Since taken on by biotechnology and pharmaceutical companies, bispecific antibodies experience a remarkable revival. In this issue of Blood, Moore et al provide evidence that antibody engineering can push the performance envelope further.1
Fueled by 5 marketed monoclonal antibodies for the therapy of B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia, B-cell malignancies have become a focal point for naked and armed monoclonal antibodies in preclinical and clinical development.2 Of particular interest are bispecific antibodies that combine antigen-binding specificities for target cells (ie, malignant B cells) and effector cells (ie, T cells, NK cells, or macrophages) in 1 molecule.3 Among these, the pursuit of bispecific antibodies that recruit and activate T cells through CD3 of the T-cell receptor (TCR) complex for redirected lysis of malignant B cells expressing CD19 has been ongoing for 2 decades.4 Greater production challenges with respect to quantity, quality, and stability of bispecific antibodies compared with conventional monoclonal antibodies hampered the clinical translation of early CD19xCD3 formats. The biotechnology company Micromet Inc developed a format termed BiTE (for Bi-specific T-cell Engager) that overcame these challenges.5 The BiTE format (see figure) recombinantly links the 4 variable domains of heavy and light chains required for 2 antigen-binding specificities like pearls on a string of polypeptide linkers. The resulting ∼ 55-kDa molecule is a single polypeptide with 1 N- and 1 C-terminus. Numerous in vitro and in vivo studies demonstrated that BiTEs combine tumor cell targeting with selective T-cell activation at low picomolar concentrations.5 Ongoing phase 1 and 2 clinical trials with the CD19xCD3 BiTE blinatumomab revealed impressive clinical activity in relapsed B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia6 as well as minimal residual disease in pre-B cell acute lymphoblastic leukemia7 at doses several orders of magnitude below those administered in conventional monoclonal antibody therapy. In addition to bypassing MHC/peptide recognition, T cells recruited by blinatumomab do not require ex vivo prestimulation or in vivo costimulation but are strictly dependent on the presence of blinatumomab-decorated normal or malignant B cells for activation. These favorable features of the BiTE format are attributed to (1) its small size that brings target and effector cells in close proximity enabling the formation of cytolytic synapses and (2) its monovalent engagement of the TCR complex preventing systemic activation of effector cells in the absence of target cells.
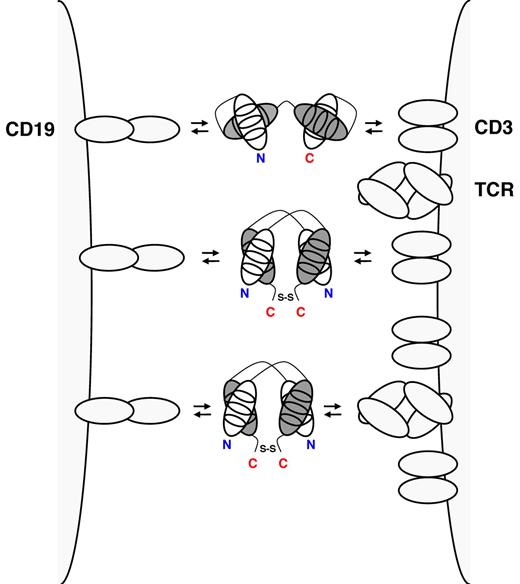
CD19xCD3 BiTE (top), CD19xCD3 DART (middle), and CD19xTCR DART (bottom) cross-link a normal or malignant B cell through CD19 (left) and a T cell through the TCR complex (right). BiTEs consist of a single polypeptide displaying 2 antigen-binding specificities through cognate heavy (gray) and light chain (white) variable domains (shown with the 3 complementarity determining regions). BiTEs have 1 N-terminus (N, shown in blue) and 1 C-terminus (C, shown in red). In DARTs, cognate heavy and light chain variable domains are on 2 separate polypeptides that associate and are stabilized by a C-terminal disulfide bridge. Thus, DARTs have 2 N-termini and 2 C-termini.
CD19xCD3 BiTE (top), CD19xCD3 DART (middle), and CD19xTCR DART (bottom) cross-link a normal or malignant B cell through CD19 (left) and a T cell through the TCR complex (right). BiTEs consist of a single polypeptide displaying 2 antigen-binding specificities through cognate heavy (gray) and light chain (white) variable domains (shown with the 3 complementarity determining regions). BiTEs have 1 N-terminus (N, shown in blue) and 1 C-terminus (C, shown in red). In DARTs, cognate heavy and light chain variable domains are on 2 separate polypeptides that associate and are stabilized by a C-terminal disulfide bridge. Thus, DARTs have 2 N-termini and 2 C-termini.
The success of the BiTE format triggered the search for intellectual property space among bispecific antibody formats of similar size and valence. A potentially competing format was recently developed by the biotechnology company MacroGenics Inc and termed DART (for Dual-Affinity Re-Targeting).8 The DART format is based on the diabody format that separates cognate variable domains of heavy and light chains of the 2 antigen binding specificities on 2 separate polypeptide chains.9 Whereas the 2 polypeptide chains associate noncovalently in the diabody format, the DART format provides additional stabilization through a C-terminal disulfide bridge (see figure). DARTs can be produced in high quantity and quality and reveal exceptional stability in both formulation buffer and human serum. In this issue of Blood, Moore et al conduct a side-by-side comparison of the in vitro performance of CD19xCD3 DART and BiTE molecules that were based on the same parental mouse anti–human CD3 and mouse anti–human CD19 monoclonal antibodies as blinatumomab.1 In various redirected cytotoxicity assays with human B-cell lines and autologous human B cells, the bispecific antibody in the DART format consistently outperformed the BiTE format with respect to the maximal level of B-cell lysis, the concentration required for half-maximal B-cell lysis, and the induction of molecular markers of T-cell activation. Neither format induced the activation or proliferation of T cells in the absence of B cells. The comparison appears legitimate as the previously reported low picomolar concentrations of the BiTE format required for half-maximal target-cell lysis were confirmed. Compared with the BiTE format, the DART format revealed a moderately higher association rate constant for CD3, a moderately lower dissociation rate constant for CD19, and an ability to cross-link T cells and B cells more efficiently. The authors discuss that the more rigid configuration of the DART format with limited flexibility between the 2 antigen-binding specificities may explain these favorable features.
In addition to comparing CD19xCD3 DART and BiTE formats, Moore et al also generated and characterized a CD19xTCR DART that engages the TCR complex through an invariant epitope displayed by the TCR rather than by CD3 (see figure).1 Notably, the CD19xTCR DART revealed virtually identical in vitro activity as the CD19xCD3 DART and demonstrated in vivo activity based on a xenograft mouse model with human effector and target cells. While this finding along with the recently published CD32BxCD16 and CD32BxCD79B DARTs8,10 underscores the adaptability of this bispecific antibody platform, it also provides an alternate T-cell recruiting and activation mechanism that may have a different activity and toxicity profile than blinatumomab.
So do DARTs BiTE better? Although this study would have been more complete by including side-by-side comparisons of the stability of DART and BiTE in formulation buffer and human serum and the in vivo activity in xenograft mouse models, Moore et al make a strong case for clinical translation of the DART format in general and CD19xCD3 and CD19xTCR DARTs in particular. Ultimately, only clinical trials can provide a comprehensive side-by-side comparison of DART, BiTE, and other bispecific antibody formats with identical antigen-binding specificities. Perhaps more importantly, the perceived commercial viability of bispecific antibody platforms developed by competing biotechnology companies has attracted resourceful pharmaceutical companies into the arena.11 This can be considered good news for cancer patients.
Conflict-of-interest disclosure: The author declares no competing financial interest. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal