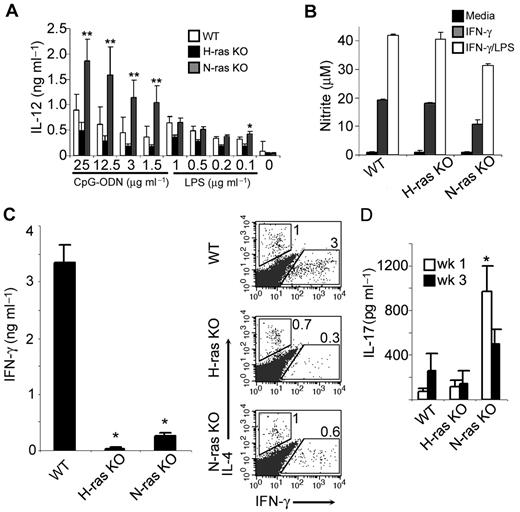
Because IL-12 is required to establish Th1-mediated responses against L major,31 and DCs are a major source of IL-12 in vivo, we next examined whether Ras-deficient DCs might be impaired in IL-12 production. As shown in Figure 6A, DCs lacking H-ras exhibited slightly reduced IL-12 production compared with WT DCs on stimulation with increasing amounts of LPS or CpG-ODNs. By contrast, N-ras–deficient DCs produced normal or significantly enhanced amounts of IL-12 in response to LPS or CpG-ODNs, respectively; the latter suggesting that N-ras could act as a negative regulator of TLR9-mediated signaling in DCs. Thus, enhanced susceptibility of Ras-deficient mice to L major infection is not seemingly associated with defects in IL-12 production by DCs or in the expression of the IL-12R on activated CD4+ T cells (Figure 4B), which is also required for resistance to L major.32 Further, LPS- or CpG-ODN–induced up-regulation of MHC class II and costimulatory molecules in DCs was not affected by the Ras deficiency (data not shown). However, macrophages from H-ras– or N-ras–deficient mice were as competent as those from WT mice to produce NO, a key effector mechanism for control and clearance of L major30 after stimulation with IFN-γ or IFN-γ plus LPS (Figure 6B), indicative of no primary defect in their function.
Unimpaired DC and macrophage function but defective Th1 differentiation in vivo in mice lacking H-ras or N-ras. (A) IL-12 production by LPS- or CpG-ODN–stimulated (48 hours) BM-derived DCs from WT, H-ras–, and N-ras–deficient mice (mean ± SD of duplicate cultures and 2 mice per group), as determined by ELISA (*P < .005 and **P < .001 vs WT). (B) BM-derived macrophages from mice of the indicated genotype were stimulated for 24 hours without or with IFN-γ (100 U/mL) or IFN-γ plus LPS (2 μg/mL), followed by assessment of NO production measured as nitrite (mean ± SD of duplicate cultures and 2 mice per group). (C) Draining LN cells (5 × 105) from L major–infected (week 3 after infection) mice of the indicated genotype were cultured with 1 × 105 WT DCs presenting SLA (12 μg/mL). Cytokine production was determined after 24 hours by intracellular cytokine staining in gated CD4+ T cells (right) or after 48 hours by ELISA (left; mean ± SD of duplicate cultures and 2 mice per group; *P < .03 vs WT). Numbers within dot plots indicate percent cells in the gated regions. (D) IL-17 response to SLA of draining LN cells from infected (weeks 1 and 3 after infection) mice, as measured by fluorescent bead assay (mean ± SD of 2 mice per group; *P < .05 vs WT).
Unimpaired DC and macrophage function but defective Th1 differentiation in vivo in mice lacking H-ras or N-ras. (A) IL-12 production by LPS- or CpG-ODN–stimulated (48 hours) BM-derived DCs from WT, H-ras–, and N-ras–deficient mice (mean ± SD of duplicate cultures and 2 mice per group), as determined by ELISA (*P < .005 and **P < .001 vs WT). (B) BM-derived macrophages from mice of the indicated genotype were stimulated for 24 hours without or with IFN-γ (100 U/mL) or IFN-γ plus LPS (2 μg/mL), followed by assessment of NO production measured as nitrite (mean ± SD of duplicate cultures and 2 mice per group). (C) Draining LN cells (5 × 105) from L major–infected (week 3 after infection) mice of the indicated genotype were cultured with 1 × 105 WT DCs presenting SLA (12 μg/mL). Cytokine production was determined after 24 hours by intracellular cytokine staining in gated CD4+ T cells (right) or after 48 hours by ELISA (left; mean ± SD of duplicate cultures and 2 mice per group; *P < .03 vs WT). Numbers within dot plots indicate percent cells in the gated regions. (D) IL-17 response to SLA of draining LN cells from infected (weeks 1 and 3 after infection) mice, as measured by fluorescent bead assay (mean ± SD of 2 mice per group; *P < .05 vs WT).
In view of the above-mentioned data, we focused on the T-cell cytokine response during early L major infection. We infected WT and Ras-deficient mice in the ear with 1000 metacyclic promastigotes of L major, and cells from the local draining LN were collected 3 weeks later, cultured with SLA-pulsed DCs from WT mice, and assessed for cytokine production. WT DCs were used as an APC to optimize the conditions necessary to show IFN-γ production by Ras-deficient T cells, thus avoiding potential adverse effects of the Ras deficiency on DC function. As shown in Figure 6C, LN T cells from Ras-deficient mice exhibited impaired parasite-specific IFN-γ but normal IL-4 responses compared with WT counterparts, as measured by intracellular cytokine staining after 24 hours. Analysis of supernatant fluids from 48-hour cultures by ELISA also showed a markedly reduced SLA-specific IFN-γ response of both H-ras– and N-ras–deficient T cells. Of interest, an augmented antiparasite IL-17 (Th17) cytokine response was observed in N-ras– but not H-ras–deficient mice (Figure 6D). This suggests that impaired Th1 differentiation in Ras mutant mice is not a common consequence of alternative development toward the Th17 lineage. In fact, T cells from mice lacking N-ras, but not H-ras, displayed markedly increased production of IL-17 early after TCR engagement in vitro, even in the absence of Th17-polarizing cytokines (L.S.-A., E.S., E.F.-M., unpublished observations, November 2007). Taken together with results shown in Figure 3, these data are consistent with an intrinsic defect of H-ras– and N-ras–deficient T cells to differentiate into Th1 effectors, which in turn could compromise the development of protective immunity against L major.
CpG-ODN administration protects H-ras– and N-ras–deficient mice from L major infection
CpG-ODNs trigger an immunomodulatory cascade that results in a Th1-biased immune milieu.27 CpG-ODN treatment has been used successfully to promote protective and curative Th1 responses to L major infection in otherwise susceptible BALB/c mice.33 In resistant C57BL/6 mice, CpG-ODNs delivered at the same site and time of infection enhanced Th1 immunity and thereby moderated the pathology associated with parasite infection.34 We therefore tested whether CpG-ODNs could protect Ras-deficient mice from L major infection. For this, mice were infected in both ears with 1000 L major metacyclic promastigotes alone or in combination with CpG-ODNs. As previously reported,34 C57BL/6 WT mice treated with CpG-ODNs developed an attenuated dermal pathology compared with untreated mice (Figure 7A). Strikingly, CpG-ODN treatment also protected H-ras– and N-ras–deficient mice from the development of dermal lesions. This protective effect correlated with an early and long-lasting containment of parasite growth in the infection site (Figure 7B) and draining LN (data not shown) in both WT and Ras-deficient mice.
CpG-ODN protects H-ras– and N-ras–deficient mice from L major infection. (A) Course of L major infection in CpG-ODN–treated WT, H-ras–, and N-ras–deficient mice. Mean induration ± SD of 2-3 mice (4-6 ears) per group are shown. Dotted line represents the evolution of infection in untreated WT mice. (B) Parasite burden per ear in infected CpG-ODN–treated mice at weeks 3 and 8 after infection. Results represent mean ± SD for 2 mice per group. (C) IFN-γ and IL-4 production, as determined by ELISA, by draining LN cells isolated from untreated (−) or CpG-ODN–treated (+) L major–infected mice at week 3 after infection and stimulated with SLA (12 μg/mL) for 48 hours. Values represent the mean ± SD of duplicate cultures and 4 mice in each group of untreated mice (*P < .01 and **P < .001 vs untreated WT) or 2 mice in the CpG-ODN–treated groups. IL-4 was not detectable in CpG-ODN–treated mice regardless of their genotype.
CpG-ODN protects H-ras– and N-ras–deficient mice from L major infection. (A) Course of L major infection in CpG-ODN–treated WT, H-ras–, and N-ras–deficient mice. Mean induration ± SD of 2-3 mice (4-6 ears) per group are shown. Dotted line represents the evolution of infection in untreated WT mice. (B) Parasite burden per ear in infected CpG-ODN–treated mice at weeks 3 and 8 after infection. Results represent mean ± SD for 2 mice per group. (C) IFN-γ and IL-4 production, as determined by ELISA, by draining LN cells isolated from untreated (−) or CpG-ODN–treated (+) L major–infected mice at week 3 after infection and stimulated with SLA (12 μg/mL) for 48 hours. Values represent the mean ± SD of duplicate cultures and 4 mice in each group of untreated mice (*P < .01 and **P < .001 vs untreated WT) or 2 mice in the CpG-ODN–treated groups. IL-4 was not detectable in CpG-ODN–treated mice regardless of their genotype.
We next analyzed the parasite-specific cytokine response at week 3 after parasite challenge, when parasite burdens were comparable in WT and Ras mutant mice either untreated (Figure 5C) or treated with CpG-ODNs (Figure 7B). As shown in Figure 7C, draining LN cells from untreated H-ras– and N-ras–deficient mice produced IFN-γ but at levels markedly lower than WT cells, further supporting a negative effect of the H-ras and N-ras deficiency on IFN-γ expression in activated T cells. Of interest, Ras-deficient T cells showed significantly higher production of IL-4 than their WT counterparts, resembling the cytokine profile of antigen-stimulated T cells from susceptible BALB/c mice.35 As previously observed in vitro (Figure 3B), CpG-ODNs partly improved IFN-γ production in Ras-deficient mice and particularly in animals lacking N-ras, probably because of enhanced IL-12 release by stimulated N-ras–deficient DCs (Figure 6A), but without reaching the levels observed in either untreated or CpG-ODN–treated WT mice. Notably, CpG-ODN treatment completely abrogated the enhanced IL-4 response observed in untreated Ras-deficient mice (Figure 7C). Thus, as previously reported for susceptible BALB/c mice,33 the Th1-promoting activity of CpG-ODNs in vivo seems to protect Ras-deficient mice from L major infection by interfering with the early establishment of a Th2-type response that facilitates parasite growth.
Discussion
Why mammals have 3 distinct Ras genes and 4 different but highly related Ras proteins is puzzling. A possible explanation might be that each isoform subsumes specific functions not shared by the other family members. In this respect, our work provides the first direct evidence that H-ras and N-ras are critically involved in the control of T-cell differentiation in the periphery but not in the thymus. Because K-ras 4A is not detectable in the mouse thymus,36 our findings point to K-ras 4B as the key isoform implicated in thymocyte development.11 In line with this, activating mutations of K-ras correlate with the appearance of thymic tumors.37 Further, mice doubly deficient for H-ras and N-ras show grossly normal intrathymic development.15
In a previous study with mice deficient for N-ras,23 a minor but consistent decrease in CD8+ SP thymocytes was interpreted as impaired positive selection because of N-ras deficiency. However, TCR transgenic models were not analyzed in that study. With the use of MHC class I– and class II–restricted transgenic TCRs expressed in the Ras-deficient backgrounds, we show here that N-ras or H-ras deficiency has no major effect on positive selection of either CD4+ or CD8+ T cells. Furthermore, peripheral TCR transgenic N-ras–deficient CD8+ T cells were capable of mounting effective antigen-specific primary responses (S.I., E.S., E.F.-M., unpublished data). Yet a role for H-ras or N-ras or both in fine-tuning thymocyte selection, particularly that mediated by low-affinity ligands,38 cannot be completely discarded.
H-ras and N-ras seem to also be dispensable for some T-cell activation events triggered by the TCR, including induction of the activation markers CD25 and CD69, IL-2 production, and proliferation. In contrast, deficiency of either H-ras or N-ras caused a defect in TCR-induced early IFN-γ and T-bet expression, resulting in affected Th1 priming of CD4+ T cells. Ultimately, Ras-deficient mice proved highly susceptible to L major infection, even though the capacity of DCs and macrophages to produce IL-12 and NO, respectively, which are also critical for a protective Th1 response to the parasite,30,31 were not overtly affected by the Ras deficiency.
Early during Th1 differentiation,25 TCR-mediated stimulation of naive CD4+ T cells results in low-level expression of IFN-γ and IL-4, and of both Th1-specific T-bet and Th2-specific GATA-3 transcription factors. Later, subsequent development along the Th1 lineage is thought to require signals via IL-12R and IFN-γR, which further up-regulates T-bet expression and drives high-level production of IFN-γ by Th1 effectors, while suppressing Th2 polarization.39 Although much progress has been made in understanding these late events of Th1 lineage commitment,2 the signaling pathways triggered early by the TCR and required for Th1 priming have remained elusive.40 Now, we show that CD4+ T cells lacking H-ras or N-ras are both intrinsically defective in inducing IFN-γ and T-bet early after TCR engagement and exhibit impaired differentiation into Th1 effectors during early L major infection, strongly supporting that the TCR signals necessarily through H-ras and N-ras to mediate early Th1 priming and polarization, which is relevant for establishment of protective Th1 responses in vivo.
In a transgenic mouse expressing a dominant-negative form of Ras in T cells, thymocyte positive selection11 and Th2 polarization12 were impaired strongly. However, because of its mechanism of action, that it, sequestration of activating GEFs,13 this dominant-negative approach cannot discriminate between contributions from different Ras isoforms.14 Of note, dominant-negative Ras markedly suppressed TCR-mediated ERK activation, but not other signaling events, in immature and mature T cells,11,12 with this probably underlying the biologic consequences of its expression. In line with this, pharmacologic ERK inhibitors mimicked the detrimental effects of dominant-negative Ras on both positive selection and Th2 differentiation.12,41 In contrast, T-lineage cells lacking H-ras or N-ras did not show, in our hands, defective TCR-mediated ERK activation, which correlated well with their unaffected positive selection and capability to mount antiparasite Th2 responses in the context of impaired Th1 immunity.
Although the mechanisms underlying the requirement for H-ras and N-ras during Th1 differentiation remains to be further determined, our study clearly places these Ras isoforms upstream from T-bet and IFN-γ, seemingly at a stage before the action of IL-1226,42 and unveils a critical function for them in the establishment of protective Th1 immunity to L major. Because Ras-deficient mice ultimately develop strong Th2 responses to L major, it could be argued that H-ras and N-ras are also required for suppression of Th2 responsiveness, as observed for instance in JNK1-deficient mice.43 This is unlikely to be the case, because in vitro TCR-activated H-ras– and N-ras–deficient T cells, in contrast to their JNK1-lacking counterparts, did not show an early bias toward a Th2 cytokine response, even when Th2-polarizing conditions are provided. Moreover, Th2 cells were gradually augmented in Ras mutants after parasite challenge, with overt Th2 responses being detected only at late phases of infection and probably as result of the failure of Ras-deficient mice to mount an effective Th1 response opposing the expansion of Th2 cells. Further supporting this notion, administration of CpG-ODNs at the time and site of parasite challenge completely protected Ras-deficient mice against an otherwise progressive infection. This protective effect is probably related to the well-established Th1-promoting properties of CpG-ODNs, including improvement of antigen presentation and production of Th1-biasing cytokines (ie, IL-12) by APCs.27 Consistent with this, Ras-deficient DCs were capable of inducing MHC, costimulatory molecules, and IL-12 expression in response to CpG-ODNs in vitro, suggesting that they, and probably other immune cells, could be also stimulated in vivo by CpG-ODNs to rescue the Th1-priming defect and to allow Th1 responses to ensue in Ras-deficient mice.33 Thus, lack of H-ras– or N-ras–mediated signaling during thymocyte development does not seemingly imprint an absolute and irreversible inability for T cells to become Th1 or Th2 effectors in the periphery. Instead, H-ras and N-ras appear to be critical for optimal TCR-mediated induction of IFN-γ in mature CD4+ T cells, but whether this trait is imprinted in the thymus remains unclear. Further progress on these issues awaits the generation and analysis of mice with disrupted Ras function specifically in mature T cells. Curiously enough, in vitro TCR-stimulated CD4+ T cells from the T cell–specific Ras–dominant-negative transgenic mice, in contrast to H-ras– and N-ras–deficient T cells shown here, displayed increased production of IFN-γ and normal Th1 polarization.12 The latter raises the question as to whether the dominant-negative Ras protein in T cells actually targets H-ras, N-ras, both, or neither isoform or other member(s) of the Ras superfamily.13
H-ras and N-ras are highly related in structure, share mechanism of action,7 and can be both activated in response to TCR engagement. Thus, it is intriguing that they cannot compensate each other for Th1 polarization leading to protective Th1 immunity. One possible explanation could be that active forms of H-ras and N-ras trigger distinct downstream pathways critical for Th1 development. Alternatively, they could engage the same Th1-promoting pathway(s) but acting in a cooperative manner.44 Further complexity in the interplay between Ras isoforms will also probably emerge from their distinct sensitivity to the TCR signal strength45 or unique function or both in particular intracellular compartments.8 Further studies are thus required to elucidate these issues.
The selective coupling of individual Ras isoforms to the TCR in mature but not immature T cells, and particularly for peripheral Th differentiation, is reminiscent of that reported for some members of other isoenzyme families, such as PKC-θ21 and the GTPase Rac2,46 which suggests a conserved mechanism allowing differential signaling from the TCR, depending of the differentiation state of the cell. Rac2-deficient T cells developed normally in the thymus but showed decreased IFN-γ production under Th1 conditions in vitro.47 Interestingly enough, Rac2-deficient mice did not display increased susceptibility to L major,48 suggesting that the role of Rac2 in Th1 responses to infection in vivo, in contrast to that of H-ras and N-ras described here, is largely redundant.
In conclusion, the identification of specific and overlapping functions of Ras isoforms,49 as shown here for H-ras and N-ras in T cells, may have important implications not only for understanding the molecular mechanisms that regulate T-cell differentiation and responses but also for development of more specific Ras modulators50 that could be promising therapeutics, individually or in combination with other immunomodulators, for the regulation of Th cells in infection, inflammation, and autoimmunity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank J. R. Regueiro, E. Martínez-Naves, and O. Williams for critically reading the manuscript.
This work was supported by Instituto de Salud Carlos III (grant FIS PI080102; E.F.-M.), Comunidad de Madrid (CAM; grant GR/SAL/0168/2004; E.F.-M.), and Universidad Complutense-CAM (grant CCG08-UCM/SAL-4215; E.F.-M.) and by Instituto de Salud Carlos III (grants FIS PI021570 and RTICC-RD06/0020/000; E.S.) and Junta de Castilla y León (grants SA044A08 and GR93; E.S.). S.I. was supported by the “Sara Borrell” Program of FIS-ISCIII.
Authorship
Contribution: S.I. performed research, analyzed and interpreted data, and drafted the manuscript; M.S., L.S.-A., and E.C. carried out some experiments; B.A., C.A., and E.S. provided vital reagents and mice; and E.F.-M. designed research, carried out some experiments, analyzed data, and drafted and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.I. is Unidad de Inmunología Viral, Centro Nacional de Microbiología, Instituto de Salud Carlos III, Madrid, Spain.
Correspondence: Edgar Fernández-Malavé, Inmunología, Facultad de Medicina, Universidad Complutense, 28040 Madrid, Spain; e-mail: edfernan@med.ucm.es.


![Figure 2. Unimpaired TCR-mediated early T-cell activation in mice lacking H-ras or N-ras. (A) Splenocytes from WT, H-ras–, and N-ras–deficient mice were stimulated without or with anti-CD3 plus anti-CD28 mAb for 48 hours and stained for CD25 and CD69 expression. Numbers indicate percentage of cells in the gated region. (B) IL-2 production by splenic T cells stimulated with anti-CD3 (solid line), concanavalin A (dotted line), or without stimulation (shaded curve), as measured after 48 hours by fluorescent bead assay. Numbers over each curve indicate IL-2 concentration (ng/mL). (C) Proliferation responses of unstimulated and anti–CD3/CD28-stimulated LN T cells. Mean [3H]-thymidine incorporation ± SD of triplicate cultures is shown (*P < .05 vs WT). Results in each panel are representative of ≥ 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/19/10.1182_blood-2010-10-315770/4/m_zh89991171350002.jpeg?Expires=1766001063&Signature=Jt86Mcg-aPV88XCdilkb39R39CUq~hwRMnnXhlKMlRb8qwSWbPs~3gZ58cG16t1rrmANLjbcOtEQ7UpEaavrVgqwQY8f7hcIf2deOZk1V6lOt72Lp5zNKioByQlmTA96v0cZrNWXqiMoMUjyr1V4xb7g40uC5mIJ8Jw-Oi3oxf5h5wdRv~a5eA~jFJOJCi65zEJIxC9hbwx-dRZEajLnZXLQiGLlycwkF13FLAnvs2yadhEXVCTZcMm3xdOBeWekLDZY9jy33VpqxTFttH~lFEvslAhbYDZnglYrIVVN3ONm81rrdDZIdcMHmz1sWmwIHrUSHinAWyCjrtJH56FLUA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal