Abstract
We describe the application of a novel, bispecific antibody platform termed dual affinity retargeting (DART) to eradicate B-cell lymphoma through coengagement of the B cell–specific antigen CD19 and the TCR/CD3 complex on effector T cells. Comparison with a single-chain, bispecific antibody bearing identical CD19 and CD3 antibody Fv sequences revealed DART molecules to be more potent in directing B-cell lysis. The enhanced activity with the CD19xCD3 DART molecules was observed on all CD19-expressing target B cells evaluated using resting and prestimulated human PBMCs or purified effector T-cell populations. Characterization of a CD19xTCR bispecific DART molecule revealed equivalent potency with the CD19xCD3 DART molecule, demonstrating flexibility of the DART structure to support T-cell/B-cell associations for redirected T cell–killing applications. The enhanced level of killing mediated by DART molecules was not accompanied by any increase in nonspecific T-cell activation or lysis of CD19− cells. Cell-association studies indicated that the DART architecture is well suited for maintaining cell-to-cell contact, apparently contributing to the high level of target cell killing. Finally, the ability of the CD19xTCR DART to inhibit B-cell lymphoma in NOD/SCID mice when coadministered with human PBMCs supports further evaluation of DART molecules for the treatment of B-cell malignancies.
Introduction
Targeted mAb-based therapies provide effective and safe treatments for hematologic malignancies. Rituximab, which specifically targets the B-cell antigen CD20, has had the greatest success, revolutionizing the treatment of the 2 most common forms of nonHodgkin lymphoma: follicular and diffuse large B-cell lymphoma. In addition, mAb-based therapies targeting CD52 (alemtuzumab) and CD33 (gemtuzumab ozogamicin) have been approved for the treatment of chronic lymphocytic leukemia and acute myelogenous leukemia, respectively. Despite the progress of these strategies, they do have limitations. Only a fraction of patients respond to rituximab, and the majority of those who do respond will eventually relapse. Treatment with alemtuzumab and gemtuzumab are limited by safety concerns, and many additional hematologic malignancies do not respond to treatment with any of these targeted therapies. Various therapies based on alternate mAbs, including second-generation anti-CD20 mAbs and those targeting alternate cell-surface proteins such as CD19, CD22, CD30, CD37, CD40, and CD74, have been developed and are at different stages of clinical testing in the hopes of providing approaches to treating a broader spectrum of hematologic malignancies that are poorly served by existing therapies.1,2
Whereas targeting of cell-surface antigens themselves can mediate antitumor activity through the induction of apoptosis, most mAb-based activity against hematologic malignancies is reliant on either Fc-mediated effector functions such as complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity3,4 or is engineered through the conjugation of an immunotoxin or radiolabeled isotope.1 Considering the potential of naturally occurring CTLs to mediate cell lysis, various strategies have also been explored to recruit CTLs to mediate tumor cell killing. Tumor-specific CTLs exert extremely potent effects through recognition of the corresponding peptide/MHC complex recognized by their TCR, and are among the most potent cells that mediate antitumor effects. A major limitation in generating tumor-specific CTLs in vivo is that their induction requires the use of vaccine strategies, such as dendritic cell–based vaccines,5 that are capable of breaking tolerance to cancer self-antigens. One alternative is ex vivo expansion and activation of rare, tumor-specific CTLs for reinfusion into cancer patients.6 However, cancer cells can down-regulate MHC expression as an escape mechanism, thus preventing the ability of CTLs to recognize their antigenic peptide. The genetic manipulation of patients' T cells to express chimeric antigen receptors comprising a tumor-specific antigen and T cell–activating properties before their adoptive transfer provides a non–MHC-restricted approach to targeting cancer, as was shown recently in the treatment of lymphoma with T cells engineered to recognize CD19.7 However, the patient-specific manipulation and risk associated with this procedure represent major limitations to its expanded use. Alternate strategies are therefore required that can overcome the above limitations and take full advantage of the cytotoxic power that T lymphocytes can exercise against cancer cells without requiring ex vivo expansion. Bispecific antibodies/fragments that can simultaneously recognize tumor antigens and invariant epitopes of the CD3/TCR complex have been shown to effectively recruit T lymphocytes to tumor targets in a noncognate fashion, effectively bypassing the MHC context required for antigen recognition by the TCR and theoretically enabling all T cells to be redirected against the tumor.
Various strategies have been developed to generate bispecific molecules capable of T-cell recruitment, including heterohybridomas,8 bispecific diabodies,9,10 and bispecific single-chain antibody molecules.11,12 Catumaxomab, a rat/murine hybrid antibody designed to coengage EpCAM on tumor cells and CD3 on T cells, was recently approved for the treatment of malignant ascites, providing clinical validation of redirected T-cell killing for therapeutic benefit.13 Regarding single-chain Fv (scFv) molecules, both monomeric (1 binding site per specificity) or homodimeric (2 sites per specificity) structures can be built by varying the VL/VH order and linker sequences. The first approach has been used to construct Bi-specific T-cell Engager (BiTE) molecules,12 and the latter strategy is the basis of a format in which 2 molecules self-assemble to form a tetravalent tandem diabody structure called tandab.14 Head-to-head comparison of these platforms in the context of CD19xCD3 bispecific molecules demonstrated the BiTE protein to be significantly more potent than the equivalent tandab.15 The CD19xCD3 BiTE molecule (blinatumomab) was further evaluated in chimpanzees, and significant B-cell depletion was observed.16 Moreover, results from early clinical studies of blinatumomab have demonstrated impressive response rates in both relapsing nonHodgkin lymphoma and B-cell acute lymphoblastic leukemia patients, supporting further evaluation of this potential therapeutic avenue.17
Despite this success, scFv-based bispecific strategies have limitations, including constraints imparted by the linker sequences that connect the V regions in a “nonnatural” manner, resulting in reduced or altered antigen recognition and potency. Furthermore, scFv-based constructs have a tendency to form aggregates due to “domain exchange” of the V regions with partners from other molecules. To address the functional and structural limitations of existing bispecific molecules, we have developed an alternative bispecific antibody platform called dual affinity retargeting (DART). In DART proteins, each Fv is formed by the association of a VL partner on one chain with a VH partner on the second chain in a VLA-VHB + VLB-VHA configuration. This configuration lacks the constraint of an intervening linker sequence and therefore is more analogous to the natural association in an IgG molecule. The covalent linkage between the 2 chains limits the freedom of the component Fv domains to undergo domain exchange, resulting in a high degree of stability that is independent of the strength of the VL-VH interface. Recent studies have demonstrated successful application of DART molecules for the targeting of B cells through either recruitment of natural killer cells18 or by co-ligation of inhibitory and activating receptor pathways.19 In the present study, we have explored the potential for DART molecules to recruit the cytolytic activity of human T cells against B-cell lymphoma using either CD3 or TCRαβ specificities as effector arms and CD19 as the tumor target. For the purpose of direct comparison, we also constructed a CD19xCD3 BiTE protein based on published sequences such that the Fv sequences of the CD19xCD3 DART and BiTE molecules were identical. Finally, the CD19xTCR DART molecule was tested for its ability to mediate tumor cytolysis in xenograft models.
Methods
Construction, expression, and purification of DART and BiTE molecules
DART molecules consist of 2 distinct polypeptides that are coexpressed to generate a covalently linked heterodimeric complex with one binding site for each of 2 specificities. DART molecules may contain either of 2 alternative carboxyterminal heterodimerization domains: a pairing of VEPKSC on one chain and FNRGEC on the other or a pairing of oppositely charged, coiled-coil domains.19,20 The CD19xCD3 DART molecule was constructed using anti-CD19 Fv sequences from HD37 and anti-CD3 Fv sequences from TR66.21,22 The CD19xCD3 BiTE molecule was constructed using the same antibody sequences with linkers from blinatumomab/MT103, as described previously.22,23 These Fv sequences are identical to those used to construct the DART molecule. HER2xTCR and 4420xTCR DART molecules were similarly constructed using anti-HER2 sequences derived from 4D5, the murine mAb from which trastuzumab is derived24 ; anti-fluorescein sequences from 4-4-2025 ; and anti-TCR sequences from hBMA031, a derivative of BMA03126,27 that was humanized by CDR grafting.28 DART- and BiTE-encoding sequences were cloned into CET1019A(S/D) UCOE vectors (Millipore). Chinese hamster ovary (CHO)-S cells (Invitrogen) were transfected, and stable expressing populations were selected and grown in fed-batch mode. Anti-CD19 and anti-CD3 proteins were purified using CD19 and CD3, respectively, coupled to CNBr-activated Sepharose (GE Healthcare). The 4420xTCR DART molecule was purified using IgG Sepharose (GE Healthcare) that was fluoresceinated using fluorescein isothiocyanate (Pierce). Proteins were further purified by size-exclusion chromatography (SEC) using a Superdex 200HR 10/30 (GE Healthcare) column equilibrated in PBS. Purified proteins were analyzed by SDS-PAGE and SEC. Reducing and nonreducing SDS-PAGE were performed using the NuPAGE Bis-Tris system (Invitrogen). SEC was used to analyze the approximate size and heterogeneity of the purified molecules. The Superdex 200HR 10/30 column (GE Healthcare) equilibrated with PBS was used to perform the analysis.
Bispecific ELISA
A MaxiSorp ELISA plate (Nunc) coated with the soluble human CD3ϵ/δ heterodimer (2 μg/mL) and incubated in BupH bicarbonate buffer (Thermo Scientific) overnight at 4°C was blocked with PBS (0.5% BSA; 0.1% Tween-20) (PBST/BSA) for 30 minutes at room temperature. DART or BiTE molecules were applied, followed by the sequential addition of sCD19-Fc (100 ng/mL), and goat anti–human IgG Fc-specific antibody (130 ng/mL; Jackson ImmunoResearch), each diluted in PBST/BSA and bound for 1 hour at room temperature after intervening plate washing. The immune complex was detected with SuperSignal ELISA Pico chemiluminescent substrate (Thermo Scientific). sCD19-Fc protein was purified from CHO-S cells expressing hCD19 (aa 1-291) fused to the human IgG1 hinge/Fc region.
SPR analysis
Binding of bispecific antibody variants to corresponding ectodomains of human CD3 and human CD19 was analyzed by surface plasmon resonance (SPR) using a BIAcore 3000 biosensor (GE Healthcare). The antigens hCD3 and hCD19-Fc fusion were immobilized on the CM-5 sensor chip using the amine coupling kit as recommended by the manufacturer at the immobilization level (∼ 500 and ∼ 1000 RU, respectively). Binding experiments were performed in HBS-EP buffer (10mM HEPES, pH 7.4, 150mM NaCl, 3mM EDTA, and 0.005% P20 surfactant). Corresponding bispecific antibodies were injected in duplicate at a flow rate of 30 μL/min for 60 seconds with a dissociation time of 180 seconds. Regeneration of antigen surfaces was performed by pulse injection of 10mM glycine, pH 1.5.
Flow cytometric analysis of fresh blood
Human whole blood (Lonza) was stained with 5 μg/mL CD19xTCR or 4420xTCR. After red blood cell removal using FACS Lysing Solution (BD Biosciences), cells were stained with 1 μg/mL biotinylated 1F5 mAb (MacroGenics), which recognizes an epitope in the DART coiled-coil heterodimerization domain, along with relevant T- and B-cell markers, anti–CD3-peridinin chlorophyll A protein (perCP), and anti–CD22-phyco-erythrin (PE) (BD Biosciences). The 1F5 mAb–positive cells were detected with streptavidin-allophycocyanin (APC) and analyzed with the FACSCalibur flow cytometer (BD Biosciences).
Cell-killing assays
All cell lines were obtained from ATCC (Manassas, VA). PBMCs were isolated from healthy donor blood using the Ficoll-Paque Plus kit (GE Healthcare), and CD8+ T cells were purified using a T-cell isolation kit (StemCell Technologies). B-lymphoma target cells (4 × 105 cells/mL) were treated with serial dilutions of CD19xCD3 BiTE, CD19xCD3 DART, or a control molecule, together with resting human PBMCs (ratio of effector cells to target cells [E:T] = 25-30:1) or IL-2–activated human PBMCs (E:T = 15:1), incubated at 37°C overnight, and lactate dehydrogenase (LDH) release was measured (Promega). For studies with purified T cells, target cells were labeled with CFSE and cell killing was monitored by propidium iodide staining using FACSCalibur flow cytometry. Data were processed and the percentage of cytotoxicity was analyzed using GraphPad Prism5 software.
CD69 expression and cytokine detection
Human T cells were purified from PBMCs, combined with 3 × 105 cells/mL Raji B lymphoma target cells (E:T = 5:1), and treated overnight with bispecific antibodies at 37°C. Cells were analyzed by flow cytometry using αCD4-APC, αCD8-FITC, and αCD69-PE mAbs (BD Biosciences), and then secreted IFNγ was detected using a cytometric bead array cytokine kit (BD Biosciences).
In vitro autologous B-cell depletion
Purified human PBMCs (100μL resuspended at 2 × 106 cells/mL) were incubated with titrated test articles for 18 hours at 37°C, and the B-lymphocyte subset was stained with APC-labeled anti-CD20 antibody (BD Biosciences), FITC-labeled annexin V, and propidium iodide (R&D Systems) for 30 minutes. Differentiation between live and dead CD20+ B cells was quantified using FACSCalibur flow cytometry.
T cell–proliferation assays
The proliferation of unstimulated human PBMCs in response to the addition of DART or BiTE molecules was measured in a standard 3H-thymidine proliferation assay, as described in Loffler et al.23 After a 24-hour incubation of human PBMCs (1 × 106 cells/mL) with bispecific antibody, cells were pulse-labeled for 18 hours with 3H-thymidine, harvested, and assayed using a TopCount (PerkinElmer). As controls, the same PBMCs depleted of B cells using Dynabeads CD19 pan B (Invitrogen) were used.
Cell-to-cell association assay
For CD19xCD3 DART- and BiTE-molecule–mediated co-binding assays, cells from the CD19-expressing Daudi cell line were labeled with CellTrace CFSE (Invitrogen). Simultaneously, the CD3-expressing T-cell line Jurkat was loaded with PKH26 dye using a cell membrane–labeling kit (Sigma). Cells (5 × 106/mL) from each line were resuspended in PBS and mixed in a 1:1 ratio in the presence of various amounts of DART or BiTE molecules for 30 minutes at room temperature. Cobinding was measured using flow cytometry and was quantified as the percentage of positive cells in the upper right quadrant of FL1 compared with FL2, representing the CFSE+PKH26+ double-positive population.
Human PBMC reconstitution xenograft studies
NOD/SCID female mice (Taconic Farm) 7-8 weeks old were randomly divided into groups of 6-8, and inoculated subcutaneously (left flank) with 1 × 106 Raji cells ± 5 × 106 human PBMCs in 50% Matrigel. One hour later, each mouse received test molecules or vehicle intravenously. An additional dose was administered on each of the 4 following days (5 doses total). Tumor dimensions were scored 1 week after the initial administration of test molecules. The arithmetic average of tumor dimensions from each group was plotted against time.
Results
Generation and characterization of CD19xCD3-bispecific antibodies
Prior studies using various bispecific antibody–based molecules have demonstrated that a broad spectrum of B-cell lymphomas can be targeted for elimination by T cells through the coengagement of CD3 on T cells and CD19 expressed on B cells.14,23,29 To determine the applicability of DART molecules to support redirected T-cell killing and to allow comparison with previously tested bispecific platforms, a CD19xCD3 DART molecule was constructed. The most advanced CD19xCD3 bispecific molecule in clinical development is blinatumomab; therefore, a DART molecule was generated using anti-CD3 and anti-CD19 variable antibody regions identical to those contained in blinatumomab (Figure 1). To gauge the bispecific binding properties and cellular activity of the CD19xCD3 DART molecule, a cognate CD19xCD3 single-chain BiTE molecule (blinatumomab) was generated in parallel to serve as a reference. Compared with the BiTE molecule, which is composed of 2 single-chain Fvs connected by a short linker, the DART molecule is a covalently linked bispecific diabody designed to overcome the limitations of prior bispecific diabodies (Figure 1A).18 Each bispecific molecule was expressed in CHO and purified to homogeneity using antigen-affinity (sCD3) and size-exclusion chromatography (Figure 1B). SDS-PAGE analysis under nonreducing conditions indicated that both BiTE and DART molecules migrate close to 50 kDa, with the majority of the DART molecules being covalently linked. Under reducing conditions, the molecules migrated as anticipated, with the separate DART chains co-migrating around 26 kDa, whereas the single-chain BiTE molecule maintained migration at 50 kDa (Figure 1C). As an alternative strategy, the BiTE molecule was purified using metal-affinity chromatography and SEC. In all assays, this material behaved identically to the CD3 affinity-purified material (data not shown). Both the CD19xCD3 BiTE and DART molecules exhibited dual specific binding in an ELISA format, with the DART molecule demonstrating slightly enhanced binding (Figure 1D). Consistent with this observation, SPR analyses revealed increased affinity of the DART molecule for both CD3 and CD19 compared with the BiTE molecule (Table 1). Equilibrium dissociation constants (Kds) obtained for BiTE-hCD19 (1.3 × 10−9M) and BiTE-hCD3 (2.63 × 10−7M) molecule interactions were consistent with those published for the BiTE molecule interaction with Nalm6 and purified T cells (1.49 × 10−9M and 2.67 × 10−7M, respectively).22 The approximately 2-fold decrease in the Kd for DART interactions with corresponding antigens was mainly due to an increased association rate for hCD3 binding and a lower dissociation rate for hCD19 binding.
CD19xCD3 DART and BiTE molecules are highly purified and capable of simultaneous engagement of both targets. (A) Schematic representation of DART (left) and BiTE (right) molecules. The DART molecule consists of 2 covalently linked polypeptide chains that heterodimerize to generate 2 specificities. The BiTE molecule is a single polypeptide that assembles to form 2 scFv domains joined by a short linker. (B) DART and BiTE molecules were purified by affinity chromatography followed by SEC. Purified material forms a single peak, as demonstrated by analytical SEC. Elution volume versus absorbance (mAU, milliabsorbance units) is plotted for both molecules. The approximately 15-mL elution volume is consistent with a 50-kDa molecular mass predicted for both DART and BiTE molecules. The black trace is the DART molecule and the gray trace is the BiTE molecule. (C) Nonreducing and reducing SDS-PAGE of purified molecules. Under nonreduced conditions, both DART and BiTE molecules migrate at approximately 50 kDa. A small amount of reduced material can be detected for the DART molecule around 25 kDa. However, these chains are associated in a dimeric complex, as indicated by the SEC profile. Under reducing conditions, the individual chains of the DART molecule migrate at approximately 25 kDa, whereas the single chain of the BiTE molecule remains at approximately 50 kDa. (D) Bispecific ELISA demonstrates simultaneous engagement of both target antigens by the molecules. ELISA plates were coated with soluble human CD3ϵ/δ, then the DART or BiTE molecule was applied at the indicated concentrations. Finally, soluble human CD19-Fc fusion protein was bound, detected with peroxidase-conjugated goat anti–human Fc polyclonal antibody, and developed using a chemiluminescent HRP substrate. The black trace is the DART molecule and the gray trace is the BiTE molecule.
CD19xCD3 DART and BiTE molecules are highly purified and capable of simultaneous engagement of both targets. (A) Schematic representation of DART (left) and BiTE (right) molecules. The DART molecule consists of 2 covalently linked polypeptide chains that heterodimerize to generate 2 specificities. The BiTE molecule is a single polypeptide that assembles to form 2 scFv domains joined by a short linker. (B) DART and BiTE molecules were purified by affinity chromatography followed by SEC. Purified material forms a single peak, as demonstrated by analytical SEC. Elution volume versus absorbance (mAU, milliabsorbance units) is plotted for both molecules. The approximately 15-mL elution volume is consistent with a 50-kDa molecular mass predicted for both DART and BiTE molecules. The black trace is the DART molecule and the gray trace is the BiTE molecule. (C) Nonreducing and reducing SDS-PAGE of purified molecules. Under nonreduced conditions, both DART and BiTE molecules migrate at approximately 50 kDa. A small amount of reduced material can be detected for the DART molecule around 25 kDa. However, these chains are associated in a dimeric complex, as indicated by the SEC profile. Under reducing conditions, the individual chains of the DART molecule migrate at approximately 25 kDa, whereas the single chain of the BiTE molecule remains at approximately 50 kDa. (D) Bispecific ELISA demonstrates simultaneous engagement of both target antigens by the molecules. ELISA plates were coated with soluble human CD3ϵ/δ, then the DART or BiTE molecule was applied at the indicated concentrations. Finally, soluble human CD19-Fc fusion protein was bound, detected with peroxidase-conjugated goat anti–human Fc polyclonal antibody, and developed using a chemiluminescent HRP substrate. The black trace is the DART molecule and the gray trace is the BiTE molecule.
Affinity measurements of the CD19xCD3 BiTE molecule and the CD19xCD3 DART molecule
| Protein . | CD3 interaction . | CD19 interaction . | ||||
|---|---|---|---|---|---|---|
| ka, 1/Ms . | kd, 1/s . | Kd, nM . | ka, 1/Ms . | kd, 1/s . | Kd, nM . | |
| CD19xCD3DART molecule | 3.2 × 106 | 0.4 | 125 | 1.2 × 106 | 0.8 × 10−3 | 0.7 |
| CD19xCD3BiTE molecule | 1.9 × 106 | 0.5 | 263 | 1.2 × 106 | 1.5 × 10−3 | 1.3 |
| Protein . | CD3 interaction . | CD19 interaction . | ||||
|---|---|---|---|---|---|---|
| ka, 1/Ms . | kd, 1/s . | Kd, nM . | ka, 1/Ms . | kd, 1/s . | Kd, nM . | |
| CD19xCD3DART molecule | 3.2 × 106 | 0.4 | 125 | 1.2 × 106 | 0.8 × 10−3 | 0.7 |
| CD19xCD3BiTE molecule | 1.9 × 106 | 0.5 | 263 | 1.2 × 106 | 1.5 × 10−3 | 1.3 |
Bispecific proteins at concentrations of 0, 12.5, 25, 50, 100, and 200nM were injected over immobilized antigens. Binding curves were analyzed using BIAevaluation 4.1 software. The association rate constant (ka) and the dissociation rate constant (kd) were estimated by global fitting analysis of the association/dissociation curves to the 1:1 Langmuir interaction model. Kd was calculated as Kd = kd/ka. Data are representative of at least 2 independent experiments.
CD19xCD3 DART molecule exhibits potent redirected killing of B-cell lymphoma
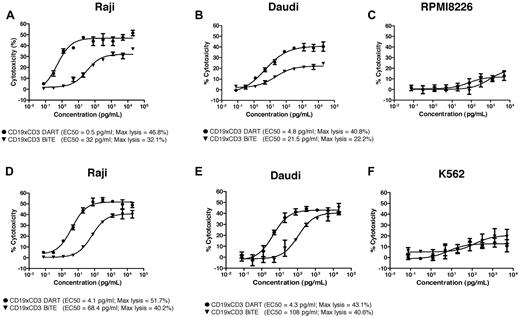
The CD19xCD3 DART molecule was evaluated for its ability to redirect immune cell killing against CD19-expressing malignant B-cell lines using either freshly isolated human PMBCs or IL-2 activated PBMCs as the effector cell population. Experiments performed using multiple independent-donor PBMCs revealed the CD19xCD3 DART molecule to be more potent than the CD19xCD3 BiTE molecule (representative donor data shown in Figure 2). With freshly isolated resting human PBMCs, the maximal level of cytotoxicity mediated by the DART molecule was considerably greater than that mediated by the BiTE molecule, whereas the concentration required to obtain 50% of maximal activity (EC50) was up to 60-fold lower (Figure 2A-B). Analyses performed with IL-2–activated human PBMCs confirmed the DART molecule to be more potent than the BiTE molecule, with a 16-25 times lower EC50, although there was less improvement in the overall level of cell lysis compared with that observed in resting PBMCs (Figure 2D-E). Neither molecule mediated lysis of CD19− cell lines with either resting or activated PBMCs (Figure 2).
CD19xCD3 DART molecule mediates enhanced redirected killing of B-cell lymphoma. CD19+ cells (Raji or Daudi) or CD19− cells (RPMI-8226 or K562) were seeded in 96-well plates. Human resting PBMCs (A-C) or IL-2–activated PBMCs (D-F) and serial dilutions of the CD19-CD3 DART molecule (●) or the CD19-CD3 BiTE molecule (▾) were added to the plate and incubated overnight. Cytotoxicity was then detected by LDH release. EC50 values and maximum lysis were determined using GraphPad Prism software. The data are representative of multiple independent experiments using independent human PBMC donors.
CD19xCD3 DART molecule mediates enhanced redirected killing of B-cell lymphoma. CD19+ cells (Raji or Daudi) or CD19− cells (RPMI-8226 or K562) were seeded in 96-well plates. Human resting PBMCs (A-C) or IL-2–activated PBMCs (D-F) and serial dilutions of the CD19-CD3 DART molecule (●) or the CD19-CD3 BiTE molecule (▾) were added to the plate and incubated overnight. Cytotoxicity was then detected by LDH release. EC50 values and maximum lysis were determined using GraphPad Prism software. The data are representative of multiple independent experiments using independent human PBMC donors.
The CD19xTCR DART molecule displays potent B-cell lymphoma killing and T-cell activation properties similar to the CD19xCD3 DART molecule
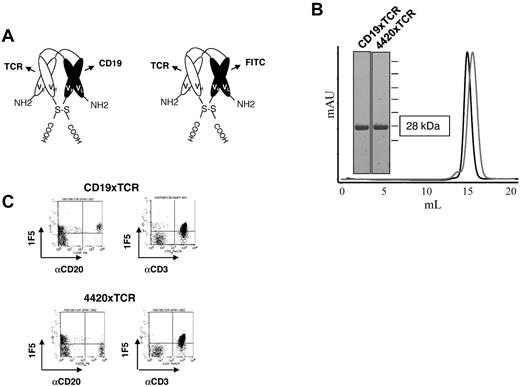
To evaluate the flexibility of DART-based molecules in redirecting T-cell killing and to explore the feasibility of using an alternative T-cell antigen, DART molecules were constructed comprising T-cell binding specificity for the TCRαβ subunits of the TCR. Specifically, a humanized Fv region derived from the murine BMA031 antibody26,27 that binds to an invariant region of the TCRαβ subunits of the TCR was used to engage the TCR. Two independent DART molecules were generated: a CD19xTCR DART molecule comprising bispecificity for CD19 and TCRαβ and a 4420xTCR DART molecule comprising specificity for fluorescein and TCRαβ (Figure 3A-B). To evaluate their cellular-binding specificities, the molecules were evaluated by flow cytometry for binding to T cells and B cells. As expected, the CD19xTCR DART molecule exhibited specificity for both B cells and T cells, whereas the 4420xTCR DART molecule only bound T cells (Figure 3C).
CD19xTCR DART molecule is highly purified and binds both of its target cells. (A) Schematic representation of the CD19xTCR DART molecule and a control DART molecule (4420xTCR) that binds TCR and fluorescein. (B) DART molecules were purified by affinity chromatography followed by SEC. Reducing SDS-PAGE and analytical SEC demonstrates proper assembly and a high degree of purity. The black trace is the CD19xTCR DART molecule and the gray trace is the 4420xTCR DART molecule. (C) Flow cytometric analyses of human PBMCs revealed bispecific binding of the CD19xTCR DART molecule (detected by biotinylated 1F5 mAb and streptavidin-APC) to both B cells (CD20) and T cells (CD3), whereas the 4420xTCR DART molecule displays binding only to T cells.
CD19xTCR DART molecule is highly purified and binds both of its target cells. (A) Schematic representation of the CD19xTCR DART molecule and a control DART molecule (4420xTCR) that binds TCR and fluorescein. (B) DART molecules were purified by affinity chromatography followed by SEC. Reducing SDS-PAGE and analytical SEC demonstrates proper assembly and a high degree of purity. The black trace is the CD19xTCR DART molecule and the gray trace is the 4420xTCR DART molecule. (C) Flow cytometric analyses of human PBMCs revealed bispecific binding of the CD19xTCR DART molecule (detected by biotinylated 1F5 mAb and streptavidin-APC) to both B cells (CD20) and T cells (CD3), whereas the 4420xTCR DART molecule displays binding only to T cells.
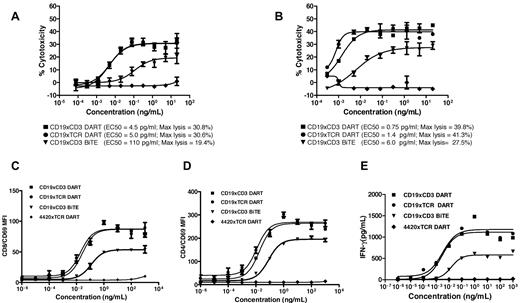
Evaluation of the CD19xTCR DART molecule to redirect immune cell killing revealed it to have essentially overlapping potency with the CD19xCD3 DART molecule regardless of target cell population, effector cell population, or the E:T (Figure 4). With resting PBMCs as the effector population, both DART molecules displayed equally potent killing of the Raji (Figure 4A) or Daudi cell lines (see supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), which was greater than that mediated by the CD19xCD3 BiTE molecule, based both on the maximal level of lymphoma cell lysis and on the EC50. The enhanced level of potency mediated by the DART molecule on Raji cells was independent of the E:T (supplemental Figure 1B). A similar pattern of response to the DART molecule was also observed against Raji cells using IL-2–activated CD8 T cells (Figure 4B). As anticipated, neither CD19xTCR- nor CD19xCD3-based bispecific molecules mediated T-cell killing of a non-CD19-expressing cell line (RPMI-8226; data not shown). Analyses of T-cell activation revealed similar responses mediated by each DART molecule, as measured by CD69 induction on either CD4 and CD8 T-cell subsets (Figure 4C-D) or IFNγ release (Figure 4E). Consistent with the redirected killing data, the level of T-cell activation mediated by the DART molecules was greater than that observed by the BiTE molecule. No cell killing, T-cell activation, or cytokine release was observed with the 4420xTCR DART molecule in any of the assays, supporting the requirement for co-ligation of both TCR and CD19 to obtain cell killing.
Enhanced redirected immune cell killing and T-cell activation by the DART molecule. The CD19xTCR DART molecule (■), CD19xCD3 DART molecule (●), CD19xCD3 BiTE molecule (▾), and control DART molecule (♦) were evaluated for cytotoxicity against Raji B-cell lymphoma cells in the presence of either resting human PBMCs (A) or purified activated T cells (B), for induction of CD69 on purified T cells in the presence of Raji B-cell lymphoma cells on either the CD4+ T-cell subset (C) or the CD8+ T-cell subset (D), and for their ability to mediate IFNγ release (E) in the presence of Raji target cells and purified T cells. Cytotoxicity was detected using LDH release (A) or FACS-based analysis (B), with EC50 values and maximum lysis determined using GraphPad Prism software. The data are representative of multiple independent experiments using independent human PBMC donors or purified T-cell populations.
Enhanced redirected immune cell killing and T-cell activation by the DART molecule. The CD19xTCR DART molecule (■), CD19xCD3 DART molecule (●), CD19xCD3 BiTE molecule (▾), and control DART molecule (♦) were evaluated for cytotoxicity against Raji B-cell lymphoma cells in the presence of either resting human PBMCs (A) or purified activated T cells (B), for induction of CD69 on purified T cells in the presence of Raji B-cell lymphoma cells on either the CD4+ T-cell subset (C) or the CD8+ T-cell subset (D), and for their ability to mediate IFNγ release (E) in the presence of Raji target cells and purified T cells. Cytotoxicity was detected using LDH release (A) or FACS-based analysis (B), with EC50 values and maximum lysis determined using GraphPad Prism software. The data are representative of multiple independent experiments using independent human PBMC donors or purified T-cell populations.
CD19-directed DART molecules mediate autologous B-cell depletion, with T-cell activation dependent on target engagement
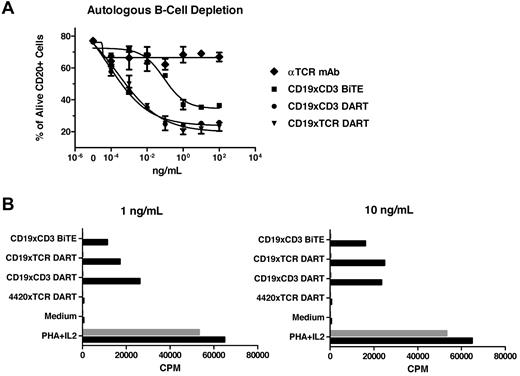
Because CD19 is expressed on normal peripheral B cells, the ability of DART molecules to mediate autologous B-cell depletion was evaluated on human PBMCs. Consistent with their ability to redirect killing of lymphoma cells, both the CD19xCD3 DART molecule and the CD19xTCR DART molecule mediated essentially the same level of autologous B-cell depletion, as measured by reduced levels of CD20+ cells (Figure 5A). The level of B-cell depletion observed with the DART molecule was greater than that mediated by the BiTE molecule, based on the maximal level of depletion and the reduced EC50, again consistent with the enhanced activity observed with the DART molecule on B-cell lymphoma. In contrast, the parental TCR mAb or 4420xTCR DART molecule (data not shown) mediated no reduction in B-cell number, indicating the requirement for B-cell engagement to obtain depletion. The ability of the bispecific molecules to induce T-cell proliferation was also examined in PBMCs (Figure 5B). Both the CD19-based DART molecule and the CD19xCD3 BiTE molecule induced T-cell proliferation. In contrast, the 4420xTCR DART molecule did not mediate any increase in T-cell proliferation, demonstrating that monovalent interaction with the TCR alone is insufficient to induce T-cell proliferation and requires a bivalent interaction provided by interaction of the second Fv region with a cell-surface antigen. To demonstrate directly that the CD19xCD3 bispecific antibodies only induce T-cell proliferation when coengaged with CD19, human PBMCs depleted of B cells were also evaluated, and no T-cell proliferation was observed with either the CD19-based DART molecule or the BiTE molecule.
Autologous B-cell depletion by the DART molecule and dependence on coengagement for T-cell proliferation. (A) The ability of the DART molecules to mediate autologous B-cell depletion was determined by incubating healthy human donor PBMCs with the panel of the indicated proteins for 18 hours and determining the percentage of viable CD20+ B cells by FACS. (B) Standard 3H-thymidine proliferation assay using unstimulated primary human PBMCs before (black bars) and after (gray bars) depletion of B cells by immunomagnetic beads. The data are representative of multiple independent experiments using independent healthy human PBMC donors. Phytohemagglutinin (PHA) and IL-2 were used as positive controls for stimulation.
Autologous B-cell depletion by the DART molecule and dependence on coengagement for T-cell proliferation. (A) The ability of the DART molecules to mediate autologous B-cell depletion was determined by incubating healthy human donor PBMCs with the panel of the indicated proteins for 18 hours and determining the percentage of viable CD20+ B cells by FACS. (B) Standard 3H-thymidine proliferation assay using unstimulated primary human PBMCs before (black bars) and after (gray bars) depletion of B cells by immunomagnetic beads. The data are representative of multiple independent experiments using independent healthy human PBMC donors. Phytohemagglutinin (PHA) and IL-2 were used as positive controls for stimulation.
DART molecules mediate enhance cell-cell association
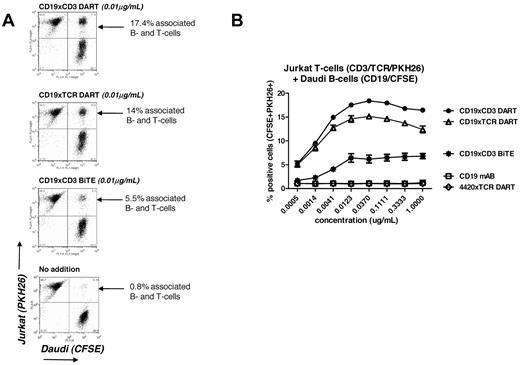
To investigate further the improvement in mediating redirected immune killing obtained with the CD19-based DART molecules, we evaluated their ability to mediate B-cell/T-cell association. We used Jurkat T cells as the CD3/TCR–expressing cell line and Daudi as the CD19-expressing B-cell line. Jurkat T cells were selected because they do not mediate cell killing but do express CD3 and TCR, and therefore evaluation of the cell-cell association can be made without the confounding complication of T-cell–mediated killing. Jurkat cells labeled with PHK26 were mixed with Daudi cells labeled with CFSE, and the level of B-cell/T-cell–associated cell complexes was determined by flow cytometric analyses of the population, which was positive for both PHK26 and CFSE fluorescent dyes compared with the respective (uncomplexed) single-positive populations (Figure 6). A clear cell-cell association was observed with both CD19-based DART molecules and the CD19-based BiTE molecule in the presence of 10 ng/mL of bispecific antibody (Figure 6A). With the CD19xCD3 and CD19xTCR DART molecules, up to 15%-17% of the total cell population was observed to be cell-cell associated. In contrast, only 5%-6% of the mixed Jurkat/Daudi cell population was found to be associated in the presence of the CD19xCD3 BiTE molecule (Figure 6A). The enhanced level of cell-cell association mediated by the DART molecules was observed consistently across a range of concentrations of bispecific antibody (Figure 6B). As expected, neither CD19 mAb alone nor the 4420xTCR DART molecule mediated the association of Jurkat T-cells with Daudi B cells at any concentration tested.
DART molecules mediate the cell-cell association. Jurkat T cells expressing surface CD3 were loaded with CellTrace CFSE dye, and cell membranes of CD19+ Daudi B cells were labeled with the fluorescent dye PKH26. (A) Cells were incubated for 30 minutes at room temperature with or without DART or BiTE molecules at 0.01 μg/mL. The Jurkat-Daudi cell-cell association was determined using flow cytometry and quantified as the percentage of positive cells in the upper right quadrant of FL1 versus FL2, representing the CFSE+PKH26+ double-positive population. (B) The experiment was repeated using increasing concentrations of the molecules. Each experimental point was set up in duplicate and the mean ± SD was plotted.
DART molecules mediate the cell-cell association. Jurkat T cells expressing surface CD3 were loaded with CellTrace CFSE dye, and cell membranes of CD19+ Daudi B cells were labeled with the fluorescent dye PKH26. (A) Cells were incubated for 30 minutes at room temperature with or without DART or BiTE molecules at 0.01 μg/mL. The Jurkat-Daudi cell-cell association was determined using flow cytometry and quantified as the percentage of positive cells in the upper right quadrant of FL1 versus FL2, representing the CFSE+PKH26+ double-positive population. (B) The experiment was repeated using increasing concentrations of the molecules. Each experimental point was set up in duplicate and the mean ± SD was plotted.
The CD19xTCR DART molecule inhibits B-cell lymphoma in a xenograft model
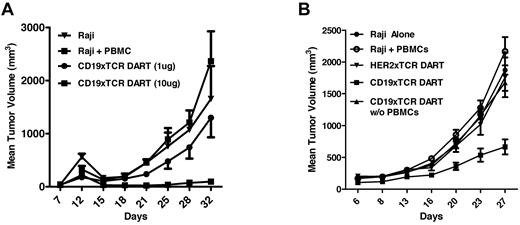
It was demonstrated previously that the ability to redirect T-cell killing can also be recapitulated in xenograft models in which human PBMCs are provided as an effector population.30 These studies have included evaluation and demonstration of efficacy for CD3-based bispecific molecules. Because we incorporated a novel T cell–targeting specificity into the DART molecules (TCRαβ), we determined if the CD19xTCR DART molecule could also block human B-cell lymphoma growth in mice. Complete protection in a Raji xenograft model was observed with the CD19xTCR DART molecule when administered at 10 μg doses (Figure 7A). Protection from tumor growth was also observed in a second study using a lower dose (3 μg per injection) and an independent human PBMC population (Figure 7B). As expected, lack of activity with the CD19xTCR DART molecule (10 μg per dose) in the absence of human PBMCs revealed the need for T-cell engagement to block tumor formation, whereas lack of protection with a TCR-based DART molecule lacking B-cell specificity (the HER2xTCR DART molecule) revealed the requirement for B-cell engagement for tumor protection.
CD19xTCR DART molecule inhibits B-cell lymphoma. NOD-SCID mice (N = 6-8) were implanted subcutaneously with 1 × 106 Raji cells and 5 × 106 human PBMCs, and then treated IV with the indicated DART molecules on days 1-5. (A) 10μg doses of the CD19xTCR DART molecule effectively blocked tumor growth. (B) With an independent human PBMC donor, 3-μg doses of the CD19xTCR DART molecule blocked tumor growth, whereas no tumor inhibition was observed with a non-B-cell lymphoma DART molecule (HER2xTCR) or in the absence of PBMCs.
CD19xTCR DART molecule inhibits B-cell lymphoma. NOD-SCID mice (N = 6-8) were implanted subcutaneously with 1 × 106 Raji cells and 5 × 106 human PBMCs, and then treated IV with the indicated DART molecules on days 1-5. (A) 10μg doses of the CD19xTCR DART molecule effectively blocked tumor growth. (B) With an independent human PBMC donor, 3-μg doses of the CD19xTCR DART molecule blocked tumor growth, whereas no tumor inhibition was observed with a non-B-cell lymphoma DART molecule (HER2xTCR) or in the absence of PBMCs.
Discussion
As an initial step in evaluating the ability of DART molecules to redirect T-cell killing, the activity of a CD19xCD3 DART molecule was compared with that of a CD19xCD3 BiTE molecule incorporating the binding specificities of blinatumomab, a molecule presently undergoing clinical evaluation.17 As anticipated, the CD19xCD3 BiTE molecule displayed potent activity against CD19+ lines, with EC50 values in the 10-100 pg/mL range, which is consistent with previously published reports using either resting or prestimulated effector populations.15,23 The CD19xCD3 DART molecule, however, exhibited even greater potency than the BiTE molecule in redirected killing, based both on the maximal level of lymphoma cell lysis obtained and on the lower EC50 values (Figures 2 and 4). The more potent activity exhibited by the DART molecule was observed with either unfractionated PBMCs (resting or activated) or purified T cells (activated) as the effector populations, and against both B-cell lymphoma and autologous B cells. Furthermore, it was detected independently of the E:T, suggesting that the more effective killing by the DART molecule was due to better T-cell/tumor-cell engagement and not to improving the effector cell availability. Neither molecule mediated any detectable killing on CD19− cells, indicating the requirement for target engagement for detectable CTL activity. Because the same antigen-recognition specificities were used, the enhancement observed with the DART molecule was presumably due to a platform-based structural difference in the molecules that promotes higher activity. These data support our hypothesis that the DART architecture provides a stable and physiologic geometry to support bispecific interactions.
Within the TCR/CD3 signaling complex, the most convenient targets for mAb-mediated T-cell activation are the invariant chains of the TCR/CD3 signaling complex, with the CD3ϵ chain being the most commonly targeted component (as in the case for the CD3 mAb used in this report). mAbs directed against a common determinant on the TCRα/β chain have also been applied, and have shown promise in the treatment of autoimmune disease,31 although only anti-CD3 mAbs are currently approved or under development.32 Because TCRα/β has a somewhat more limited expression than CD3ϵ, and in some studies has shown a lower potential for side effects when targeted therapeutically,33 it was of interest to ascertain whether redirecting killing via TCR retargeting would have similar or different effects as CD3. The CD3-signaling subunit of the TCR is composed of 4 elements in a δϵ/γϵ/ζζ configuration.34 However, the stoichiometric relationship between the CD3 chains and the TCRα/β heterodimer is not fully understood, with evidence suggesting either a 1:134,35 or a more complex, dynamic stoichiometric relationship.36 It was therefore also of interest to ascertain whether redirecting killing via the TCR has identical or different effects as CD3 activation. We constructed a DART molecule that binds to an invariant portion of the TCRβ chain using the Fv region from the antibody BMA031.26,27 To allow direct comparison with CD3 engagement, the TCR DART molecule incorporated the identical anti-CD19 specificity used for the CD19xCD3 DART molecule. In redirected killing assays, the activity of the CD19xTCR molecule overlapped that observed with the CD19xCD3 DART molecule, irrespective of whether PBMCs or purified T cells were used as the effector populations. Both DART molecules also mediated similar levels of T-cell activation of either CD4 and CD8 T-cell subsets. As expected, no activity was observed on either B-cell lymphoma or normal peripheral B cells with a control DART molecule (4420xTCR) that only engages TCRαβ, indicating the requirement for CD19 coengagement for the observed T cell–mediated killing of B cells. These observations indicate that, irrespective of whether CD3 or TCR is coengaged with CD19, the DART architecture supports an enhanced level of redirected T-cell killing, suggesting an opportunity to incorporate alternative T-cell specificities to CD3 (such as TCRαβ) in the design of therapeutic bispecific antibodies with a potentially more favorable efficacy and safety profile. Data from a human PBMC reconstitution model demonstrate that the CD19xTCR DART molecule is indeed efficient at protecting tumor formation in mice, supporting the use of such a bispecific molecule for the treatment of lymphoma.
The ability of T cell–directed bispecific molecules to attack lymphoma cells relates to both the molecules' propensity to promote direct T-cell/tumor contact and lysis and their ability to promote T-cell activation and expansion within the tumor environment. Occurrence of the latter phenomenon in a target-independent fashion, however, should be limited or absent, because it relates to the propensity of producing systemic side effects such as cytokine storm. In the presence of target cells, both the CD19xTCR and the CD19xCD3 DART molecules mediated T-cell expansion. Consistent with the need for target coengagement to observe killing activity, no T-cell expansion was observed when the target B-cell population was depleted. This dependence on coengagement was also observed for the BiTE molecule, which is consistent with previous data demonstrating the strict requirement for T-cell activation on coengagement of the bispecific molecule.37
Both CD3- and TCR-based DART molecules were significantly more potent in redirected killing assays than the BiTE molecule. Several factors appear to contribute to explain this enhanced potency. The modest but reproducible improved affinity for target recognition by the DART molecules may be a contributing element. T-cell activity is generally thought to be very sensitive to the half-life of the antigen/receptor interaction. A higher association rate can result in significant increased engagement time with a target through rapid rebinding, leading to an increase of the T-cell cytotoxic response.38 A remaining factor may be the difference in the ability to adopt alternative structures, such as the degrees of freedom of the 2 formats. Because productive engagement is likely to require multiple individual interactions at the immune synapse, the individual affinities or kinetic properties and the orientation of the binding sites are likely to be critical factors in determining the “productivity” of the reaction between the opposing cells at a given concentration of bispecific reagent. The DART format has a fixed distance and orientation between the 2 binding domains, whereas the BiTE format is likely to allow for a great deal of flexibility of the T-cell–binding site relative to the tumor-binding domain. In this model, there could be a limited number of orientations favorable to optimal engagement, T-cell activation, and cytolysis. The predicted more rigid and more compact structure of the DART molecule would appear to have a highly favorable orientation for this activity. Alternatively, a fraction of BiTE molecules could require more time to adopt such a favorable orientation at any one time. Because this must occur within a finite time of engagement due to the intrinsic affinities of each binding partner, the probability of productive engagement would be comparatively diminished. As predicted by this model, both the CD19xCD3 and the CD19xTCR DART molecules mediated a more efficient and stable cell-to-cell association than the BiTE molecule in this assay system, providing correlative evidence for the increased level of potency observed with DART-based molecules.
In summary, the favorable functional and biophysical properties of DART molecules indicates an opportunity for the development of a new therapeutic modalities to treat lymphoma through redirected T-cell mechanisms. The flexibility to explore different effector arms, as shown here by TCRαβ targeting, provides an opportunity to recruit and activate T cells in alternative approaches to CD3 activation, and the efficient killing activity and ability to mediate cell-cell associations indicate an optimal architecture to support redirected immune-mediated cell killing mechanisms.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Shelley Butler, Arin Whiddon, and Wanhua Yan for technical assistance, and Melinda Hanson, Jeff Nordstrom, Tim Mayer, and Ralph Alderson for review of the manuscript.
Authorship
Contribution: P.A.M. designed research, interpreted data, and wrote the paper; W.Z., H.L., S.G., M.C.V., and S.A. performed research, analyzed data, and edited the paper; G.J.R. performed research, contributed vital new reagents, analyzed data, and edited the paper; S.B. contributed vital new reagents and edited the paper; L.H., S.Z., and V.C. contributed vital new reagents; Y.Y., K.S., L.J., L.H., and T.Z. performed research and analyzed data; S.K. designed research; E.B. designed research, interpreted data, and edited the paper; and S.J. designed research and new reagents, interpreted data, and edited the paper.
Conflict-of-interest disclosure: All authors are employed by MacroGenics, a privately held company, and as a condition of employment have received MacroGenics stock options.
Correspondence: Paul A. Moore, PhD, MacroGenics, 1500 East Gude Dr, Rockville MD 20850; e-mail: moorep@macrogenics.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal