Abstract
Mesenchymal stem cells (MSCs) are characterized by their manifold immunomodulatory and regenerative properties. The stress-responsive, cytoprotective, and immunoregulatory molecule heme oxygenase-1 (HO-1) was recently identified as a key contributor for MSC-mediated suppression of alloactivated T cells. As HO-1 has also been implicated in the induction of regulatory T cells (Tregs), we sought to examine its impact on MSC-driven promotion of Tregs. Human MSCs were shown to induce, in a HO-1–dependent fashion, IL-10+ Tr1 and transforming growth factor-β+ Th3 Treg-subsets in allo- and T-cell receptor-activated lymphocytes. Because inflammatory stimuli modulate (“license”) human MSCs, we were interested in whether an in vitro alloreactive micro-milieu within mixed lymphocyte reactions (MLRs) alters the HO-1 expression. We observed a substantial down-regulation of HO-1 facilitated by yet unidentified soluble factor(s) produced in an MLR, and most probably occurring at the level of its major transcription-factor NF-E2–related factor 2. Interestingly, HO-1 lost its impact regarding suppressiveness, Treg induction, and promotion of IL-10 production for MSCs, which were prelicensed in an MLR environment. Taken together, we show that HO-1 produced by human MSCs beyond its direct suppressive function promotes formation of Tr1 and Th3 Tregs and IL-10 production, functions, which are taken over by other molecules, among them COX-2, after an alloreactive priming.
Introduction
Mesenchymal stem cells (MSCs) are a heterogeneous population of fibroblast-like, multipotent cells characterized by their ability to differentiate in vitro and in vivo into tissues of the mesodermal lineage. Nowadays, it is well established that human MSCs possess a plethora of immunomodulatory properties.1 Their regulatory impact on allogeneic immune reactions, such as graft-versus-host disease (GVHD), occurring after bone marrow transplantation makes MSCs an attractive tool of low toxicity for cell-based therapies.2 As yet, several key mechanisms have been described contributing to their direct or indirect alteration of T-, NK-, B-, and dendritic-cell functions. These multifactorial processes require various contact as well as contact-independent signals, among them prostaglandin E2 (PGE2), IL-6, IL-10, indoleamine 2,3 dioxygenase (IDO), and transforming growth factor-β (TGF-β).1,3-5 To some degree, it has been implicated that full suppressive activity depends on a so-called proinflammatory “licensing” of MSCs composed of interferon-γ (IFN-γ) in concert with IL-1α, IL-1β, or tumor necrosis factor-α.6,7
Recently, a stress-responsive pathway was found to be strongly involved in MSC-mediated T-cell suppression.8 The stress-inducible enzyme heme-oxygenase-1 (HO-1), which catalyzes the rate-limiting step of the heme degradation to biliverdin, has a suppressive effect on T-cell proliferation in human and rat MSCs, which is similar to previous findings in regulatory T cells (Tregs).8,9 Furthermore, HO-1 is a potent cytoprotective enzyme that exerts strong anti-inflammatory, antioxidative, and antiapoptotic activities through its products, especially carbon monoxide and biliverdin.10,11 This versatility, coupling direct tissue protection with immunosuppression, is of substantial relevance for transplantation immunology, where several aggressive pathologic processes have to be faced simultaneously.12,13 There are various preclinical transplantation models evaluating HO-1, altogether suggesting beneficial effects with regards to transplantation tolerance and tissue regeneration.14,15 Treg induction is one of the proposed pathways how HO-1 exerts its tolerogenic effects.11,13,15 Tregs are pivotal for the maintenance of self-tolerance and of extraordinary interest for transplantation research because of their capability of controlling autoreactive immune cells.16 On successful engraftment, frequency of circulating Tregs has been shown to be of diagnostic as well as of prognostic value in GVHD.17 Similar to the adoptive transfer of MSCs, administration of Tregs in GVHD patients is currently evaluated within clinical trials.18 Naturally occurring Tregs are of thymic origin, but it has become apparent that, under various conditions, induced or adaptive forms can be generated extrathymically.19 To date, several phenotypically and functionally distinct induced Treg subsets have been described with the most delineated cell populations, including IL-10+ T regulatory (Tr) 1, TGF-β+ T helper (Th) 3, and CD25+FOXP3+ nTreg-like CD4+ cells. Several recent in vivo and in vitro studies indicate that Treg activation, expansion, and/or induction from conventional CD4+ T cells are important components of the MSC-mediated immunomodulation.5,20-23
In the present study, we sought to determine the importance of HO-1 in the induction of major Treg subsets by human MSCs. Furthermore, as MSCs, despite their known hypo-immunogenicity, respond to signals of local and systemic inflammation, we evaluated the effects of the milieu generated by alloactivated lymphocytes on HO-1 expression.2,7,24 These “prelicensed” MSCs were subsequently assessed with regards to their ability to induce Tregs and to suppress T cells.
Methods
Reagents
DMEM-LG, RPMI 1640 cell-medium, fetal bovine serum, and trypan blue (0.4%) were purchased from Invitrogen; dimethyl sulfoxide, hydrogen peroxide (H2O2), penicillin-streptomycin, and trypsin-ethylenediaminetetraacetic acid solution were obtained from Sigma-Aldrich. IFN-γ was obtained from Boehringer Ingelheim.
Cell isolation
The Ethics Committee of the Karolinska University Hospital approved this study. MSCs were isolated from bone marrow aspirates taken from the iliac crest of 13 healthy donors and expanded as previously described in detail while fulfilling uniformly the minimal MSC criteria.25,26 All MSCs used in this study were harvested in passage 3. Peripheral blood mononuclear cells were isolated from healthy donors by density gradient-based Ficoll-Paque (GE Healthcare), and CD4+ T cells from healthy donors were subsequently isolated immunomagnetically (cell purity ≥ 90%) using a CD4+ T-cell isolation kit II according to the manufacturer's instructions (Miltenyi Biotec).
Antibodies and flow cytometry
Cells were stained according to the manufacturer's recommendations using fluorochrome-coupled monoclonal antibodies. CD3 Pacific Blue (HIT3a), CD4 peridinin chlorophyll protein (RPA-T4), FOXP3 Alexa Fluor-488 (150D), TGF-β AlexaFluor-647 (BG/hLAP) were purchased from BioLegend, CD25 phycoerythrin (PE)/allophycocyanin (M-A251), CD45 fluorescein isothiocyanate (FITC; HI30), CD73 PE (AD2), CD90 PE (5E10), CD105 PE (266), and IL-10 PE (JES3-19F1) from BD Biosciences, and HO-1z from Abcam. Intracellular oxidation levels were quantified upon labeling with 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein-diacetate-acetylester (Invitrogen), extracellular and intracellular thiol content upon staining with Alexa Fluor-633-coupled maleimide (ALM-633; Invitrogen), and mercury orange (Sigma-Aldrich) respectively, as described in detail elsewhere.27
Cells were analyzed using an LSRII flow cytometer (BD Biosciences) and FlowJo Version 9.0.2 software (TreeStar).
Cell death
Cell death was determined by 7-amino-actinomycin D (7-AAD; BD Biosciences) and annexin V–FITC (eBioscience) costaining. Viable cells were defined as 7-AAD−/annexin V− in the flow cytometric analysis.
Coculture experiments
For alloactivated, mixed lymphocyte reactions (MLRs), pooled irradiated allogeneic peripheral blood lymphocytes (PBLs) from 5 healthy donors were used as stimulators of nonirradiated PBLs. Proliferation was measured by assessment of thymidine uptake after 5 days. MSCs were cocultured in a cell-to-cell ratio of 1:10 to the responder PBLs. Alternatively, T cells from a single donor were activated in a T-cell receptor (TCR)/B7 manner using stimulating anti-CD2/CD3/CD28 bead-coupled antibodies (Miltenyi Biotec) in a one bead per cell ratio. In particular experiments, HO-1, COX-2, inducible nitric oxide synthase, IDO, or secreted TGF-β in/by MSCs were specifically inhibited/neutralized by (pre)treatment with 50μM tin protoporphyrin (SnPP; Frontier Scientific), 10μM NS398 (Cayman Chemicals), 1mM NG-monomethyl-L-arginine (Sigma-Aldrich), 1mM 1-methyl-tryptophan (Sigma-Aldrich), and 10 μg/mL neutralizing anti–TGF-β antibodies (αTGF-β, R&D Biosystems), respectively.
Cytokine assays
IL-10 and PGE2 were measured in cell culture supernatants by standard sandwich ELISA kits (R&D Systems) according to the manufacturer's instructions.
RNA preparation and quantitative RT-PCR
Total RNA was extracted (RNeasy mini kit; QIAGEN) and cDNA prepared (iScript cDNA synthesis kit; Bio-Rad). Messenger RNA levels were quantified by real-time quantitative PCR (SYBR Green Supermix; ABI 7500 Detection System; Applied Biosystems). Relative gene expression was determined by normalizing the expression of each target to β-actin. The following gene-specific primers were used (forward and reverse sequence): catalase, 5′-CATTCGATCTCACCAAGGTTTGGCC-3′ and 5′-AGCAGGTAGGGACAGTTCACAGG-3′; foxp3, 5′-GAAACAGCACATTCCCAGAGTTC-3′ and 5′-ATGGCCCAGCGGATGAG-3′; gclc, 5′-ATGGAGGTGCAATTAACAGAC-3′ and 5′-ACTGCATTGCCACCTTTGCA-3′; ho1, 5′-CTTCTTCACCTTCCCCAACA-3′ and 5′-AGCTCCTGCAACTCCTCAAA-3′; ido, 5′-GCATTTTTCAGTGTTCTTCGCATA-3′ and 5′-TCATACACCAGACCGTCTGATAGC-3′; il10, 5′-ACGGCGCTGTCATCGATT-3′ and 5′-GGCATTCTTCACCTGCTCCA-3′; mnsod, 5′-CTTCAGCCTGCACTGAAGTTCAAT-3′ and 5′-CTGAAGGTAGTAAGCGTGCTCCC-3′; nrf2, 5′-AAACCAGTGGATCTGCCAAC-3′ and 5′-GACCGGGAATATCAGGAACA-3′; and TGF-β, 5′-GGACATCAACGGGTTCACTACC-3′ and 5′-AGCAGGAAAGGCCGGTTC-3′.
Statistical analysis
Differences in means and correlation analyses were evaluated with parametric (2-tailed student or paired t test and Pearson test) or nonparametric (Mann-Whitney U or Wilcoxon and Spearman ρ test) tests. All analyses were performed using SPSS Version 16.0 (SPSS).
Results
Human MSCs express the immunoregulatory molecule HO-1
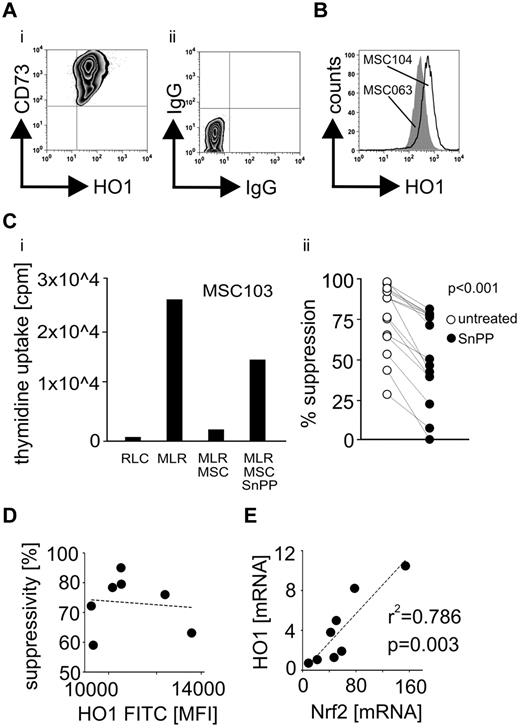
In line with previous findings,8 HO-1 was expressed in MSCs derived from the bone marrow of all assessed 9 healthy donors (Figure 1A-B). Levels of HO-1 in MSCs depicted a strong interindividual variability (Figure 1B,D), and its pharmacologic inhibition by SnPP resulted in a substantial decrease, but not in a total abrogation of the capacity to suppress alloreactive T cells (Figure 1C). Despite the evident role of HO-1 in MSC-mediated T-cell regulation, cellular HO-1 levels were not associated with the absolute suppressive potency (Figure 1D), which is in accordance with the multitude of studies implying a network of several contributing factors. The gene expression levels of HO-1 and NF-E2–related factor 2 (Nrf2), its major regulating transcription factor,28 correlated significantly with each other in the evaluated cells (Figure 1E).
Human MSCs express the immunoregulatory molecule HO-1. HO-1 expression was assessed in 9 human bone marrow–derived MSCs by flow cytometry as shown in the representative (A) dot plot and (B) histogram depicting quantitatively an interindividual variability. (C) Prior MLR HO-1 was blocked in 6 different MSCs (in a minimum of 2 independent 5-day MLRs for every investigated MSC) using 50μM of the protoporphyrin SnPP. The ability of MSCs to suppress T-cell alloreactions was assessed as (i) representatively shown for MSC103 by measuring the thymidine incorporation in responder PBLs. RLC indicates responder PBLs alone. (ii) Suppression by individual untreated MSCs was compared with their SnPP-pretreated counterparts. (D) Expression levels of HO-1 in the MSCs defined as the mean fluorescence intensity (MFI) were correlated with their ability to suppress alloactivated T cells. (E) The relative basal gene expression levels (mRNA) of Nrf2 and HO-1 were correlated in the MSCs from different donors (n = 8). All experimental settings were conducted with MSCs derived from at least 3 individual donors, and n refers to the number of repeated experiments. Bar represents SD.
Human MSCs express the immunoregulatory molecule HO-1. HO-1 expression was assessed in 9 human bone marrow–derived MSCs by flow cytometry as shown in the representative (A) dot plot and (B) histogram depicting quantitatively an interindividual variability. (C) Prior MLR HO-1 was blocked in 6 different MSCs (in a minimum of 2 independent 5-day MLRs for every investigated MSC) using 50μM of the protoporphyrin SnPP. The ability of MSCs to suppress T-cell alloreactions was assessed as (i) representatively shown for MSC103 by measuring the thymidine incorporation in responder PBLs. RLC indicates responder PBLs alone. (ii) Suppression by individual untreated MSCs was compared with their SnPP-pretreated counterparts. (D) Expression levels of HO-1 in the MSCs defined as the mean fluorescence intensity (MFI) were correlated with their ability to suppress alloactivated T cells. (E) The relative basal gene expression levels (mRNA) of Nrf2 and HO-1 were correlated in the MSCs from different donors (n = 8). All experimental settings were conducted with MSCs derived from at least 3 individual donors, and n refers to the number of repeated experiments. Bar represents SD.
HO-1 is involved in the MSC-mediated induction of Tr1 and Th3 cells
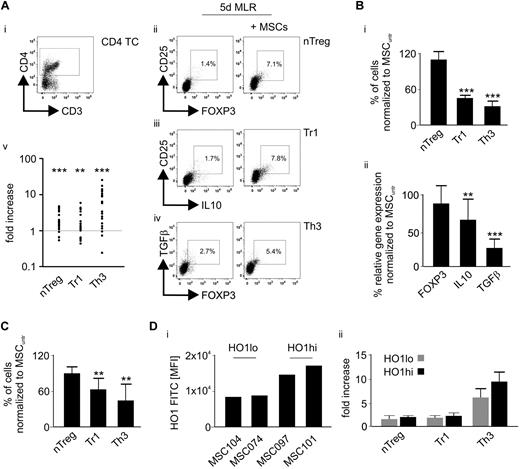
When MSCs were present in a 5-day MLR, the numbers of nTreg-like, Tr1-, and Th3-cell subsets significantly increased (Figure 2A). Inhibition of HO-1 in MSCs markedly reduced their ability to induce Tr1 and Th3 cells in alloactivated T cells, whereas the nTreg-like frequency was slightly (but not significantly) elevated (Figure 2Bi). Accordingly, gene expression analyses in the retrieved lymphocytes revealed a concomitant down-regulation of IL-10 and TGF-β, which are characteristic molecules for Tr1 and Th3 cells, whereas FOXP3 levels were stable (Figure 2Bii). Similarly, HO-1 was involved in the MSC-mediated Treg induction of TCR/B7 (by CD2, CD3, and CD28 bead-coupled antibodies) activated single donor CD4+ T cells (Figure 2C). Next, we comparatively assessed Treg promotion by MSCs expressing high (MSC097 and MSC101) versus low (MSC104 and MSC074) HO-1 levels (Figure 2Di) without observing any significant differences (Figure 2Dii).
HO-1 is involved in the MSC-mediated induction of Tr1 and Th3 cells. (A) After an MLR for 5 days in the presence or absence of MSCs, the (v) fold increase of (i) CD3+CD4+ T cells expressing (ii) CD25+FOXP3+ (nTreg-like), (iii) CD25+IL-10+ (Tr1), and (iv) TGF-β+FOXP3+ (Th3) was assessed by flow cytometry (n = 20). (B) HO-1 in MSCs was blocked before primary MLR using 50μΜ SnPP. Subsequently, (i) frequencies of nTreg-like, Tr1, and Th3 cell subsets were determined by flow cytometry (n = 6) and (ii) gene expression of FOXP3, IL-10, and TGF-β in the retrieved lymphocytes by quantitative real-time PCR (n = 8). The Treg frequency and the gene expression levels detected in the presence of untreated MSCs (MSCuntr) represent the 100% baseline. (C) MSCs treated with 50μΜ SnPP were subjected to a 5-day coculture with purified CD4+ T cells from single healthy donors (n = 6), activated by CD2, CD3, and CD28 bead-coupled antibodies; and subsequently, frequencies of nTreg-like, Tr1, and Th3 cell subsets were determined by flow cytometry. The Treg frequency detected in the presence of untreated MSCs (MSCuntr) represents the 100% baseline. (Di) MSCs expressing high (MSC097 and MSC101) and low (MSC104 and MSC074) HO-1 levels as assessed by flow cytometry were (ii) subjected to MLR for 5 days and Treg promotion was evaluated subsequently. Results are representative for 2 independent MLRs performed with each MSC. All experimental settings were conducted with MSCs derived from at least 3 individual donors, and n refers to the number of repeated experiments. Bars represent SD. **P ≤ .01. ***P ≤ .001.
HO-1 is involved in the MSC-mediated induction of Tr1 and Th3 cells. (A) After an MLR for 5 days in the presence or absence of MSCs, the (v) fold increase of (i) CD3+CD4+ T cells expressing (ii) CD25+FOXP3+ (nTreg-like), (iii) CD25+IL-10+ (Tr1), and (iv) TGF-β+FOXP3+ (Th3) was assessed by flow cytometry (n = 20). (B) HO-1 in MSCs was blocked before primary MLR using 50μΜ SnPP. Subsequently, (i) frequencies of nTreg-like, Tr1, and Th3 cell subsets were determined by flow cytometry (n = 6) and (ii) gene expression of FOXP3, IL-10, and TGF-β in the retrieved lymphocytes by quantitative real-time PCR (n = 8). The Treg frequency and the gene expression levels detected in the presence of untreated MSCs (MSCuntr) represent the 100% baseline. (C) MSCs treated with 50μΜ SnPP were subjected to a 5-day coculture with purified CD4+ T cells from single healthy donors (n = 6), activated by CD2, CD3, and CD28 bead-coupled antibodies; and subsequently, frequencies of nTreg-like, Tr1, and Th3 cell subsets were determined by flow cytometry. The Treg frequency detected in the presence of untreated MSCs (MSCuntr) represents the 100% baseline. (Di) MSCs expressing high (MSC097 and MSC101) and low (MSC104 and MSC074) HO-1 levels as assessed by flow cytometry were (ii) subjected to MLR for 5 days and Treg promotion was evaluated subsequently. Results are representative for 2 independent MLRs performed with each MSC. All experimental settings were conducted with MSCs derived from at least 3 individual donors, and n refers to the number of repeated experiments. Bars represent SD. **P ≤ .01. ***P ≤ .001.
MSCs preconditioned by an MLR-generated milieu exhibit decreased HO-1 levels
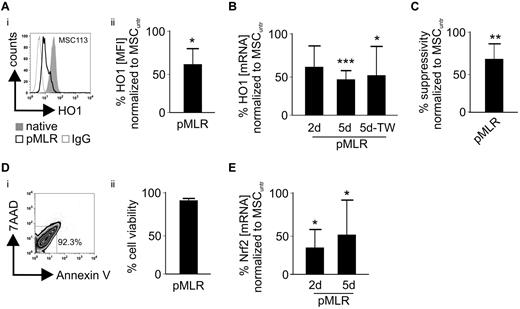
MSCs cocultured in an MLR for 2 or 5 days showed a significant decrease in HO-1 on the mRNA as well as on the protein level (Figure 3A-B). Cell-to-cell contact was not necessary for HO-1 down-regulation as it was similarly observed in cocultures of MSCs and lymphocytes separated by trans-wells (Figure 3B).
MSCs preconditioned by an MLR-milieu exhibit decreased HO-1 levels. (A) After 5 days of a priming MLR (pMLR) and removal of the PBLs (MSC purity ≥ 90%), (i-ii) HO-1 levels (MFI) were determined in the MSCs as shown in the representative histogram for MSC113 cells and compared with nonprimed (native) MSCs (n = 5). (B) Relative gene expression (mRNA) levels of HO-1 in the MSCs were determined after 2 and 5 days of MLR, respectively (n = 5). Equivalent experiments were carried out in regular or trans-wells (TW). (C) After 5 days of initial pMLR, the suppressive activity of the primed MSCs was evaluated compared with nonprimed (native) cells in a subsequent MLR (n = 5). (D) After 5 days of MLR, MSCs were evaluated with regard to apoptosis and necrosis by flow cytometry. As shown (i) in the representative dot plot analysis (ii) for 6 independent experiments, viable cells were defined as 7-AAD−/annexin V−. (E) Relative gene expression levels (mRNA) of Nrf2 in MSCs were determined after 2 and 5 days of MLR, respectively (n = 5). All experimental settings were conducted with MSCs derived from at least 3 individual donors, and n refers to the number of repeated experiments. Bars represent SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
MSCs preconditioned by an MLR-milieu exhibit decreased HO-1 levels. (A) After 5 days of a priming MLR (pMLR) and removal of the PBLs (MSC purity ≥ 90%), (i-ii) HO-1 levels (MFI) were determined in the MSCs as shown in the representative histogram for MSC113 cells and compared with nonprimed (native) MSCs (n = 5). (B) Relative gene expression (mRNA) levels of HO-1 in the MSCs were determined after 2 and 5 days of MLR, respectively (n = 5). Equivalent experiments were carried out in regular or trans-wells (TW). (C) After 5 days of initial pMLR, the suppressive activity of the primed MSCs was evaluated compared with nonprimed (native) cells in a subsequent MLR (n = 5). (D) After 5 days of MLR, MSCs were evaluated with regard to apoptosis and necrosis by flow cytometry. As shown (i) in the representative dot plot analysis (ii) for 6 independent experiments, viable cells were defined as 7-AAD−/annexin V−. (E) Relative gene expression levels (mRNA) of Nrf2 in MSCs were determined after 2 and 5 days of MLR, respectively (n = 5). All experimental settings were conducted with MSCs derived from at least 3 individual donors, and n refers to the number of repeated experiments. Bars represent SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
MSCs were cocultured (primed) in an MLR for 5 days and the nonadherent lymphocytes subsequently removed by washing. Next, when the primed MSCs were subjected to a second 5-day MLR, their suppressive capacity was significantly reduced compared with that of their unprimed, native counterparts (Figure 3C). These phenomena were not the result of an increased cell death of the MSCs occurring during the conditioning MLR (Figure 3D), and messenger Nrf2 transcript levels were altered similarly to HO-1 (Figure 3E).
IFN-γ administration rescues HO-1 expression and partially the suppressive potency of MSCs
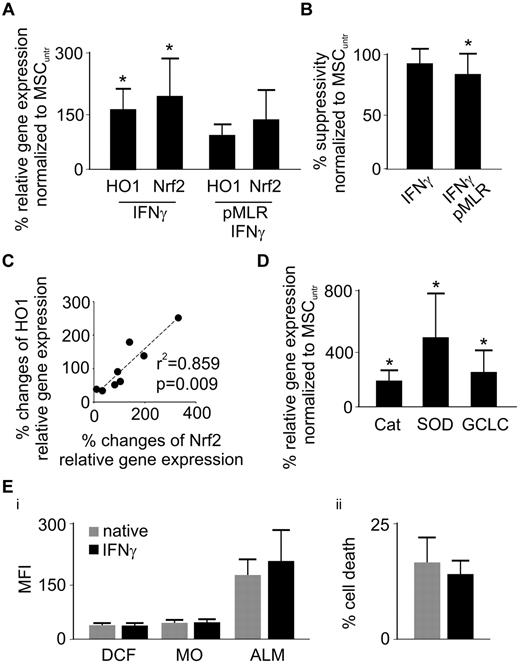
Unconditioned, native MSCs treated with IFN-γ significantly increased their expression of both HO-1 and Nrf2 (Figure 4A). Despite this substantial gain of HO-1 (Figure 4A), suppressive activity remained constant (Figure 4B), in line with the results shown in Figure 1D. Coadministration of IFN-γ during MLR conditioning efficiently prevented the HO-1 and Nrf2 diminution in MSCs (Figure 4A) while partially rescuing their suppressive capacity (Figures 3D, 4B). Again, up- and down-regulation of Nrf2 and HO-1 was closely associated in all tested conditions (Figure 4C).
IFN-γ administration rescues HO-1 expression and partially the suppressive potency of MSCs. (A) Relative gene expression (mRNA) of HO-1 and Nrf2 was measured in IFN-γ-treated MSCs with or without priming MLR (n = 7). (B) After an initial pMLR for 5 days with or without IFN-γ (100 U/mL), MSCs were subjected to a secondary MLR for 5 days. Suppressive activity was measured by thymidine incorporation (in responder PBLs) and compared with nonprimed (native) cells (MSCuntr) or MSCs solely treated with 100 U/mL IFN-γ (n = 6). (C) Percentage up- or down-regulation of HO-1 and Nrf2 relative gene expression was assessed in MSCs upon priming with MLRs or IFN-γ and correlation (HO-1/Nrf2) analysis performed (n = 8). (D) Relative gene expression (mRNA) of catalase (Cat), superoxide-dismutase (SOD), and catalytic subunit of glutamylcysteine ligase (GCLC) was assessed in MSCs 5 days after IFN-γ treatment (n = 7). (Ei) MSCs were stained with 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein-diacetate-acetylester, mercury orange (MO), and ALM 5 days after IFN-γ treatment to determine intracellular oxidative stress (DCF), intracellular (PE-channel/MO) and extracellular (allophycocyanin-channel/ALM) thiols, respectively, as assessed by flow cytometry (n = 4). (ii) After 5 days of IFN-γ treatment, MSCs were subjected to treatment with 2mM H2O2 for 8 hours and subsequently analyzed regarding cell death by 7-AAD and annexin V FITC staining (n = 6). All experimental settings were conducted with MSCs derived from at least 3 individual donors, and n refers to the number of repeated experiments. Bars represent SD. *P ≤ .05.
IFN-γ administration rescues HO-1 expression and partially the suppressive potency of MSCs. (A) Relative gene expression (mRNA) of HO-1 and Nrf2 was measured in IFN-γ-treated MSCs with or without priming MLR (n = 7). (B) After an initial pMLR for 5 days with or without IFN-γ (100 U/mL), MSCs were subjected to a secondary MLR for 5 days. Suppressive activity was measured by thymidine incorporation (in responder PBLs) and compared with nonprimed (native) cells (MSCuntr) or MSCs solely treated with 100 U/mL IFN-γ (n = 6). (C) Percentage up- or down-regulation of HO-1 and Nrf2 relative gene expression was assessed in MSCs upon priming with MLRs or IFN-γ and correlation (HO-1/Nrf2) analysis performed (n = 8). (D) Relative gene expression (mRNA) of catalase (Cat), superoxide-dismutase (SOD), and catalytic subunit of glutamylcysteine ligase (GCLC) was assessed in MSCs 5 days after IFN-γ treatment (n = 7). (Ei) MSCs were stained with 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein-diacetate-acetylester, mercury orange (MO), and ALM 5 days after IFN-γ treatment to determine intracellular oxidative stress (DCF), intracellular (PE-channel/MO) and extracellular (allophycocyanin-channel/ALM) thiols, respectively, as assessed by flow cytometry (n = 4). (ii) After 5 days of IFN-γ treatment, MSCs were subjected to treatment with 2mM H2O2 for 8 hours and subsequently analyzed regarding cell death by 7-AAD and annexin V FITC staining (n = 6). All experimental settings were conducted with MSCs derived from at least 3 individual donors, and n refers to the number of repeated experiments. Bars represent SD. *P ≤ .05.
Because Nrf2 is a key transcription factor in orchestrating the cellular antioxidative machinery,28 we evaluated the effect of IFN-γ on the expression of the enzymatic antioxidants catalase and superoxide-dismutase as well as well the catalytic subunit of glutamylcysteine ligase, the rate-limiting molecule in the glutathione synthesis. The expression of all investigated molecules was highly up-regulated on IFN-γ treatment (Figure 4D) but without any apparent effect on the levels of cellular oxidation, of intracellular and surface thiols, as well as protection from oxidative stress-induced cell death (Figure 4E).
MSCs preconditioned in an MLR depict an increased Treg-promoting capability despite HO-1 down-modulation
MSCs cultured under normal conditions, treated with IFN-γ or preconditioned in an MLR with or without IFN-γ for 5 days, were harvested and subsequently added to a new MLR (Figure 5A). After 5 additional days, we quantified the Treg induction as shown in Figure 5B. IFN-γ treatment alone increased HO-1 levels (Figure 4A) without any additional boost on the Treg formation (Figure 5B). In contrast, preconditioning of MSCs with MLRs increased their capability of Treg promotion (compared with native MSCs), in particular of the nTreg-like and Tr1 subsets (Figure 5B), despite HO-1 down-modulation (Figure 3A-C). Sustaining HO-1 during the preconditioning MLR by IFN-γ treatment (Figure 4A) did not provide any further Treg-promoting effect (Figure 5B). In line with the findings on IL-10+ Tr1 cells (Figure 5B), supernatants of MLRs, performed with preconditioned MSCs, contained significantly higher amounts of IL-10 (Figure 5C). It is doubtful that these MSCs produced the surplus IL-10 because their IL-10 expression is almost completely abolished within 48 hours of coculture in an MLR (Figure 5D). Inhibiting HO-1 in native MSCs with SnPP resulted in a significant reduction of IL-10 production in the following MLR. In contrast, IL-10 levels remained stable in MLRs performed with MSCs that had been preconditioned (Figure 5A) before SnPP treatment (Figure 5Ei). In addition, HO-1 inhibition had no effect on the suppressive potency of these preconditioned cells (Figure 5Eii), which again is in opposition to the findings in native MSCs (Figure 1C).
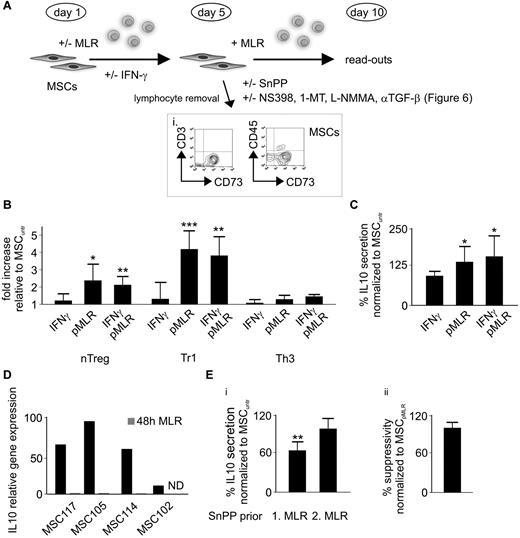
MSCs conditioned in an MLR depict an increased Treg-promoting capability despite HO-1 down-regulation. (A) Schematic model depicting the experimental setup and time frame for conditioning (priming) of native MSCs in an initial MLR (for 5 days) followed by subjection to a second MLR (for 5 days), including various treatments (INF-γ/SnPP/NS398/1-methyl-tryptophan (1-MT)/NG-monomethyl-L-arginine (L-NMMA)/αTGF-β). (i) Purity of MSCs (> 90%) was assessed after primary MLR for 5 days and subsequent removal of PBLs by flow cytometry as shown in the representative dot plots, MSCs being the CD73+/CD3−/CD45− cells. (B-C) After initial priming of MSCs in an alloreactive milieu (pMLR) with or without addition of IFN-γ or solely IFN-γ pretreatment of native MSCs for 5 days, harvested MSCs were subjected to a second MLR. After 5 days of coculturing, (B) fold increase of Treg subpopulations and (C) percentile alteration of IL-10 secretion were assessed by flow cytometry and ELISA, respectively (n = 6). (D) Relative gene expression of IL-10 in 4 different MSCs after 48 hours of culturing with or without MLR was determined by real-time PCR. (Ei) The impact of HO-1 inhibition on the IL-10 production was determined by ELISA in the supernatants of MLRs performed in the presence of with SnPP-pretreated native (SnPP prior 1. MLR) or preconditioned (SnPP prior 2. MLR) MSCs (n = 6). (ii) Effects of the HO-1 inhibition on the suppressive potency of by MLR preconditioned (pMLR) MSCs was evaluated by the thymidine incorporation (in responder PBLs) during secondary MLR (n = 6). All experimental settings were conducted with MSCs derived from at least 3 individual donors, and n refers to the number of repeated experiments. Bars represent SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
MSCs conditioned in an MLR depict an increased Treg-promoting capability despite HO-1 down-regulation. (A) Schematic model depicting the experimental setup and time frame for conditioning (priming) of native MSCs in an initial MLR (for 5 days) followed by subjection to a second MLR (for 5 days), including various treatments (INF-γ/SnPP/NS398/1-methyl-tryptophan (1-MT)/NG-monomethyl-L-arginine (L-NMMA)/αTGF-β). (i) Purity of MSCs (> 90%) was assessed after primary MLR for 5 days and subsequent removal of PBLs by flow cytometry as shown in the representative dot plots, MSCs being the CD73+/CD3−/CD45− cells. (B-C) After initial priming of MSCs in an alloreactive milieu (pMLR) with or without addition of IFN-γ or solely IFN-γ pretreatment of native MSCs for 5 days, harvested MSCs were subjected to a second MLR. After 5 days of coculturing, (B) fold increase of Treg subpopulations and (C) percentile alteration of IL-10 secretion were assessed by flow cytometry and ELISA, respectively (n = 6). (D) Relative gene expression of IL-10 in 4 different MSCs after 48 hours of culturing with or without MLR was determined by real-time PCR. (Ei) The impact of HO-1 inhibition on the IL-10 production was determined by ELISA in the supernatants of MLRs performed in the presence of with SnPP-pretreated native (SnPP prior 1. MLR) or preconditioned (SnPP prior 2. MLR) MSCs (n = 6). (ii) Effects of the HO-1 inhibition on the suppressive potency of by MLR preconditioned (pMLR) MSCs was evaluated by the thymidine incorporation (in responder PBLs) during secondary MLR (n = 6). All experimental settings were conducted with MSCs derived from at least 3 individual donors, and n refers to the number of repeated experiments. Bars represent SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
Preconditioning MSCs within an MLR leads to a dramatic increase of COX-2 expression
As it has been previously described, inflammatory licensing of the MSCs with IFN-γ led to an increase in COX-2 expression.7,29 This effect was even more dramatic for the MSCs preconditioned in an MLR (Figure 6A) and resulted in a significant increase of PGE2 production during subsequent MLRs (Figures 5A, 6B). Blocking the up-regulated COX-2 in the preconditioned MSCs before the following MLR led to a reduced IL-10 production and suppressiveness (Figure 6C-D). Induction of nTreg-like and Tr1 cells, which was highly promoted by preconditioned MSCs (Figure 5B), was weakened (by ∼ 30% and 20%, respectively; Figure 6E). Blocking COX-2 by NS398 had no impact on the inherent HO-1 expression30 in MSCs (Figure 6F). In contrast to HO-1 (Figures 1Cii, 5E), other main mediators of human MSC suppression,1 including COX-2, IDO, inducible nitric oxide synthase, and TGF-β, were still effective after inflammatory priming (Figure 6G). However, inhibiting COX-2 and IDO was significantly less effective in restoring proliferation in prelicensed compared with native MSCs (Figure 6G).
Induction of COX-2 in MSCs during conditioning in MLRs. (A) MSCs were treated with IFN-γ or cocultured with MLRs (pMLR) in regular or trans-wells. Relative gene expression of COX-2 was evaluated after 2 and 5 days (n = 9). (B) After initial priming of MSCs in an alloreactive milieu (pMLR) with or without administration of IFN-γ (100 IU/mL) or solely IFN-γ pretreatment for 5 days, MSCs were applied to a secondary MLR. After 5 days, levels of secreted PGE2 were assessed using ELISA (n = 6). (C-E) After initial alloreactive priming (pMLR), COX-2 in the MSCs was inhibited by NS398 (10μM) before performing secondary MLR. After 5 days, (C) IL-10 secretion, (D) suppressive activity, and (E) nTreg-like/Tr1 induction were evaluated by ELISA, thymidine incorporation (responder PBLs), and flow cytometry, respectively (n = 6). (F) To rule out any potential effect of NS398 on the HO-1 expression in MSCs, cells were analyzed with regard to HO-1 levels 5 days on treatment by flow cytometry (n = 6). (G) The effect of blocking COX-2 (NS398, 10μM), IDO (1-methyl-tryptophan [1-MT], 1mM), inducible nitric oxide synthase (NG-monomethyl-L-arginine [L-NMMA], 1mM), or neutralizing TGF-β (anti–αTGF-β antibodies, 10 μg/mL) on the suppressive activity of native versus prelicensed MSCs (n = 12) was assessed by thymidine incorporation in a 5-day (secondary) MLR. All experimental settings were conducted with MSCs derived from at least 3 individual donors, and n refers to the number of repeated experiments. Bars represent SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
Induction of COX-2 in MSCs during conditioning in MLRs. (A) MSCs were treated with IFN-γ or cocultured with MLRs (pMLR) in regular or trans-wells. Relative gene expression of COX-2 was evaluated after 2 and 5 days (n = 9). (B) After initial priming of MSCs in an alloreactive milieu (pMLR) with or without administration of IFN-γ (100 IU/mL) or solely IFN-γ pretreatment for 5 days, MSCs were applied to a secondary MLR. After 5 days, levels of secreted PGE2 were assessed using ELISA (n = 6). (C-E) After initial alloreactive priming (pMLR), COX-2 in the MSCs was inhibited by NS398 (10μM) before performing secondary MLR. After 5 days, (C) IL-10 secretion, (D) suppressive activity, and (E) nTreg-like/Tr1 induction were evaluated by ELISA, thymidine incorporation (responder PBLs), and flow cytometry, respectively (n = 6). (F) To rule out any potential effect of NS398 on the HO-1 expression in MSCs, cells were analyzed with regard to HO-1 levels 5 days on treatment by flow cytometry (n = 6). (G) The effect of blocking COX-2 (NS398, 10μM), IDO (1-methyl-tryptophan [1-MT], 1mM), inducible nitric oxide synthase (NG-monomethyl-L-arginine [L-NMMA], 1mM), or neutralizing TGF-β (anti–αTGF-β antibodies, 10 μg/mL) on the suppressive activity of native versus prelicensed MSCs (n = 12) was assessed by thymidine incorporation in a 5-day (secondary) MLR. All experimental settings were conducted with MSCs derived from at least 3 individual donors, and n refers to the number of repeated experiments. Bars represent SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.
Discussion
HO-1 holds an important protective role in various aspects of normal physiology. High embryonic lethality and the development of a progressive inflammatory state could be found in HO-1−/− mice.31 One recorded pediatric case of HO-1 deficiency was characterized by growth retardation, hemolytic anemia, coagulopathy, and premature atherosclerosis.32 On grounds of its prominent cytoprotective and immunoregulatory properties, HO-1 is of notable interest for the field of regenerative medicine. Several studies have highlighted the amenities of the HO-1 (over)expression in MSCs with regard to tissue recovery.33 In addition, HO-1 has emerged as a major factor inducing tolerance, featuring considerable implications in the transplantation of solid organs and hematopoietic stem cells.8,12,14,15,34,35 The tolerogenic MSCs use HO-1 to control alloactivated lymphocytes; and in line with previous observations, virtually all evaluated human MSCs8 expressed HO-1 at various levels. Despite the evident role of HO-1 in MSC-mediated suppression, cellular levels were not associated with suppressive potency, underlining the multitude of yet not completely identified contributing factors.1,3,4,8 Beyond its direct suppressive functions, HO-1 has been also implicated in the activation as well as the induction of Tregs.11,15,35,36 Similar to MSCs, Tregs are under extensive preclinical and clinical evaluation for the treatment of GVHD occurring in bone marrow transplanted patients.2,17,18
Several in vivo and in vitro studies have shown an MSC-mediated induction and expansion of Tregs.5,22,23,37,38 PGE2 and TGF-β derived from the MSCs could be identified as major facilitators of these effects.5,37 Noticeably, most of the studies do not differentiate between Treg subsets, which possess diverse functional properties and require different stimuli to be generated and thrive.16,19 We show that human MSCs promote the induction of Tregs, resembling the phenotype of naturally occurring thymic forms (nTreg-like), IL-10+ Tr1, and TGF-β+ Th3 cells. Promotion of the Tr1 and Th3, but not nTreg-like, subtypes is HO-1 dependent in alloactivated as well as in TCR/B7-stimulated lymphocytes. The exact mechanism by which HO-1 supports Treg induction remains elusive. Two of its metabolic products, namely, the heavy-chain ferritin and bilirubin, have been linked to Treg activation and expansion.39-41 In analogy to the suppressiveness, the capacity for Treg induction among the various MSCs was not determined by their absolute HO-1 levels, thus representing a further indication for the multifactorial nature of this process.5,37
The steadily growing interest in the clinical application of MSCs involves several diseases characterized by chronic inflammation, among them systemic lupus erythematosus or GHVD.2,20 However, it remains open how exactly MSCs respond to inflammatory stimuli, and hitherto existing results are rather contradictory.7,29 As HO-1 is known to be a stress-responsive molecule, we sought to investigate the impact of an alloreactive environment24 on its expression in human MSCs. Coculturing human MSCs with MLRs led, unlike in cells derived from rats,8 to a significant reduction of HO-1 and Nrf2 expression, which was mediated by soluble factors. Changes in the transcriptional signature were accompanied by a weakened suppressive potency. Because Nrf2 is its major transcription factor, regulation by the inflammatory milieu is suggested to occur at this level.28,42 Treatment with IFN-γ during MLR efficiently rescued Nrf-2 and HO-1 expression, an observation that was similarly made in renal cells.43 The suppressive potency could only be partly preserved and was, according to recent observations,7 not further boosted in MSCs subjected only to IFN-γ treatment. However, these observations remain to be investigated for higher doses as dosing and duration of IFN-γ treatment represent major determinants for the effects exerted in human MSCs.
Plain IFN-γ administration led to a substantial up-regulation of Nrf2 and HO-1 in normally cultured MSCs. In addition to HO-1, Nrf2 controls a whole battery of molecules involved in the cellular antioxidative protection.28 Despite the substantial increase of antioxidant expression, we did not observe any gain of antioxidative capacity after 5 days of culture. This could be the result of a short observational time or an already existing very efficient, saturated antioxidative machinery in human MSCs.44
Conditioning human MSCs within an inflammatory MLR environment potentiated their competence of inducing nTreg-like and IL-10+ Tr1 cells as well as driving IL-10 production, despite the accompanying loss of HO-1. These prelicensed MSCs were excluded as a potential (additional) source for IL-10 because their inherent IL-10 expression decreased dramatically during the priming MLR. Interestingly, in prelicensed MSCs, HO-1 was no longer the mediator of Treg induction, promotion of IL-10 production, and suppressiveness. Our data suggest a “reprogramming” of the human MSCs, first encountering a strong in vitro alloreactive environment24 that inter alia results into a switch of functions initially mediated by HO-1.
Several studies have reported that inflammatory stimuli, such as IFN-γ or tumor necrosis factor-α, lead to COX-2 up-regulation in human MSCs.7,29 Meanwhile, PGE2 plays a nonredundant role in MSC- as well as tumor-promoted Treg induction and IL-10 production5,45 and is involved in several checkpoints of MSC-mediated immunosuppression.3,4 Upon MLR coculture, we noticed a dramatic increase of COX-2 expression in the MSCs. Inhibition of COX-2 in these prelicensed cells led, in contrast to blocking HO-1, to a diminished generation of IL-10, Tregs, and suppressive potency during subsequent, secondary MLR. Decrease of Treg induction, albeit significant, was at a range (20%-30%) that definitely implies the considerable participation of accessory mechanisms. Additional candidate molecules, including IDO and TGF-β, which can regulate HO-1 activity46 as well as Treg induction,5,19 were investigated without detecting significant alterations (data not shown).
In addition to lymphocytes, dendritic cells represent a major cellular target of the MSC-mediated immunomodulation.1,3 MSCs induce a tolerogenic (Treg-promoting) dendritic cell phenotype. The role of HO-1 in such a process remains to be elucidated, as it has been repeatedly shown that HO-1 reduces the antigen-presenting and stimulatory capacity of dendritic cells.47
Despite the existing consensus on the role of human MSCs in Treg induction/expansion, the impact of these cells on the MSC-mediated immunosuppression remains rather controversial. On the one hand, certain studies suggest that the immunoregulatory effects exerted by MSCs are strongly associated with Treg expansion.22,38 In several other models, the suppressive effect of MSCs shows no link to Treg induction.20,48,49 In our in vitro model, Treg induction did not correspond to suppressive potency of MSCs. One of the potential explanations may be that the suppressive Treg function is “overpowered” by the dominant presence of MSCs. It may also be speculated that MSCs perform a multistep inhibition: (1) they execute their direct effects preferably at the site of inflammation; and (2) they promote weaker, but longer lasting, responses among others by promoting adaptive Tregs. In future studies, it will be of interest to determine the homeostasis of the Treg (subsets) in GVHD patients treated with MSCs. However, concomitant immunosuppressive treatment may affect the MSC function,50 and potential alterations in this respect, especially of the HO-1 expression, have to be taken into consideration and further investigated.
Altogether, our findings point toward a strong role of HO-1 in the promotion of Tr1 and Th3 cell induction as well as the suppressive activity when MSCs initially encounter an alloreactive milieu in vitro. After this priming, it appears that MSCs are relicensed by as of yet not defined soluble factors. Within this process, HO-1 loses its prominent role in suppressiveness and IL-10 and Treg promotion, functions that are partly resumed by PGE2. Our results emphasize the significance of the proinflammatory microenvironment on shaping the immunomodulatory repertoire of human MSCs. Their plasticity endows them with the capability of adaptation to external conditions by even switching suppressive mechanisms.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Cancer Society of Stockholm, the Children's Cancer Foundation, the Juvenile Diabetes Research Foundation, Karolinska Institutet, the Stockholm City Council, the Swedish Cancer Society, the Swedish Research Council, the Swedish Society of Medicine, the Sven an Ebba-Christina Hagbergs Foundation, and the Tobias Foundation. D.M. was supported by a grant from the German Research Foundation.
Authorship
Contribution: D.M. and R.J. designed and performed research, analyzed data, and wrote the manuscript; C.C.J. designed and performed research; R.O. performed research; and R.K. and K.L.B. designed research, supervised data analysis, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dimitrios Mougiakakos, Department of Oncology and Pathology, Cancer Center Karolinska R8:01, Karolinska Institutet, 171 76 Stockholm, Sweden; e-mail: Dimitrios.Mougiakakos@ki.se.
References
Author notes
D.M. and R.J. contributed equally to this study.

![Figure 6. Induction of COX-2 in MSCs during conditioning in MLRs. (A) MSCs were treated with IFN-γ or cocultured with MLRs (pMLR) in regular or trans-wells. Relative gene expression of COX-2 was evaluated after 2 and 5 days (n = 9). (B) After initial priming of MSCs in an alloreactive milieu (pMLR) with or without administration of IFN-γ (100 IU/mL) or solely IFN-γ pretreatment for 5 days, MSCs were applied to a secondary MLR. After 5 days, levels of secreted PGE2 were assessed using ELISA (n = 6). (C-E) After initial alloreactive priming (pMLR), COX-2 in the MSCs was inhibited by NS398 (10μM) before performing secondary MLR. After 5 days, (C) IL-10 secretion, (D) suppressive activity, and (E) nTreg-like/Tr1 induction were evaluated by ELISA, thymidine incorporation (responder PBLs), and flow cytometry, respectively (n = 6). (F) To rule out any potential effect of NS398 on the HO-1 expression in MSCs, cells were analyzed with regard to HO-1 levels 5 days on treatment by flow cytometry (n = 6). (G) The effect of blocking COX-2 (NS398, 10μM), IDO (1-methyl-tryptophan [1-MT], 1mM), inducible nitric oxide synthase (NG-monomethyl-L-arginine [L-NMMA], 1mM), or neutralizing TGF-β (anti–αTGF-β antibodies, 10 μg/mL) on the suppressive activity of native versus prelicensed MSCs (n = 12) was assessed by thymidine incorporation in a 5-day (secondary) MLR. All experimental settings were conducted with MSCs derived from at least 3 individual donors, and n refers to the number of repeated experiments. Bars represent SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/18/10.1182_blood-2010-12-324038/4/m_zh89991170410006.jpeg?Expires=1767772424&Signature=l8xdAutmI5WvU62cojFvY3KsRHPwBxXXZ1HGsxyOrccr7X1VIFn4vCpT5rLW4jB3RSkKa1L6RdKCeoUl7ikkQJOGXp0Ub3XSddWtZ2Wz4DtbYLVor458VmBHqDCy-x1OflgEAc~oGvjX9PEJithwM1TiOvLKiw5kh-cg4XBkaZ9hHmIJTBAev5LnjJC9JDarmi-nWLnDhK~JGOqgFaRopdw7fmCjr-rVwF3KSQi1m3wul~-ppK1-na4YtHMPGUQ0VfwC161qEhgHztFvfn5i9sRGLhNEaTrvz1~tLKahR9gYifqkVjrIYGgoORCsalNNPf1eHPVcQMaOS35d8Z4UZg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal