To the editor:
Human genetic studies have been instrumental in understanding the trans-acting regulation of the human globin genes and particularly the fetal-to-adult hemoglobin switch.1 Important insight has come from studies of common genetic variation underlying fetal hemoglobin (HbF) expression in adults, resulting in the identification of BCL11A as a critical regulator of HbF silencing.2 In addition, rare human mutations that disrupt transcription factors including KLF13,4 and GATA15 highlight the importance of these factors in HbF expression and human erythropoiesis more generally. Hence, human genetic studies of globin gene regulation are critical to better understand the in vivo requirement for many putative regulators of this process.
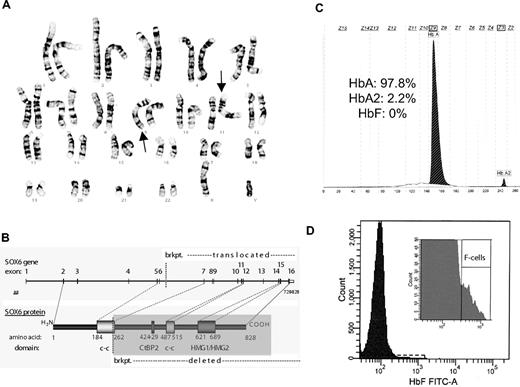
Recent studies suggest that the transcription factor SOX6 has a critical role in silencing of the γ-globin (HbF) and related mouse embryonic globin genes.6-8 This activity appears to occur in collaboration with BCL11A. We studied a 10-year-old male patient with a disruption of the SOX6 gene from a balanced translocation (karyotype of 46,XY, t(9;11)(q33.1;p15.1)) found in the course of a genetic workup for craniosynostosis (Figure 1A-B).9 The craniosynostosis appeared to be attributable to this heterozygous disruption that likely results in haploinsufficiency, which is supported by the finding of a SOX6 missense mutation in another patient with craniosynostosis and evidence for a role of this gene in joint and cartilage development.9 The patient had release surgery of the lambdoid and posterior sagittal sutures as an infant and has since been clinically asymptomatic. Approval for this study was obtained from the Ethics Review Board of the Medical Faculty at the University of Cologne. Informed consent was provided according to the Declaration of Helsinki. We were able to obtain a blood sample from this patient for hematologic analysis.
Heterozygous disruption of SOX6 does not result in elevations of HbF levels. (A) Conventional G-banding of chromosomes analyzed from the patients lymphocytes showing a balanced translocation with a karyotype of 46,XY,t(9;11)(q33.1;p15.1) that could be confirmed with fine mapping. (B) A schematic showing the resulting breakpoint (brkpt.) on chromosome 11 at position 16195,423 (from hg18) that disrupts the SOX6 gene. Domains are shown from SOX6, including the coiled-coil (c-c), CtBP2 binding, and HMG domains. (C) HbF content was measured using capillary electrophoresis (Capillarys 2; Sebia). No HbF was detected, which should be detectable in zone 7 (Z7). (D) The number of F cells were quantitated by flow cytometry (BD FACSCanto II; Becton Dickinson). A total of 50 000 erythrocytes were analyzed. The patient had 0.7% F cells (normal range: 0.4%-4%).
Heterozygous disruption of SOX6 does not result in elevations of HbF levels. (A) Conventional G-banding of chromosomes analyzed from the patients lymphocytes showing a balanced translocation with a karyotype of 46,XY,t(9;11)(q33.1;p15.1) that could be confirmed with fine mapping. (B) A schematic showing the resulting breakpoint (brkpt.) on chromosome 11 at position 16195,423 (from hg18) that disrupts the SOX6 gene. Domains are shown from SOX6, including the coiled-coil (c-c), CtBP2 binding, and HMG domains. (C) HbF content was measured using capillary electrophoresis (Capillarys 2; Sebia). No HbF was detected, which should be detectable in zone 7 (Z7). (D) The number of F cells were quantitated by flow cytometry (BD FACSCanto II; Becton Dickinson). A total of 50 000 erythrocytes were analyzed. The patient had 0.7% F cells (normal range: 0.4%-4%).
A complete blood count was within normal limits and showed no evidence of anemia or other abnormalities (white blood cells: 5900 cells/mL; red blood cells: 4.53 × 106 cells/mL; Hb: 12.8 g/dL; Hct: 38.5%; and platelets: 212 000/μL). Loss of SOX6 activity derepresses human fetal or mouse embryonic globin gene expression in several experimental systems.6-8 However, capillary electrophoresis of the patient's blood showed no detectable HbF expression present (0% HbF, 97.8% HbA, 2.2% HbA2) (Figure 1C). We additionally analyzed the percentage of red blood cells containing a significant fraction of HbF, using the F-cell assay (Figure 1D). This revealed that the patient had F cells within the normal range (0.7% for this patient, with a normal range of 0.4%-4%).
These results suggest that heterozygous SOX6 disruption is insufficient to impair normal erythropoiesis or silencing of the HbF genes in vivo. In contrast, lowering the level of SOX6 by ∼80% increases HbF expression in cultured human erythroblasts.7 Thus, repression of γ-globin expression may require reduction of SOX6 expression below a critical threshold that is not attained by haploinsufficiency. However, of note, the effect of SOX6 on erythropoiesis appears to be sensitive to the level of expression present.10 It is also possible that dosage compensation of SOX6 gene expression occurs from the intact allele in erythroid cells, in contrast to bone/cartilage cells where heterozygous gene disruption gives rise to the craniosynostosis phenotype. We could not differentiate between these possibilities, because we were unable to get consent to obtain appropriate tissue or cells for expression studies. Regardless, analysis of this patient shows that in contrast to other transcription factors, such as KLF1, heterozygous disruption of SOX6 does not alter erythropoiesis or γ-globin gene expression in vivo. Thus, distinct gene dosage requirements exist for 2 different erythroid transcription factors that function in a common pathway to repress γ-globin expression. Our findings have important implications for developing therapeutic strategies to target the KLF1-BCL11A-SOX6 HbF silencing pathway2,4,7 and emphasize the utility of using rare human mutations to better understand aspects of globin gene regulation and erythropoiesis.
Authorship
Contribution: All authors performed the research, analyzed the data, and wrote the final version of the letter.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vijay G. Sankaran, Children's Hospital of Boston, 300 Longwood Ave, Boston, MA 02115; e-mail: vijay.sankaran@childrens.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal