Abstract
Targeted irradiation of the bone marrow with radiolabeled monoclonal antibodies (radioimmunotherapy) represents a novel therapeutic approach with both myeloablative and antileukemic potential. In an open-label, single-center pilot study, 30 pediatric and adolescent patients undergoing hematopoietic cell transplantation for malignant (n = 16) and nonmalignant (n = 14) disorders received treatment with a 90Y-labeled anti-CD66 monoclonal antibody. Patients with a high risk of relapse (n = 7) received additional treatment with standard conditioning based on either total body irradiation or busulfan to intensify the antileukemic effect. In patients with comorbidities (n = 23), radioimmunotherapy was combined with a reduced-intensity conditioning regimen to reduce systemic toxicity. Preferential irradiation of the bone marrow was achieved in all patients. Nonrelapse mortality was 4 (13%) of 30 patients. In patients with malignant diseases, the probabilities of overall and disease-free survival at 2 years were 0.69 (95% confidence interval 0.37-0.87) and 0.46 (95% confidence interval 0.19-0.70), respectively. In patients with nonmalignant diseases, the probability of both overall and disease-free survival at 2 years was 0.94 (95% confidence interval 0.63-0.99). This pilot study demonstrates that radioimmunotherapy is effective in achieving myeloablation with low additional toxicity when used in combination with standard or reduced-intensity conditioning in young patients.
Introduction
Hematopoietic cell transplantation (HCT) is used in a variety of malignant and nonmalignant disorders and has substantially improved long-term survival in recent decades.1-4 This therapeutic approach, however, is associated with significant toxicity of the conditioning regimens, as well as risk of disease recurrence. The risks of toxicity and relapse are strongly dependent on the intensity of the preparative regimen applied to eradicate or reduce the tumor burden, to facilitate the engraftment of hematopoietic cells in the marrow, or to prevent graft rejection. Unfortunately, the efficacy and toxicity of conditioning regimens are inversely correlated with intensity. For example, increasing the dose of total body irradiation (TBI) from 12 to 15.75 Gy significantly reduces the relapse rate; however, survival is not improved because of a higher nonrelapse mortality (NRM).5,6
Reduced-intensity conditioning (RIC) increasingly is being used for conditioning before HCT in elderly and heavily pretreated patients to limit NRM. Indeed, the RIC approach facilitates HCT in patients with high comorbidity and leads to significant reduction of NRM.7-11 However, intensive immunosuppression is required to prevent transplant rejection and to control graft-versus-host disease (GVHD), particularly after donor lymphocyte infusions (DLIs), which are commonly necessary to reach sustained donor cell engraftment. This is associated with complications, in particular serious infections, and results in substantial morbidity of the RIC approach.12,13 Furthermore, RIC has limitations with respect to stable donor cell engraftment, particularly in HLA-nonidentical HCT and in young patients with nonmalignant diseases who have an intact immune system. For instance, in patients with β-thalassemia and high comorbidity, NRM remains elevated,14 and sustained donor cell chimerism and control of β-thalassemia is achieved only in a minority of cases.15
Taken together, there is a need to further modify conditioning with regimens characterized by sufficient myeloablative and antileukemic potential but low toxicity. This goal may be achieved by targeted therapy that avoids the severe side effects of unselective chemotherapy or irradiation. Recently, a novel antibody-based, minimal-intensity conditioning regimen that uses 2 anti-CD45 monoclonal antibodies for myelosuppression has been explored in children with severe immunodeficiencies.16 Although this regimen was well tolerated and 69% of patients achieved full donor chimerism, a more effective myeloablative approach is required in most instances, particularly in the more common hematologic and neoplastic diseases.
One possibility for such a targeted therapy is radioimmunotherapy with radiolabeled monoclonal antibodies. The anti-CD66 monoclonal antibody is a murine IgG1 antibody; CD66 is expressed at a high density on normal myelopoietic cells from the promyelocyte onward up to mature granulocytes.17-19 The antibody has been explored for use in radioimmunotherapy in phase 1 or 2 studies in adults by labeling with rhenium-188 (188Re), which is a γ-photon and β-particle emitter, and more recently with yttrium-90 (90Y), which is a pure β-particle emitter. In these studies with patients who had high-risk acute leukemia or myelodysplastic syndrome, radioimmunotherapy was combined mainly with high-dose TBI or busulfan-based conditioning regimens.20-22 In 1 study, radioimmunotherapy was used as part of a dose-reduced, fludarabine-containing conditioning for patients older than 55 years of age.23 Relapse rates between 20% and 55% and NRMs between 20% and 59% have been observed in these adult patients, resulting in leukemia-free survival rates between 20% and 60%.20-25 On the basis of these encouraging results, we used radioimmunotherapy with 90Y–anti-CD66 for conditioning in 30 pediatric and adolescent patients undergoing HCT. The aim was to evaluate the usefulness and applicability of radioimmunotherapy in this age group, when applied together with standard conditioning in patients at high risk of relapse and together with RIC in patients with high comorbidity. This is the first report on a series of younger patients with malignant and nonmalignant disease treated by this approach.
Methods
Patients
Thirty patients were recruited between April 2003 and September 2007 for this phase 2, open-label, single-center study (Table 1). Patients were not suitable for conventional (myeloablative) conditioning protocols because of either high comorbidity or advanced malignant disease with very poor prognosis. Inclusion criteria to the study were as follows: (1) acute leukemia, myelodysplastic syndrome, malignancies with poor responses to the primary induction therapy (partial remission = 1), leukemia with early relapse (complete remission > 1), and leukemia with poor response to secondary induction therapy (partial remission > 1); (2) malignant and nonmalignant disorders with significant organ dysfunction or ongoing infection before conditioning or after a preceding HCT. The upper age limit was 21 years.
Patient characteristics
| Variable . | Number of patients . | ||
|---|---|---|---|
| Group A . | Group B . | All . | |
| Male/female, n | 9/5 | 11/5 | 20/10 |
| Median age (range), y | 10 (5-21) | 9 (1-20) | 9 (1-21) |
| Donor, n | |||
| MSD/MFD | 5 | 4 | 9 |
| MUD | 6 | 9 | 15 |
| MMD/haplo | 3 | 3 | 6 |
| Stem cell source, n | |||
| BM | 9 | 10 | 19 |
| PBSCs | 3 | 4 | 7 |
| T-depleted PBSCs | 2 | 2 | 4 |
| Conditioning, n* | |||
| TBI-based | 7 | 0 | 7 |
| Busulfan-based | 1 | 0 | 1 |
| Fludarabine/melphalan | 5 | 12 | 17 |
| Other | 1 | 4 | 5 |
| Transplantation, n | |||
| First HSCT | 11 | 12 | 23 |
| Second HSCT | 3 | 4 | 7 |
| Risk score, n† | |||
| 1 | 3 | 12 | 15 |
| 2 | 6 | 4 | 10 |
| 3 | 5 | 0 | 5 |
| Disease status, n‡ | |||
| Standard risk | 5 | Not applicable | 5 |
| High risk | 9 | Not applicable | 9 |
| Variable . | Number of patients . | ||
|---|---|---|---|
| Group A . | Group B . | All . | |
| Male/female, n | 9/5 | 11/5 | 20/10 |
| Median age (range), y | 10 (5-21) | 9 (1-20) | 9 (1-21) |
| Donor, n | |||
| MSD/MFD | 5 | 4 | 9 |
| MUD | 6 | 9 | 15 |
| MMD/haplo | 3 | 3 | 6 |
| Stem cell source, n | |||
| BM | 9 | 10 | 19 |
| PBSCs | 3 | 4 | 7 |
| T-depleted PBSCs | 2 | 2 | 4 |
| Conditioning, n* | |||
| TBI-based | 7 | 0 | 7 |
| Busulfan-based | 1 | 0 | 1 |
| Fludarabine/melphalan | 5 | 12 | 17 |
| Other | 1 | 4 | 5 |
| Transplantation, n | |||
| First HSCT | 11 | 12 | 23 |
| Second HSCT | 3 | 4 | 7 |
| Risk score, n† | |||
| 1 | 3 | 12 | 15 |
| 2 | 6 | 4 | 10 |
| 3 | 5 | 0 | 5 |
| Disease status, n‡ | |||
| Standard risk | 5 | Not applicable | 5 |
| High risk | 9 | Not applicable | 9 |
MSD/MFD indicates HLA-matched sibling donor/HLA-matched family donor; MUD, HLA-matched unrelated donor; MMD, HLA-mismatched donor; haplo, HLA-haploidentical family donor; PBSCs, peripheral blood stem cells; and TBI, total body irradiation.
Risk score was adapted from Matthes-Martin et al26 ; risk factors were age > 10 years, advanced disease, or alternative donor.
Disease status in malignant diseases: standard risk, complete remission (CR1/CR2); high risk, complete remission beyond CR2 or no remission (NR).
Altogether, the study included 14 case subjects with malignant disease who ranged in age from 5 to 20 years (median 10 years; group A) and 16 case subjects with nonmalignant disorders who ranged in age from 8 months to 21 years (median 9 years; group B). Patients' characteristics are summarized in Table 1, and details for each patient are shown in Tables 2 and 3. In summary, the following risk factors led to inclusion in the present study: advanced malignant disease in all 14 cases in group A; ongoing severe infections in 9 cases (1 case in group A, 8 cases in group B); second transplantation in 7 cases (3 in group A, 4 in group B); significant organ damage in 5 cases in group B; and a high risk of NRM according to Matthes-Martin et al26 in 15 cases (11 in group A and 4 in group B). This risk score was based on multivariate analysis that demonstrated a significant correlation of NRM with age > 10 years, advanced disease, and use of alternative donors.
Transplantation characteristics of group A (patients with malignant diseases)
| No . | Age, y/sex . | Disease . | Indication for RIT . | Stem cell source* . | RIT BM dose, Gy† . | Conditioning regimen (dose)‡ . | Clinical course after HCT§ . | Disease status after HCT . | Survival status . |
|---|---|---|---|---|---|---|---|---|---|
| A-1 | 17/F | AML M5 CR3 | Advanced disease (2nd relapse); 2nd HCT | MUD/PBSC | 28 | TBI (12 Gy), Flu (160), ATG | Pericardial effusion; resuscitation; apallic syndrome; died of infection | CR | Dead (d +129) |
| A-2 | 9/M | AML M1/2 CR2 | Advanced disease (early relapse); pulmonary disease | MSD/BM | 19 | TBI (12 Gy), Flu (160) | Acute GVHD (4th degree); chronic GVHD (extended/severe) | CR | Alive (> 72 mo) |
| A-3 | 5/M | ALL CR2 (cortical T) | Advanced disease (early combined relapse) | MFD/BM | 15 | TBI (12 Gy), VP16 (60) | CMV reactivation | CR | Alive (> 63 mo) |
| A-4 | 15/M | AML M0 CR2 | Advanced disease (early relapse) | MUD/BM | 18 | TBI (12 Gy), Flu (160), ATG | Relapse; bacterial sepsis; aspergillosis; died of these complications | Relapse | Dead (d +94) |
| A-5 | 12/M | ALL PR2 | Advanced disease (refractory disease, 10% BM blasts) | MFD/BM | 23 | TBI (12 Gy), VP16 (60) | Fourth-degree toxicity; aspergillus pneumonia; died of multiorgan failure | CR | Dead (d +36) |
| A-6 | 11/M | ALL PR1 | Advanced disease (refractory disease, 25% BM blasts) | MSD/BM | 36 | TBI (14 Gy), VP16 (40) | Died of early relapse | Relapse | Dead (d +37) |
| A-7 | 6/M | ALL NR | Advanced disease (refractory disease, 50% BM blasts) | MMUD/PBSC | 26 | TBI (14 Gy), VP16 (60), ATG | CMV reactivation; chronic GVHD (extended/moderate) after preemptive DLI | CR | Alive (> 25 mo) |
| A-8 | 6/M | AML M0 | Advanced disease (refractory disease, 5% BM blasts) | Haplo/TDPBSC | 46 | Bu Flu (160), Cy (120), ATG OKT3 | Rejection of first graft; re-HSCT from 2nd haplo donor; CMV reactivation; EBV-LPS; BK cystitis | CR | Alive (> 45 mo) |
| A-9 | 21/F | AML M2 PR4 | Advanced disease (3rd relapse, in poor remission) | Haplo/TDPBSC | 11 | Flu (160), Cy (120), ATG OKT3 | CMV reactivation; BK cystitis; acute and chronic GVHD after preemptive DLI | CR | Alive (> 53 mo) |
| A-10 | 7/M | ALL CR2 | Advanced disease (early relapse); systemic aspergillosis | MUD/BM | 35 | Flu (160), Mel (140), ATG | Extramedullary relapse (bone, meninges); CMV reactivation; in treatment (2nd HCT) | Relapse | Alive (> 38 mo) |
| A-11 | 10/M | ALL PR3 | Advanced disease (refractory disease, 10% BM blasts); 2nd HCT | MUD/BM | 34 | Flu (160), Mel (140), ATG | Extramedullary relapse (bone, lymph nodes); died after 3rd haploidentical HCT from relapse | Relapse | Dead (27 mo) |
| A-12 | 14/F | AML M2 CR2 | Advanced disease (early relapse) | MUD/BM | 36 | Flu (160), Mel (140), ATG | Adenovirus infection; BK cystitis; acute third-degree GVHD after preemptive DLI | CR | Alive (> 30 mo) |
| A-13 | 14/F | sMDS | Advanced disease (secondary refractory disease) | MUD/PBSC | 35 | Flu (160), Mel (140), ATG | Early relapse; on treatment | Relapse | Alive (> 20 mo) |
| A-14 | 7/F | Neuroblastoma stage IV | Advanced disease (refractory disease); 2nd HCT | MFD/BM | 26 | Flu (160), Mel (140) | Late relapse; on treatment | Relapse | Alive (> 21 mo) |
| No . | Age, y/sex . | Disease . | Indication for RIT . | Stem cell source* . | RIT BM dose, Gy† . | Conditioning regimen (dose)‡ . | Clinical course after HCT§ . | Disease status after HCT . | Survival status . |
|---|---|---|---|---|---|---|---|---|---|
| A-1 | 17/F | AML M5 CR3 | Advanced disease (2nd relapse); 2nd HCT | MUD/PBSC | 28 | TBI (12 Gy), Flu (160), ATG | Pericardial effusion; resuscitation; apallic syndrome; died of infection | CR | Dead (d +129) |
| A-2 | 9/M | AML M1/2 CR2 | Advanced disease (early relapse); pulmonary disease | MSD/BM | 19 | TBI (12 Gy), Flu (160) | Acute GVHD (4th degree); chronic GVHD (extended/severe) | CR | Alive (> 72 mo) |
| A-3 | 5/M | ALL CR2 (cortical T) | Advanced disease (early combined relapse) | MFD/BM | 15 | TBI (12 Gy), VP16 (60) | CMV reactivation | CR | Alive (> 63 mo) |
| A-4 | 15/M | AML M0 CR2 | Advanced disease (early relapse) | MUD/BM | 18 | TBI (12 Gy), Flu (160), ATG | Relapse; bacterial sepsis; aspergillosis; died of these complications | Relapse | Dead (d +94) |
| A-5 | 12/M | ALL PR2 | Advanced disease (refractory disease, 10% BM blasts) | MFD/BM | 23 | TBI (12 Gy), VP16 (60) | Fourth-degree toxicity; aspergillus pneumonia; died of multiorgan failure | CR | Dead (d +36) |
| A-6 | 11/M | ALL PR1 | Advanced disease (refractory disease, 25% BM blasts) | MSD/BM | 36 | TBI (14 Gy), VP16 (40) | Died of early relapse | Relapse | Dead (d +37) |
| A-7 | 6/M | ALL NR | Advanced disease (refractory disease, 50% BM blasts) | MMUD/PBSC | 26 | TBI (14 Gy), VP16 (60), ATG | CMV reactivation; chronic GVHD (extended/moderate) after preemptive DLI | CR | Alive (> 25 mo) |
| A-8 | 6/M | AML M0 | Advanced disease (refractory disease, 5% BM blasts) | Haplo/TDPBSC | 46 | Bu Flu (160), Cy (120), ATG OKT3 | Rejection of first graft; re-HSCT from 2nd haplo donor; CMV reactivation; EBV-LPS; BK cystitis | CR | Alive (> 45 mo) |
| A-9 | 21/F | AML M2 PR4 | Advanced disease (3rd relapse, in poor remission) | Haplo/TDPBSC | 11 | Flu (160), Cy (120), ATG OKT3 | CMV reactivation; BK cystitis; acute and chronic GVHD after preemptive DLI | CR | Alive (> 53 mo) |
| A-10 | 7/M | ALL CR2 | Advanced disease (early relapse); systemic aspergillosis | MUD/BM | 35 | Flu (160), Mel (140), ATG | Extramedullary relapse (bone, meninges); CMV reactivation; in treatment (2nd HCT) | Relapse | Alive (> 38 mo) |
| A-11 | 10/M | ALL PR3 | Advanced disease (refractory disease, 10% BM blasts); 2nd HCT | MUD/BM | 34 | Flu (160), Mel (140), ATG | Extramedullary relapse (bone, lymph nodes); died after 3rd haploidentical HCT from relapse | Relapse | Dead (27 mo) |
| A-12 | 14/F | AML M2 CR2 | Advanced disease (early relapse) | MUD/BM | 36 | Flu (160), Mel (140), ATG | Adenovirus infection; BK cystitis; acute third-degree GVHD after preemptive DLI | CR | Alive (> 30 mo) |
| A-13 | 14/F | sMDS | Advanced disease (secondary refractory disease) | MUD/PBSC | 35 | Flu (160), Mel (140), ATG | Early relapse; on treatment | Relapse | Alive (> 20 mo) |
| A-14 | 7/F | Neuroblastoma stage IV | Advanced disease (refractory disease); 2nd HCT | MFD/BM | 26 | Flu (160), Mel (140) | Late relapse; on treatment | Relapse | Alive (> 21 mo) |
RIT indicates radioimmunotherapy; F, female; AML, acute myeloid leukemia (M0, M1, M2, and M5 denote subtypes of AML according to the French-American-British classification); CR, complete remission (numbers indicate number of complete remissions; eg, CR2 is second complete remission); MUD, HLA-matched unrelated donor; PBSC, peripheral blood stem cell; Flu, fludarabine; ATG, anti-thymocyte globulin; d +, number of days after transplantation; M, male; MSD, HLA-matched sibling donor; MFD, HLA-matched family donor; VP16, etoposide; PR, partial remission (numbers indicate number of partial remissions; eg, PR2 is second partial remission); fourth-degree toxicity, according to the Bearman classification36 ; NR, no remission; MMUD, HLA-mismatched undated donor; haplo, HLA-haploidentical family donor; TDPBSC, T cell–depleted peripheral blood stem cells; Bu, busulfan; Mel, melphalan; OKT3, anti-CD3 monoclonal antibody; Cy, cyclophosphamide; EBV-LPS, EBV-induced lymphoproliferative syndrome; and sMDS, secondary myelodysplastic syndrome.
TDPBSCs were < 3 × 104 CD3 per kilogram of body weight.
Calculated absorbed doses in BM.
Drugs used in the conditioning regimen were as follows, with doses given in parentheses in the table: fludarabine (mg/m2); etoposide (mg/kg); melphalan (mg/m2); and intravenous busulfan (applied dose was weight adapted according to the prescription of the manufacturer).
GVHD greater than second grade.
Transplantation characteristics of group B (patients with nonmalignant diseases)
| No . | Age, y/sex . | Disease . | Indication for RIT . | Stem cell source . | RIT BM dose, Gy* . | Conditioning regimen† . | Clinical course after HCT . | Chimerism after HCT . | Survival status . |
|---|---|---|---|---|---|---|---|---|---|
| B-1 | 20/M | β-Thalassemia | Severe hemosiderosis (liver fibrosis) | MUD/PBSC | 17 | Flu (160), Mel (140), ATG | Uneventful course | 100% donor | Alive (> 49 mo) |
| B-2 | 14/M | Congenital hemolytic anemia | Severe hemosiderosis (liver fibrosis) | MFD/BM | 16 | Flu (160), Mel (70), ATG | Uneventful course | 100% donor | Alive (> 47 mo) |
| B-3 | 4/M | RAG1-SCID (B-low T-low NK+) | 2nd HCT, active infection (pneumonitis) | Haplo/TDPBSC | 20 | Flu (160), ATG | Graft failure; 3rd HSCT from 2nd haplo donor; rejection; 4th HSCT from 3rd haplo donor; GVHD; died of adenovirus pneumonitis | Rejection | Dead (13 mo) |
| B-4 | 6/F | RAG1-CID (B-low T-low NK+) | Active infection (aspergillosis, EBV lymphoma) | MUD/PBSC | 20 | Flu (160), ATG | Uneventful course | 100% donor | Alive (> 46 mo) |
| B-5 | 10/M | Hyper-IgE syndrome (AR) | Active infection (pneumonitis) | MUD/PBSC | 16 | Flu (160), Mel (140), ATG | EBV infection | 100% donor | Alive (> 43 mo) |
| B-6 | 8/F | RAG1-CID (B-low, T-low NK+) | Active infection (pneumonitis) | MUD/BM | 17 | Flu (160), Mel (140), ATG | Adenovirus and EBV infection; chronic GVHD (extended/severe); tricytopenia; died of infection | 100% donor | Dead (36 mo) |
| B-7 | 19/M | Congenital hemolytic anemia | Severe hemosiderosis (liver fibrosis) | MUD/PBSC | 16 | Flu (160), Mel (140), ATG | Candida sepsis; Candida meningitis/osteomyelitis; CMV reactivation | 100% donor | Alive (> 40 mo) |
| B-8 | 7/F | β-Thalassemia | 2nd HCT (autologous reconstitution) | MFD/BM | 20 | Mel (140) | Bacterial sepsis (Escherichia coli); CMV reactivation | 100% donor | Alive (> 35 mo) |
| B-9 | 4/M | CGD | Active infection (aspergillosis) | MUD/BM | 18 | Flu (160), Mel (70), ATG | Uneventful course | 30% donor | Alive (> 30 mo) |
| B-10 | 9/M | CGD | Active infection (pneumonia) | MUD/BM | 17 | Flu (160), Mel (70), ATG | Bacterial sepsis; acute pancreatitis; severe colitis; pneumonitis (ongoing oxygen requirement) | 100% donor | Alive (> 27 mo) |
| B-11 | 10/M | IFN-γ–receptor deficiency | Active infection (mycobacteriosis) | MUD/BM | 17 | Flu (160), Mel (140), ATG | Bacterial sepsis; CMV reactivation; colitis (adenovirus and rotavirus); mycobacteriosis | 100% donor | Alive (> 26 mo) |
| B-12 | 10/M | Hyper-IgM syndrome | Hepatopathy (cryptosporidiosis) | MFD/BM | 16 | Flu (160), Mel (70) | Prolonged FUO; deterioration of liver function/cholestasis; enteropathy | 100% donor | Alive (> 25 mo) |
| B-13 | 5/F | Griscelli syndrome | 2nd HCT (autologous reconstitution) | Haplo/TDPBSC | 17 | Nothing | Uneventful course | 100% donor | Alive (> 24 mo) |
| B-14 | 1/M | SCID (ZAP70 deficiency) | Active infection (PCP pneumonitis, cholestasis) | MUD/BM | 17 | Flu (160), Mel (140), ATG | Cholestasis/liver cirrhosis; chronic GVHD (extended/moderate) | 100% donor | Alive (> 23 mo) |
| B-15 | 14/F | IFN-γ–receptor deficiency | 2nd HSCT (autologous reconstitution) | MFD/BM | 17 | Flu (160), Mel (70), ATG | Adenovirus infection, chronic GVHD (extended/moderate) | 100% donor | Alive (23 mo) |
| B-16 | 1/M | Hyper-IgM syndrome | Hepatopathy | MMFD/BM | 18 | Flu (160), Mel (140), ATG | EBV-associated lymphoproliferative syndrome | 10% donor | Alive (22 mo) |
| No . | Age, y/sex . | Disease . | Indication for RIT . | Stem cell source . | RIT BM dose, Gy* . | Conditioning regimen† . | Clinical course after HCT . | Chimerism after HCT . | Survival status . |
|---|---|---|---|---|---|---|---|---|---|
| B-1 | 20/M | β-Thalassemia | Severe hemosiderosis (liver fibrosis) | MUD/PBSC | 17 | Flu (160), Mel (140), ATG | Uneventful course | 100% donor | Alive (> 49 mo) |
| B-2 | 14/M | Congenital hemolytic anemia | Severe hemosiderosis (liver fibrosis) | MFD/BM | 16 | Flu (160), Mel (70), ATG | Uneventful course | 100% donor | Alive (> 47 mo) |
| B-3 | 4/M | RAG1-SCID (B-low T-low NK+) | 2nd HCT, active infection (pneumonitis) | Haplo/TDPBSC | 20 | Flu (160), ATG | Graft failure; 3rd HSCT from 2nd haplo donor; rejection; 4th HSCT from 3rd haplo donor; GVHD; died of adenovirus pneumonitis | Rejection | Dead (13 mo) |
| B-4 | 6/F | RAG1-CID (B-low T-low NK+) | Active infection (aspergillosis, EBV lymphoma) | MUD/PBSC | 20 | Flu (160), ATG | Uneventful course | 100% donor | Alive (> 46 mo) |
| B-5 | 10/M | Hyper-IgE syndrome (AR) | Active infection (pneumonitis) | MUD/PBSC | 16 | Flu (160), Mel (140), ATG | EBV infection | 100% donor | Alive (> 43 mo) |
| B-6 | 8/F | RAG1-CID (B-low, T-low NK+) | Active infection (pneumonitis) | MUD/BM | 17 | Flu (160), Mel (140), ATG | Adenovirus and EBV infection; chronic GVHD (extended/severe); tricytopenia; died of infection | 100% donor | Dead (36 mo) |
| B-7 | 19/M | Congenital hemolytic anemia | Severe hemosiderosis (liver fibrosis) | MUD/PBSC | 16 | Flu (160), Mel (140), ATG | Candida sepsis; Candida meningitis/osteomyelitis; CMV reactivation | 100% donor | Alive (> 40 mo) |
| B-8 | 7/F | β-Thalassemia | 2nd HCT (autologous reconstitution) | MFD/BM | 20 | Mel (140) | Bacterial sepsis (Escherichia coli); CMV reactivation | 100% donor | Alive (> 35 mo) |
| B-9 | 4/M | CGD | Active infection (aspergillosis) | MUD/BM | 18 | Flu (160), Mel (70), ATG | Uneventful course | 30% donor | Alive (> 30 mo) |
| B-10 | 9/M | CGD | Active infection (pneumonia) | MUD/BM | 17 | Flu (160), Mel (70), ATG | Bacterial sepsis; acute pancreatitis; severe colitis; pneumonitis (ongoing oxygen requirement) | 100% donor | Alive (> 27 mo) |
| B-11 | 10/M | IFN-γ–receptor deficiency | Active infection (mycobacteriosis) | MUD/BM | 17 | Flu (160), Mel (140), ATG | Bacterial sepsis; CMV reactivation; colitis (adenovirus and rotavirus); mycobacteriosis | 100% donor | Alive (> 26 mo) |
| B-12 | 10/M | Hyper-IgM syndrome | Hepatopathy (cryptosporidiosis) | MFD/BM | 16 | Flu (160), Mel (70) | Prolonged FUO; deterioration of liver function/cholestasis; enteropathy | 100% donor | Alive (> 25 mo) |
| B-13 | 5/F | Griscelli syndrome | 2nd HCT (autologous reconstitution) | Haplo/TDPBSC | 17 | Nothing | Uneventful course | 100% donor | Alive (> 24 mo) |
| B-14 | 1/M | SCID (ZAP70 deficiency) | Active infection (PCP pneumonitis, cholestasis) | MUD/BM | 17 | Flu (160), Mel (140), ATG | Cholestasis/liver cirrhosis; chronic GVHD (extended/moderate) | 100% donor | Alive (> 23 mo) |
| B-15 | 14/F | IFN-γ–receptor deficiency | 2nd HSCT (autologous reconstitution) | MFD/BM | 17 | Flu (160), Mel (70), ATG | Adenovirus infection, chronic GVHD (extended/moderate) | 100% donor | Alive (23 mo) |
| B-16 | 1/M | Hyper-IgM syndrome | Hepatopathy | MMFD/BM | 18 | Flu (160), Mel (140), ATG | EBV-associated lymphoproliferative syndrome | 10% donor | Alive (22 mo) |
RAG1-SCID indicates RAG1-related severe combined immunodeficiency; B-low T-low NK+, B and T cells below the normal range, normal NK-cells; AR, autosomal recessive; RAG1-CID, RAG1-related combined immunodeficiency disease; CGD, chronic granulomatous disease; IFN-γ, interferon-γ; FUO, fever of unknown origin; ZAP70, ζ-chain–associated protein kinase 70; and PCP, pneumocystis pneumonia. Other abbreviations are defined as in Table 2.
Calculated absorbed doses in BM.
Drugs used in the conditioning regimen were as follows, with doses given in parentheses in the table: fludarabine (mg/m2); etoposide (mg/kg); melphalan (mg/m2); and intravenous busulfan (applied dose was weight adapted according to the prescription of the manufacturer).
All parents and adolescent patients gave written informed consent before inclusion in the pilot study. The treatment protocol was approved by the local ethics committee of the University of Ulm.
Drug administration and preparative regimen before HCT
Details of the biology and the principles of administration of radiolabeled monoclonal antibodies have been published previously.23,27 The anti-CD66 antibody (antigranulocyte, anti–nonspecific cross-reacting antigen 95; BW 250/183) is well characterized with respect to biokinetic data and clinical application in bone marrow (BM) scintigraphy.28-30 Yttrium-90 (90Y) is a β-particle emitter with a high energy (2.3 MeV), a maximum range of the β particles of 11 mm, and a half-life of 2.7 days.31 90Y administration does not require isolation of patients because it does not emit γ radiation. The conjugation process of antibody and nuclide is described in detail in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
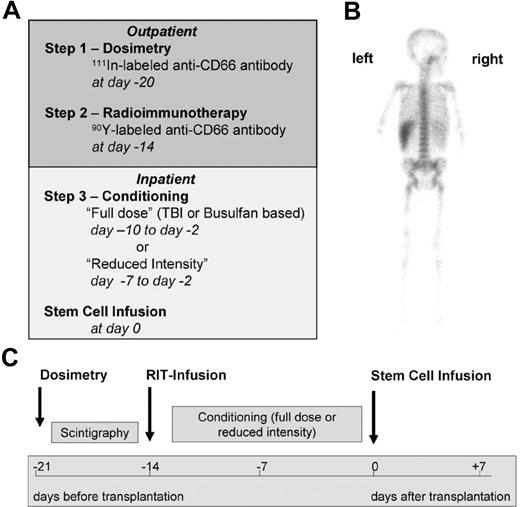
The preparative regimen consisted of 3 steps (Figure 1). In step 1 (dosimetry), patients received 71 ± 28 MBq of indium 111 (111In)-labeled anti-CD66 antibody as a single dose by slow intravenous push. Dosimetry must be performed with the surrogate tracer 111In because 90Y is a pure β emitter that cannot be monitored easily by gamma cameras. Patients were required to have a favorable dosimetry result, defined as a BM-absorbed dose higher than that of any other organ (except for the spleen). In step 2 (radioimmunotherapy), patients received a therapeutic dose of 90Y-labeled anti-CD66 antibody as a single dose by slow intravenous push. The therapeutic 90Y activity was calculated on the basis of the serial total-body gamma camera counts32 with the use of adequate phantoms provided by OLINDA/EXM software33 to deliver a dose to the BM of 30-35 Gy in patients in complete remission, 35 to 40 Gy in patients not in complete remission in the malignant group, and 16-20 Gy in the nonmalignant group. In addition, the dose to the liver and to the kidneys had to be less than 12 and 6 Gy, respectively (details of dosimetry are described in the Supplemental Materials). Steps 1 and 2 were commonly performed on an outpatient basis. In step 3, patients underwent additional conditioning (Tables 2–3). Eight patients with advanced malignant diseases (patients A-1 through A-8) received either TBI-based (12-14 Gy, n = 7) or, in 1 case of myeloid leukemia, intravenous busulfan-based (12.8 mg/kg) conditioning. Twenty-one patients with high comorbidity and either malignant (n = 5, patients A-9 through A14) or nonmalignant (n = 15) diseases were treated with melphalan (70-140 mg/m2) and fludarabine (160 mg/m2) in addition to radioimmunotherapy. Two patients (B-8, B13) received melphalan (140 mg/m2) only or nothing else in addition to radioimmunotherapy before secondary transplantation after autologous reconstitution after the first transplantation. To prevent graft rejection, patients with HLA-mismatched donors or unrelated donors received anti-thymocyte globulin, either ATG-Fresenius (Fresenius Biotec) at 50-60 mg/kg or Thymoglobulin (Genzyme GmbH) at 10 mg/kg (see Tables 2–3 for details of conditioning). HCT was performed 14 days after infusion of the radiolabeled antibody (step 2) to avoid relevant irradiation of the graft.
Treatment regimen. (A) General schedule of dosimetry, radioimmunotherapy, and further conditioning. (B) Posterior view of a γ-camera image of patient A-8 demonstrating preferential accumulation in red BM 1 day after injection of 111In-labeled anti-CD66 monoclonal antibody. (C) Time schedule of the radioimmunotherapy (RIT) conditioning regimen.
Treatment regimen. (A) General schedule of dosimetry, radioimmunotherapy, and further conditioning. (B) Posterior view of a γ-camera image of patient A-8 demonstrating preferential accumulation in red BM 1 day after injection of 111In-labeled anti-CD66 monoclonal antibody. (C) Time schedule of the radioimmunotherapy (RIT) conditioning regimen.
Transplantation, GVHD prophylaxis, and supportive care
Details of donors and stem cell sources are depicted in Tables 2–3. HLA-matched sibling donors and HLA-matched family donors were genotypically HLA identical; HLA-matched unrelated donors (MUD) were defined as identical in HLA-A, -B, -C, -DRB1, and -DQB1 loci by high-resolution genetic typing. Mismatched donors comprised 1 unrelated donor with a single-locus mismatch and 5 HLA-haploidentical family donors. Grafts used were G-CSF–mobilized peripheral blood stem cells in 11 patients and BM in 19 patients.
T cell–depleted peripheral blood stem cells were prepared by CD34+ selection with the immunomagnetic CliniMACS device (Miltenyi Biotec).34,35 In HLA-haploidentical transplants, the targeted dose of T cells was < 3 × 104 CD3+ cells per kilogram. In 4 transplants from MUDs, the T-cell dose was adjusted to 1 × 107 CD3+ cells per kilogram by T-cell add backs. T-cell depletion was the sole GVHD prophylaxis in all 4 HLA-haploidentical transplants. In patients who received grafts from MUDs, cyclosporine A and methotrexate or cyclosporine A and mycophenolate mofetil were given as GVHD prophylaxis. In patients who received matched family donor BM grafts, cyclosporine A was the sole GVHD prophylaxis. Four patients with very high-risk leukemia (Table 2: A-7, A-9, A-11, and A-12) received preemptive DLIs to prevent relapse.
Supportive care and treatment of complications followed recommendations and standard procedures of the European Group for Blood & Marrow Transplantation for pediatric HCT.
Assessment of toxicity, GVHD, and outcome
Engraftment, toxicity, and GVHD were assessed by consensus criteria.36,37 Chimerism analysis of peripheral blood and BM cells was performed by repeated analysis of short tandem repeat DNA markers, by HLA typing, or by XY-fluorescence in situ hybridization.35,38 NRM was defined as death due to causes other than relapse of malignant disease, and event-free survival was defined as the interval from transplantation until death due to any cause.
Statistical analysis
Data compiled between April 2003 and September 2007 were analyzed. Incidences are given as absolute and relative frequencies. Median and range were documented for continuous variables because of unknown normal distributions. The estimated absorbed dose is shown in Figure 2 by box-and-whisker plots. Probabilities of overall survival and disease-free survival were calculated according to the Kaplan-Meier method.39 Median survival and estimated survival rates at 24 months together with the respective 95% confidence intervals (CIs) are given. All analyses were performed separately for group A and group B. No comparative subgroup analyses were used because of the small number of observations. Statistical analysis was performed with SAS 9.1.3 (SAS Inc) software.
Results
Dosage and organ distribution of radioimmunotherapy
Mean therapeutic indices, ie, the ratio of absorbed doses in BM versus other organs, were 5.0 for the liver (range, 1.1–10.0), 13.3 for the kidneys (range, 2.2–46.1), and 85.4 for the lungs (range, 6.2–347.0), respectively (Figure 2). In all but 3 patients, absorbed doses delivered by 90Y–anti-CD66 radioimmunotherapy to the BM were more than 2 times higher than doses delivered to other organs except the spleen. A preferential irradiation of the BM with absorbed doses that ranged from 11 to 46 Gy in malignant diseases and from 16 to 20 Gy in nonmalignant diseases was achieved. The envisaged BM dose as described in “Drug administration and preparative regimen before HCT” was reached in all but 3 patients. In these 3 patients with malignant diseases (A-3, A-9, and A-14), the BM dose was reduced in order not to exceed the maximum tolerated dose to the liver. In 1 patient (A-8), a BM dose of 46 Gy, which was applied accidentally, was tolerated without side effects. The estimated absorbed doses in BM, spleen, liver, kidney, and lung are shown in supplemental Figure 1.
Therapeutic indices (ratio of absorbed doses in BM versus other organs). Box-and-whisker plot showing median, lower, and upper quartiles and sample minimum and maximum. A indicates group A, malignant diseases; and B, group B, nonmalignant diseases.
Therapeutic indices (ratio of absorbed doses in BM versus other organs). Box-and-whisker plot showing median, lower, and upper quartiles and sample minimum and maximum. A indicates group A, malignant diseases; and B, group B, nonmalignant diseases.
Myeloablative efficacy, engraftment, and relapse of disease
BM aplasia, defined as leukocyte counts < 200/μL in the peripheral blood, developed in all patients after conditioning. The myeloablative capacity of the radioimmunotherapy approach could be demonstrated clearly in 1 patient (B-13) who received only radioimmunotherapy as conditioning before a second transplantation. This girl, who had Griscelli syndrome, had experienced a secondary graft failure after first conventional HLA-haploidentical transplantation. All of her hematopoietic cells had returned to host type with the exception of a small proportion of T cells that remained of donor type. The latter finding suggested that this patient actually had not rejected the graft but rather that recovering autologous hematopoietic cells had replaced donor cells in the marrow. After radioimmunotherapy and infusion of T cell–depleted hematopoietic cells from the same HLA-haploidentical parental donor, she developed sustained, complete donor cell chimerism in the absence of any toxicity.
All but 2 patients achieved stable primary engraftment. Time to engraftment was delayed in some patients with malignant diseases and in some patients who received a cumulative radiation dose to the BM of > 20 Gy (supplemental Table 1; supplemental Figures 2-3). However, there was no significant difference in mean time to engraftment when TBI conditioning was compared with no TBI conditioning and when cumulative radiation doses of more or less than 40 Gy were compared (supplemental Table 1). Two patients rejected their primary haploidentical grafts; patient A-8 received a successful second transplant from the other parent after reconditioning, but patient B-3 died of adenovirus pneumonia after repeated transplants. The median time to achieve > 0.5 × 109/L neutrophils was 22 days (range, 12-32 days) in patients in group A and 16 days (range 11 to 40 days) in patients in group B; the median time to reach > 20.0 × 109/L platelets was 62 days (range, 19-125 days) in group A and 25 days (range, 15-119 days) in group B. Normalization of blood cell counts was achieved in all surviving patients within a time frame from 1 to 15 months.
Relapses of malignant diseases occurred in 6 (43%) of 14 patients, with 2 of 7 patients being in remission and 4 of 7 patients not in remission at HCT. Two relapses occurred among 8 patients (A-1 through A-8) after myeloablative conditioning that included TBI or busulfan in addition to radioimmunotherapy, and 4 relapses were observed among 6 patients (A-9 through A-14) treated with fludarabine and melphalan in addition to radioimmunotherapy. Interestingly, 2 of 7 patients treated for a myeloid malignancy experienced a relapse, whereas 4 of 7 treated for acute lymphoblastic leukemia (ALL) or neuroblastoma, which also regularly affect extramedullary sites, relapsed. Two of 6 relapses occurred primarily in extramedullary sites (patient A-10, relapse of ALL in bone and meninges; patient A-11, relapse of ALL in bone and lymph nodes). Three relapsed patients are alive and continuing treatment to date (A-10, A-13, and A-14). In group B (nonmalignant diseases), 1 (6%) of 16 patients experienced recurrence of disease after primary graft rejection (B-3). Two patients with nonmalignant disease developed mixed chimerism, with a proportion of 30% and 10% donor cells in both myeloid and lymphoid lineages, respectively (patient B-9, chronic granulomatous disease, MUD transplant after reduced dose of melphalan 70 mg/m2; patient B-18, hyper-IgM syndrome, HLA-mismatched family donor transplant). Thirteen (81%) of 16 patients with nonmalignant disease have sustained complete chimerism of all blood cells after conditioning with radioimmunotherapy and reduced-intensity chemotherapy.
Complications related to toxicity, infection, and GVHD
There were no immediate side effects and no acute toxicity related to the infusion of the radiolabeled antibodies, and the procedure was well tolerated. Major complications after transplantation are shown in Tables 2–3 and summarized in Table 4. Patient A-1 experienced polyserositis of unknown origin with a pericardial effusion that resulted in cardiac failure at day +7 after HCT. The patient was treated with gemtuzumab ozogamicin (Mylotarg, Wyeth Pharma) twice before HCT, which might have contributed, in conjunction with the radioimmunotherapy antibody, to this uncommon event. No other unusual severe toxic complications were observed in any patient. Most patients developed transient fever of unknown origin during aplasia. Three patients experienced bacterial sepsis, and 3 had systemic fungal infections. Patients A-4 and A-5 with active aspergillosis died of multiorgan failure; patient B-7 developed multifocal osteomyelitis due to Candida dubliniensis that required transient ventilation and prolonged antimycotic therapy (published in a report from Wellinghausen et al40 ). Complications from viral infections were observed in a high number of patients (Tables 2–3) and included transient CMV reactivation in 8 patients; symptomatic adenovirus infections in 5 patients, which caused prolonged enteritis in patient A-12 and fatal pneumonitis in patient B-3; transient EBV lymphoproliferative diseases in 4 patients; and symptomatic but transient BK virus cystitis in 3 patients. Atypical mycobacteriosis and cryptosporidiosis related to the underlying immunodeficiency were observed in 2 patients and 1 patient, respectively. All but 5 patients (A-1, A-4, A-5, B-3, and B-6) recovered from severe bacterial, fungal, or viral infections.
The incidence of acute and chronic GVHD is shown in Table 4. Eleven patients (37%) developed second- to fourth-grade acute GVHD, and 2 patients (7%) in the malignant disease group developed acute GVHD greater than second grade. Extended chronic GVHD was documented in 5 patients (17%) and was moderate in 4 patients and severe in 1 patient (B-6). Three of these patients developed GVHD after preemptive DLIs: patient A-7, who had refractory ALL, received 7 DLIs between day +39 and day +192 of his HLA-mismatched unrelated donor, which resulted in a cumulative dose of 208.3 × 106 T cells per kilogram of body weight; patient A-9, who had acute myeloid leukemia in partial remission after these relapses, received 12 DLIs between day +25 and day +109 of her haploidentical donor, which resulted in a cumulative dose of 0.21 × 106 T cells per kilogram of body weight; and patient A-12, who had early relapsed acute myeloid leukemia, received 9 DLIs between day +155 and day +392 of her MUD, which resulted in a cumulative dose of 70.1 × 106 T cells per kilogram of body weight. These DLIs resulted in acute GVHD in all 3 patients and in a sclerodermiform chronic GVHD in 1 patient (A-7). Taken together, the incidence of GVHD was not different from what would be expected after myeloablation without radioimmunotherapy.
Complications, GVHD, and outcome*
| Toxicity . | No. of patients . | ||
|---|---|---|---|
| Group A . | Group B . | All . | |
| Stomatitis | |||
| < 3rd degree | 5 | 15 | 20 |
| 3rd or 4th degree | 9 | 1 | 10 |
| Gastrointestinal tract | |||
| < 3rd degree | 9 | 11 | 20 |
| 3rd or 4th degree | 5 | 5 | 10 |
| Liver† | |||
| < 3rd degree | 8 | 8 | 16 |
| 3rd or 4th degree | 6 | 8 | 14 |
| Kidney‡ | |||
| < 3rd degree | 12 | 12 | 24 |
| 3rd or 4th degree | 2 | 4 | 6 |
| Lung | |||
| < 3rd degree | 12 | 11 | 23 |
| 3rd or 4th degree | 2 | 5 | 7 |
| Heart | |||
| < 3rd degree | 12 | 15 | 27 |
| 3rd or 4th degree | 2 | 1 | 3 |
| Acute GVHD | |||
| < grade 2 | 8 | 11 | 19 |
| Grade 2 | 4 | 5 | 9 |
| Grade 3 | 1 | 0 | 1 |
| Grade 4 | 1 | 0 | 1 |
| Chronic GVHD (in evaluable patients) | |||
| None | 7 | 11 | 18 |
| Limited | 2 | 2 | 4 |
| Extended | 2 | 3 | 5 |
| Rejection and relapse | |||
| Graft rejection | 1 | 1 | 2 |
| Relapse of disease | 6 | 1 | 7 |
| Treatment-related mortality | 2 | 2 | 4 |
| Dead | 5 | 2 | 7 |
| Alive | 9 | 14 | 23 |
| Toxicity . | No. of patients . | ||
|---|---|---|---|
| Group A . | Group B . | All . | |
| Stomatitis | |||
| < 3rd degree | 5 | 15 | 20 |
| 3rd or 4th degree | 9 | 1 | 10 |
| Gastrointestinal tract | |||
| < 3rd degree | 9 | 11 | 20 |
| 3rd or 4th degree | 5 | 5 | 10 |
| Liver† | |||
| < 3rd degree | 8 | 8 | 16 |
| 3rd or 4th degree | 6 | 8 | 14 |
| Kidney‡ | |||
| < 3rd degree | 12 | 12 | 24 |
| 3rd or 4th degree | 2 | 4 | 6 |
| Lung | |||
| < 3rd degree | 12 | 11 | 23 |
| 3rd or 4th degree | 2 | 5 | 7 |
| Heart | |||
| < 3rd degree | 12 | 15 | 27 |
| 3rd or 4th degree | 2 | 1 | 3 |
| Acute GVHD | |||
| < grade 2 | 8 | 11 | 19 |
| Grade 2 | 4 | 5 | 9 |
| Grade 3 | 1 | 0 | 1 |
| Grade 4 | 1 | 0 | 1 |
| Chronic GVHD (in evaluable patients) | |||
| None | 7 | 11 | 18 |
| Limited | 2 | 2 | 4 |
| Extended | 2 | 3 | 5 |
| Rejection and relapse | |||
| Graft rejection | 1 | 1 | 2 |
| Relapse of disease | 6 | 1 | 7 |
| Treatment-related mortality | 2 | 2 | 4 |
| Dead | 5 | 2 | 7 |
| Alive | 9 | 14 | 23 |
Two patients with preexisting liver disease developed fourth-degree toxicity of the liver; there were no other patients with fourth-degree toxicity of the liver.
No fourth-degree toxicity of the kidney was observed.
Causes of death and survival
Four of the 6 patients with relapse died, which resulted in relapse-attributed mortality of 29% in group A. Causes of death not related to relapse were untreatable infection in 1 patient with an apallic syndrome after resuscitation for cardiac arrest (patient A-1), multiorgan failure because of toxicity and aspergillus pneumonia (A-5), adenovirus pneumonia after rejection of the third haploidentical graft (B-3), and an infection in the context of extended chronic GVHD and pancytopenia after MUD transplant (B-6). Altogether, 4 patients (13%) died of causes not related to relapse 1-36 months after HCT, respectively, which emphasizes the low overall toxicity of the radioimmunotherapy approach. Remarkably, none of the 6 patients who received a transplant from an alternative non–HLA-matched donor and only 1 (patient A-1) of 7 patients with previous transplants died of NRM. The correlation of NRM with age, type of disease, graft, or donor was not significant, possibly because of low patient numbers and the heterogeneity of diseases.
Twenty-three (77%) of the 30 patients were alive between 19 and 71 months (median 35 months) after transplantation, with 9 (64%) of 14 patients in group A with malignant diseases and 14 (88%) of 16 patients in group B with nonmalignant diseases surviving. Of the surviving patients, 7 of 9 patients in group A and 13 of 14 patients in group B were free of disease. The resulting probability of survival at 24 months was 0.83 (95% CI 0.63-0.92) in the group as a whole, 0.69 (95% CI 0.37-0.87) in group A, and 0.94 (95% CI 0.63-0.99) in group B. The probability of disease-free survival at 24 months was 0.72 (95% CI 0.52-0.85) in the whole group, 0.46 (95% CI 0.19 to 0.70) in group A, and 0.94 (95% CI 0.63-0.99) in group B. The Kaplan-Maier estimates of overall survival and disease-free survival for groups A and B are shown in Figure 3A-B.
Kaplan-Maier estimates of overall and disease-free survival. (A) Overall survival. (B) Disease-free survival (DFS). Group A is patients who have diseases ( ); group B, patients with nonmalignant diseases (
); group B, patients with nonmalignant diseases ( ).
).
Kaplan-Maier estimates of overall and disease-free survival. (A) Overall survival. (B) Disease-free survival (DFS). Group A is patients who have diseases ( ); group B, patients with nonmalignant diseases (
); group B, patients with nonmalignant diseases ( ).
).
Discussion
In this report, we present a series of pediatric and adolescent patients with malignant or nonmalignant disease treated with radioimmunotherapy with a 90Y-labeled anti-CD66 monoclonal antibody in the context of HCT. The aim of the study was to explore the feasibility and tolerability of this approach in young patients with high risk factors for standard transplantation procedures (advanced disease, high relapse risk, or major pretransplantation complications). We are fully aware of the fact that the limited number and highly heterogeneous nature of the patients treated in this pilot study limit our ability to draw definitive conclusions from our findings. The 3 major findings of the present study are as follows. First, radioimmunotherapy is feasible in young children and adolescents. Second, radioimmunotherapy with 90Y-labeled anti-CD66 antibody treatment in combination with RIC results in consistent myeloablation and stable complete donor chimerism in children with nonmalignant hematologic diseases. This can be achieved with very low toxicity. This is an entirely new finding and has not been reported previously. Third, radioimmunotherapy with 90Y-labeled anti-CD66 antibody treatment in combination with either myeloablative conditioning or RIC can be applied safely in children with malignant diseases who have a very high risk of relapse or mortality after a conventional transplant. The risk of relapse observed is similar to that observed in adults and higher in patients who received RIC.
The primary aim of the present study was to evaluate the feasibility of radioimmunotherapy in children and adolescents. The application of radioimmunotherapy was feasible in all 30 patients regardless of age. The use of 90Y as a therapeutic nuclide enabled us to perform radioimmunotherapy as an outpatient procedure because 90Y, as a pure β emitter, does not expose family members or caregivers to γ irradiation and thus eliminates the need for protective isolation. This constitutes a significant advantage compared with radioimmunotherapy approaches that use other nuclides such as 131I or 188Re, which emit a significant amount of γ irradiation. However, dosimetry in very young patients may require sedation to ensure the collection of valid scintigraphic data. Both the dosimetric and therapeutic infusions of the conjugate were very well tolerated, with no evidence of a cytokine release reaction as reported in the studies by Matthews and colleagues, who used a 131I-labeled anti-CD45 antibody.41,42
The target dose to the marrow of 16-20 Gy was achieved in all patients with nonmalignant diseases, with a highly favorable ratio of marrow dose to normal organ dose. The maximal targeted absorbed dose to the marrow of 30 to 40 Gy in malignant diseases was reached in all but 3 cases. In these 3 cases, the predefined maximum tolerated absorbed dose to the liver (12 Gy) was dose limiting. The biodistribution of the 90Y conjugate was different from that of the 188Re conjugate used in previous studies, with the liver as the normal organ that received the highest dose, not the kidney. This altered dosimetry presumably reflects the superior in vivo stability of the 90Y conjugate.
We observed no excess acute organ toxicity in any of the patient groups. In particular, no cases of radiation nephropathy or veno-occlusive disease were detected. This is in marked contrast to the experience of Bunjes et al21 and Koenecke et al,43 in which radiation nephropathy and veno-occlusive disease were the dose-limiting toxicities when 188Re was used as the therapeutic nuclide.44,45 This low organ toxicity is both remarkable and reassuring if one considers the fact that 22 of the 30 patients were considered to be at high risk for severe toxicity and that this was the primary reason for their inclusion in the study.
Radioimmunotherapy appears not to have increased the incidence of other transplant-related complications such as GVHD or opportunistic infections. The good tolerability of radioimmunotherapy in the setting of myeloablative conditioning and RIC is best reflected in the NRM data. The overall NRM of 13% was considerably lower than predicted (28%-53%) for standard conditioning by the Matthes-Martin risk scores of our cohort of patients. These data on short-term toxicity are clearly encouraging, but obviously, long-term toxicity must be evaluated in larger studies with a longer follow-up.
All patients developed complete marrow aplasia. The median time to engraftment (neutrophils > 500/μL at day 18, platelets > 20 000/μL at day +44 for both groups) appeared to be prolonged compared with conventional conditioning (supplemental Figures 2-3). This raises the issue of whether marrow doses of up to 46 Gy might have resulted in stromal damage. It is reassuring that all surviving patients eventually achieved normal blood counts in line with results of the study by Bunjes et al in adults.21 These authors demonstrated rapid and durable engraftment in all 36 patients in their first published study,21 as well in further studies that used anti-CD66 antibodies with BM doses up to 40 Gy (Donald Bunjes, Ulm University Hospital, oral personal communication June 2010).
Failure of stable engraftment is a major concern in patients with nonmalignant diseases who receive RIC. The addition of radioimmunotherapy with marrow doses between 15 and 20 Gy resulted in stable engraftment and complete donor chimerism in 13 (81%) of 16 patients. Furthermore, the myeloablative nature of radioimmunotherapy could be demonstrated clearly in 1 patient who had developed an autologous recovery with return of the primary disease after her first transplantation and who achieved stable complete donor chimerism with radioimmunotherapy alone at a marrow dose of 17 Gy. Thus, the 90Y-labeled anti-CD66 antibody is a potent myeloablative agent with low intrinsic organ toxicity and therefore a potentially useful tool for the conditioning of this highly vulnerable group of patients.
A further major goal of the present study was to evaluate the safety of selective intensification of standard conditioning and RIC in patients with malignant diseases who are at very high risk of relapse after a conventional transplant. Our study demonstrates that radioimmunotherapy with our conjugate can be used safely to target doses of 30-40 Gy to the marrow in children. Because antileukemic efficacy was not the primary end point of this phase 2 study, only a few preliminary remarks with respect to this issue can be made. Radioimmunotherapy appeared to be reasonably effective in reducing the rate of relapse in the subgroup of patients grafted with a low tumor burden at transplantation, similar to the experience of Bunjes et al.21 This to some extent reflects the fact that radioimmunotherapy exerts its antileukemic effect via the cross-fire effect, and in the case of the anti-CD66 antibody, this requires the presence of a large number of binding sites on normal granulopoietic cells in the marrow, that is, a marrow in complete remission or good partial remission. The same mechanisms explain why radioimmunotherapy with anti-CD66 is unlikely to be effective at extramedullary sites, hence the significant incidence of extramedullary relapse in both the present study and the study by Bunjes et al.21 Finally, these data indicate that radioimmunotherapy at the marrow doses achieved in the present study may be less effective in the RIC than in the myeloablative setting. A similar experience has been reported by Ringhoffer et al,23 Koenecke et al,43 and Lauter et al46 using the 188Re-labeled anti-CD66 antibody in adults.
In summary, radioimmunotherapy is a feasible therapeutic approach in adolescents and children, even very young ones. Its short-term toxicity profile appears to be favorable. Further studies should be performed in the setting of both nonmalignant and malignant diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sandra Steinmann for excellent data management, Dr Klaus Schwarz and Professor Tim Niehues for many helpful discussions, Dr Thomas Kull for dosimetric assistance, the staff of the radiochemistry department for the production of the labeled antibody, and the staff of the pediatric transplant ward for their open mind to accept this new treatment modality.
This work was supported in part by grants from the Deutsche José Carreras Leukämie-Stiftung e.V. (DJCLS00/09 to A.S.S., M.S.-S., K.-M.D., S.R., and W.F.).
Authorship
Contribution: A.S.S. designed and performed the research, analyzed data, and wrote the paper; G.G. designed and performed research, contributed vital analytical tools, and analyzed data; M.H., C.S., S.A.G., S.G., and M.S.-S. performed research; R.M. analyzed data; G.K. and M.S. performed research; D.B. designed research and wrote the paper; K.-M.D. designed research; S.R. designed research and contributed new reagents and analytical tools; and W.F. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ansgar S. Schulz, Department of Pediatrics and Adolescent Medicine, University Hospital Ulm, Eythstr 24, D-89075 Ulm, Germany; e-mail: ansgar.schulz@uniklinik-ulm.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal