Abstract
CD73 is involved in the extracellular ATP metabolism by dephosphorylating extracellular AMP to adenosine and thus regulating permeability of the blood vessels and leukocyte traffic into the tissues. It is also present on lymphatic vessels where its distribution and function have not been characterized. We found that CD73 is expressed on a subpopulation of afferent lymph vessels but is absent on efferent lymphatics, unlike LYVE-1 and podoplanin, which are expressed on both types of lymphatics. The extracellular nucleotide metabolism on lymphatic endothelium differs from that on blood vessel endothelium as lymphatic endothelium has lower NTPDase and higher ecto-5′-nucleotidase/CD73 activity than blood vascular endothelium. In knockout mice, the lack of CD73 on lymphocytes decreases migration of lymphocytes to the draining lymph nodes more than 50% while CD73-deficient lymph vessels mediate lymphocyte trafficking as efficiently as the wild-type lymphatics. Thus, although endothelial CD73 is important for permeability and leukocyte extravasation in blood vessels, it does not have a role in these functions on lymphatics. Instead, lymphocyte CD73 is intimately involved in lymphocyte migration via afferent lymphatic vessels.
Introduction
Continuous recirculation of lymphocytes between blood and lymphoid tissues is central for proper functioning of the immune system, and leukocyte entrance to the sites of infections is required for elimination of intruding pathogens. Proper interaction between leukocytes and endothelial cells both in blood and lymphatic vessels is central for leukocyte trafficking. Although blood vessel and lymphatic endothelium share many characteristics including expression of certain same molecules, they also have striking differences regarding their morphology, function, and molecular profile.1-3
Balance of extracellular ATP metabolism on endothelium controls the inflammatory status of the microenvironment as ATP is highly proinflammatory and ADP procoagulative while adenosine is anti-inflammatory.4 CD73 is a key enzyme in extracellular ATP metabolism producing adenosine by dephosphorylating AMP.5-7 Besides on blood vessels,8 CD73 is also expressed on a subpopulation of lymphocytes.9 Regulatory T cells are especially CD73 positive.10 Although CD73 on the endothelial cells and lymphocytes are structurally similar, they are differently regulated. Triggering of lymphocyte CD73 with antibodies results in cleavage of CD73 and activation of LFA-1 leading to increased adhesion of lymphocytes to endothelium. Engagement of endothelial CD73, in contrast, does not cause cleavage of the molecule from the cell surface.11
The central role of CD73 in vascular permeability is well demonstrated in mice deficient with CD73. They show normal lymphocyte homing in nonchallenged conditions, but in hypoxic and inflammatory conditions increased endothelial leakiness and leukocyte extravasation via high endothelial venules (HEV) are observed12 with a concordant exacerbation of the acute inflammatory and immune responses.13
Previous histochemical studies with 5′-AMP as a substrate have demonstrated abundant expression of 5′-nucleotidase activity in human lymphatic vessels.14 However, the molecular identity of this lymphatic (ecto)enzyme and its functional role and distinctions from the well-characterized blood vessel ecto-5′-nucleotidase/CD73 have remained unknown. Therefore, this work was designed to analyze expression and functional characteristics of CD73 on lymphatic endothelium in comparison to blood vessel CD73 using both human and mouse systems. Besides significant differences in ATP metabolism between lymphatic and blood vessel endothelium in general, the results also revealed marked heterogeneity among the lymphatics and striking species-specific differences regarding CD73 expression on lymphatics. Moreover, they demonstrated a central role for lymphocyte CD73 in lymphocyte migration into the draining lymph nodes.
Methods
Animals and tissues
CD73-deficient mice were generated and backcrossed for 9 generations to C57/B16/J strain as described.15 The CD73-deficient and the wild-type B6 background strain (wt) mice were identified using one PCR reaction for the wt allele, and one for the recombined allele. Skin samples (n = 3) were obtained from punch biopsies. Human lymph nodes (n = 4) and intestinal samples (n = 6) were collected in connection to surgical operations and tested to be macroscopically and microscopically normal. Permissions for the animal studies, and to obtain human tissues, were approved by the ethical committee of the University of Turku.
Immunostainings
Double and triple immunofluorescence stainings were performed on 5-μm-thick normal human fresh-frozen skin, lymph node, and intestinal tissue sections. For detection of CD73 on blood vessels double stainings were performed with anti-CD73 monoclonal antibody (mAb) 4G4 followed by Alexa Fluor 546 goat anti–mouse IgG1 (Invitrogen). Thereafter, the sections were incubated with FITC-conjugated anti–PV-1 (174/2 mAb). FITC-conjugated NS-1 and 3G6 mAb (against chicken T cells) were used as negative controls.
CD73-positive lymphatic vessels were detected by double stainings with primary antibodies against CD73 (4G4) and LYVE-1 (RELIAtech GmbH) or podoplanin (a kind gift of Dontscho Kerjaschki, Vienna, Austria). As controls an isotype-matched mAb (AK-1; In Vivo Biotech Services GmbH) and normal rabbit serum were used. The tissue sections were incubated with primary antibodies for 40 minutes except for the anti-podoplanin Ab which was incubated overnight at +4°C. Second-stage antibodies Alexa Fluor 546 goat anti–mouse IgG1 or IgG (Invitrogen) and Alexa Fluor 488 goat anti–rabbit IgG were incubated at room temperature for 40 minutes.
To discriminate LYVE-1–positive macrophages from LYVE-1–positive small lymphatics in the quantification analyses, triple immunofluorescence stainings were done with anti-CD73 mAb 4G4, anti-LYVE-1 Ab (RELIAtech GmbH) and Alexa Fluor 647–conjugated anti-CD68 mAb (Santa Cruz Biotechnology). AK-1 and normal rabbit serum served as negative controls. Alexa Fluor 546 goat anti–mouse IgG and Alexa Fluor 488 goat anti–rabbit IgG were used as second stage antibodies.
In addition, anti-CD73 and anti–LYVE-1 double stainings were performed on normal mouse skin, lymph node, and intestinal tissue sections by using anti–mouse CD73 Ab TY/23 (BD Pharmingen) and anti–mouse LYVE-1 Ab (RELIAtech GmbH) and normal rat serum and normal rabbit serum as negative controls. Alexa Fluor 546 goat anti–rat IgG and Alexa Fluor 488 goat anti–rabbit IgG were used as secondary antibodies. Furthermore, anti-CD73 and podoplanin double stainings were done on normal mouse skin tissue samples with anti–mouse CD73 Ab TY/23 (BD Pharmingen) and biotinylated anti–mouse podoplanin Ab (BioLegend). As second stage antibodies Alexa Fluor 488 goat anti–rat IgG and streptavidin conjugated Alexa Fluor 546 were used. All samples were mounted with Prolong Gold antifade reagent (Invitrogen).
The immunofluorescence stainings were analyzed using a Zeiss LSM 510 META laser-scanning confocal microscope (Carl Zeiss). Pictures were taken with Plan-Neofluar ×20/0.5 and ×40/0.75 objectives at room temperature. The acquisition software used was Zeiss LSM software and image processing was performed with Zeiss LSM Image Browser (Carl Zeiss MicroImaging). The total number of blood and lymphatic vessels was counted from the pictures and thereafter, the quantification of CD73+PV-1+ double-positive blood vessels and the CD73+LYVE+ and CD73+Podoplanin+ double-positive lymphatic vessels was performed.
Analyses of purine-converting activities
For enzyme assays, 8000 endothelial cells were cultured overnight in appropriate Endothelial Cell Growth Medium MV with Supplement Mix (complete medium; PromoCell Gmbh) in clear 96-well plates. Purinergic activities were determined at 37°C in a final volume of 80 μL of RPMI 1640 medium containing 4mM β-glycerophosphate in the following ways: (1) for ATPase, ADPase, and ecto-5′-nucleotidase assays, cultured cells were incubated 25-30 minutes with 500μM [2,8-3H]ATP (PerkinElmer), 500μM [2,8-3H]ADP (PerkinElmer), and 300μM [2-3H]AMP (Amersham Biosciences), respectively; (2) adenylate kinase was assayed by incubating the cells for 40 minutes with 500μM [3H]AMP in the presence of 750μM γ-phosphate–donating ATP. Catalytic reactions were terminated by applying aliquots of the mixture onto Alugram SIL G/UV254 sheets (Macherey-Nagel). Radiolabeled nucleotides and nucleosides were separated by thin layer chromatography (TLC) and quantified by scintillation β-counting, as described earlier.16 Enzymatic activities are expressed as nanomoles of 3H-substrate metabolized by 106 cells per 1 hour.
Lymphocyte migration via lymphatics
These experiments were performed as described.17 Peripheral and mesenteric lymph nodes and spleens were collected from CD73-deficient and wt mice and homogenized to obtain single-cell suspensions. After lysis of erythrocytes, lymphocytes were labeled for 20 minutes with 0.5μM CFSE (Molecular Probes) at 37°C. Labeled lymphocytes were washed 3 times with the HEC medium (RPMI 1640 supplemented with 10% FCS, 1% 4mM l-glutamine, and 0.128% penicillin/streptomycin) and resuspended to RPMI 1640. To study migration under physiologic conditions, the labeled lymphocytes were injected subcutaneously into the hind-leg footpads of CD73-deficient and age-matched wt recipients. In the second set of experiments, the migration in inflammatory conditions was studied by injecting LPS to the footpads 12 hours before the lymphocyte injection. In both set-ups, after 12 hours from lymphocyte injection, popliteal lymph nodes were harvested from the recipients and passed through the wire mesh to obtain single-cell suspensions. Cell suspensions were analyzed by flow cytometry (FACSCalibur; BD Biosciences).
In the third set of experiments the CFSE-labeled lymphocytes from CD73-deficient and wt mice were incubated either with anti–LFA-1 antibody (TIB237 against CD11a; ATCC) or a class-matched negative control antibody (HB-151; ATCC) both at 10 μg/5 × 106 cells for 30 minutes. Then, the lymphocytes were injected subcutaneously with the antibodies into the hind-leg footpads of wt recipients. After 12 hours, popliteal lymph nodes were harvested from the recipients and single-cell suspensions were stained with 10 μg/mL PE rat anti-CD73 (BD Pharmingen) for 20 minutes and analyzed by flow cytometry (FACSCalibur).
Lymphocyte homing assay (intravenously)
Lymphocytes from CD73-deficient and wt mice were collected as described and wt lymphocytes were labeled for 20 minutes with 0.5μM CFSE and CD73-deficient lymphocytes with 5μM TRITC (tetramethylrhodamine-5-isothiocyanate; Molecular Probes) at 37°C. Then, labeled lymphocytes were washed 3 times with HEC medium and resuspended to RPMI 1640. wt and CD73-deficient lymphocytes were pooled (1:1 ratio) and injected into the tail vein of CD73-deficient and age-matched wt recipients. Cells were allowed to circulate for 4 hours and then peripheral and mesenteric lymph nodes and spleens were collected. Harvested tissues were homogenized and lymphocyte suspensions analyzed by flow cytometry (FACScan; BD Biosciences). The homing index (HI) was calculated from the formula: HI = [CD73-deficient cells]tissue/[wt cells]tissue:[CD73-deficient cells]input/[wt cells]input to correct the ratio of 2 injected cell populations.
FITC painting
Dorsal sides of ears of CD73-deficient and wt mice were painted with 25 μL of 1% FITC (Sigma-Aldrich) in acetone/dibutylphthalate (1:1). After 48 hours, auricular skin-draining lymph nodes were collected and transferred into Hanks medium supplemented with 2% FCS. Lymph nodes were digested with 120 μg/mL DNase I (Roche) and 0.5 mg/mL collagenase P (Roche) for 25 minutes at 37°C. Digestion was stopped by adding EDTA to a final concentration of 10mM. After digestion, the tissue was pressed through metal strainers to obtain single-cell suspensions. Cell suspensions were washed with PBS buffer supplemented with 1% BSA, 20 μg/mL DNase, 5mM EDTA, and stained with anti-CD103 PE (clone M290), CD11c PerCP-Cy5.5 (clone HL3), CD40 PE (clone 3/23), and MHC II PE (clone M5/114.15.2; all BD Pharmingen). For intracellular staining with anti-Langerin-Alexa 647 (clone 929F3.01; Dendritics), cells were fixed in 2% paraformaldehyde and stained in 0.1% saponin buffer. Stained cell suspensions were analyzed by flow cytometry (FACSCalibur).
Skin explant culture
Mice were killed and ears of CD73-deficient and wt mice were cutoff at the base, disinfected with 70% ethanol and divided into dorsal and ventral halves. The dorsal halves were cultured in 24-well plates in RPMI 1640 medium supplemented with 10% FCS (EuroClone), 2mM l-glutamine (EuroClone), 50nM 2-mercaptoethanol (Fluka), and 50 μg/mL gentamicin (Gibco) at +37°C for 48 hours. Dendritic cells emigrated into the culture medium were collected, counted with the hemocytometer and stained for CD73 expression using appropriate control stainings.
Results
CD73 is expressed on a subpopulation of lymphatic vessels in humans
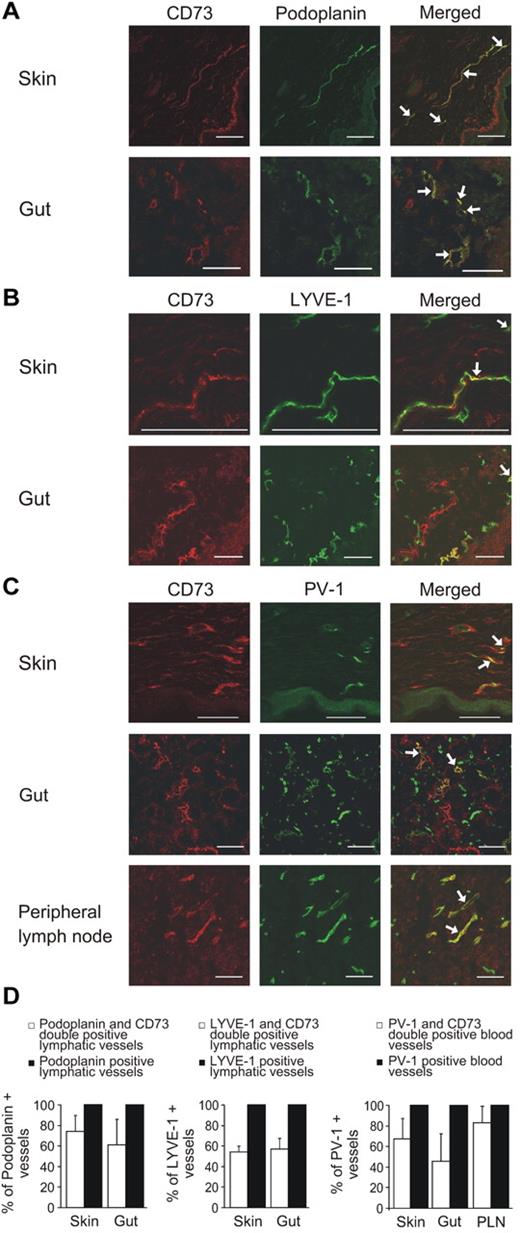
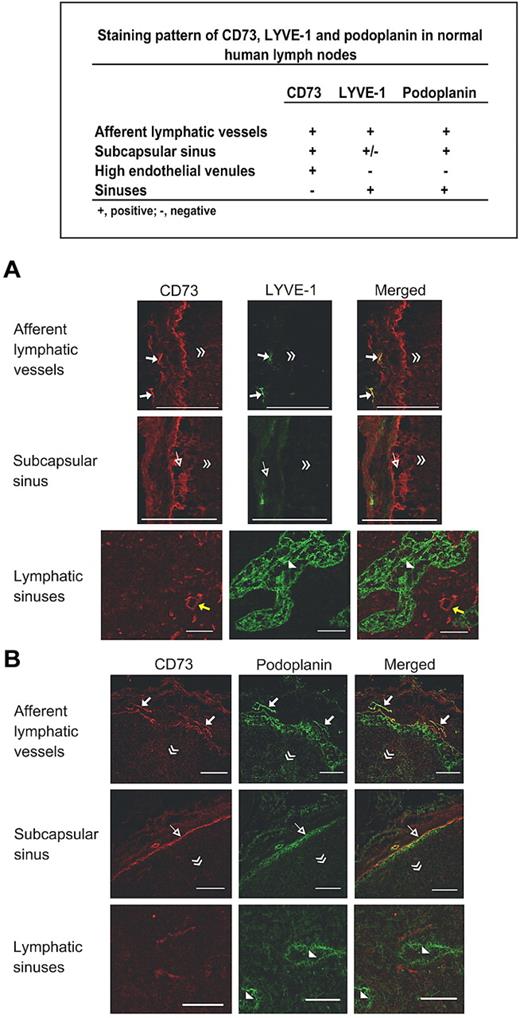
We first tested the expression of CD73 on lymphatics of human skin, gut and lymph nodes by comparing it to the established lymphatic endothelial markers in double immunofluorescence stainings. In the skin and the gut, the majority of the afferent podoplanin-positive lymphatics also expressed CD73 (Figure 1A). Approximately half of the LYVE-1–positive lymphatics in the skin and in the gut were also CD73 positive (Figure 1B). Double stainings with a blood vessel marker PV-1 revealed that percentages of CD73-positive blood vessels varied markedly between skin, gut, and peripheral lymph nodes (Figure 1C). A summary of the expression patterns of CD73, LYVE-1, podoplanin, and PV-1 is presented in Figure 1D. In lymph nodes the subcapsular sinuses were brightly CD73 positive, whereas the lymphatic sinuses, which are the exit sites for lymphocytes in the lymph nodes, were CD73 negative. This is in a striking contrast to LYVE-1 and podoplanin, which both are highly expressed in lymphatic sinuses (Figure 2).
Only a subpopulation of lymphatic and blood vessels express CD73. Double immunofluorescence stainings of human skin and gut with anti-CD73 and anti-podoplanin antibodies (recognizing lymphatic vessels; A), with anti-CD73 and anti–LYVE-1 antibodies (recognizing lymphatic vessels; B) and skin, gut, and lymph nodes with CD73 and anti–PV-1 antibodies (recognizing blood vessels; C). (D) Summary of the results in different organs. Podoplanin-, LYVE-1–, and PV-1–positive vessels have been set as 100% by definition and CD73-positive vessels of those have been presented as mean percentages ± SEM; 35-269 vessels/sample were counted depending on the size of the sample. Arrows point to some double-positive vessels. Scale bar, 100 μm.
Only a subpopulation of lymphatic and blood vessels express CD73. Double immunofluorescence stainings of human skin and gut with anti-CD73 and anti-podoplanin antibodies (recognizing lymphatic vessels; A), with anti-CD73 and anti–LYVE-1 antibodies (recognizing lymphatic vessels; B) and skin, gut, and lymph nodes with CD73 and anti–PV-1 antibodies (recognizing blood vessels; C). (D) Summary of the results in different organs. Podoplanin-, LYVE-1–, and PV-1–positive vessels have been set as 100% by definition and CD73-positive vessels of those have been presented as mean percentages ± SEM; 35-269 vessels/sample were counted depending on the size of the sample. Arrows point to some double-positive vessels. Scale bar, 100 μm.
CD73 discriminates afferent and efferent lymphatics. Double stainings of human lymph nodes with anti-CD73 and anti–LYVE-1 antibodies (A) and anti-CD73 and anti-podoplanin antibodies (B). Examples of afferent lymphatics (arrows), endothelium of subcapsular sinuses (open arrows) and HEV (yellow arrows) are pointed out in the appropriate panels. Lymphatic sinuses are indicated with arrowheads. The direction of the lymph node center is indicated with double arrowheads. Scale bars, 100 μm.
CD73 discriminates afferent and efferent lymphatics. Double stainings of human lymph nodes with anti-CD73 and anti–LYVE-1 antibodies (A) and anti-CD73 and anti-podoplanin antibodies (B). Examples of afferent lymphatics (arrows), endothelium of subcapsular sinuses (open arrows) and HEV (yellow arrows) are pointed out in the appropriate panels. Lymphatic sinuses are indicated with arrowheads. The direction of the lymph node center is indicated with double arrowheads. Scale bars, 100 μm.
Lymphatic endothelium shows higher ecto-5′-nucleotidase/CD73 and lower NTPDase activity than blood vessel endothelium
To compare the patterns of lymphatic and vascular nucleotide metabolism, we cultured human microvascular endothelial cells, which contain both lymphatic and vascular endothelial cells, and separated them by using anti-podoplanin and magnetic bead separation. Thus, this allowed us to compare the different cell types of the same individuals. First, autoradiographic analysis of major AMP-converting pathways was conducted and these images are illustrated in Figure 3A. Incubation of vascular endothelial cells with 20μM [3H]AMP caused its dephosphorylation into [3H]adenosine (lane 2) and this catalytic reaction can be prevented by specific ecto-5′-nucleotidase inhibitor APCP (lane 3). Joint addition of [3H]AMP and ATP to the cells caused their transphosphorylation into 3H-phosphoryls ADP and ATP (lane 4), which can be prevented by the inhibitor of adenylate kinase Ap5A (lane 5). It is noteworthy that lymphatic endothelial cells are characterized by substantially higher [3H]AMP-dephosphorylating capability in comparison with vascular endothelium (lanes 6, 7). These findings corroborate our previous kinetic and substrate-specificity data showing the presence of extensive network of endothelial nucleotide-converting enzymes with the elements of inactivating cascade including NTPDase1/CD39 and ecto-5′-nucleotidase/CD73 and the counteracting phosphorylating enzymes comprising adenylate kinase and NDP kinase on human umbilical vein endothelium.18
Nucleotide metabolism is different in vascular and lymphatic endothelial cells. (A) Vascular human dermal microvascular endothelial cells (HDMEC) were incubated 45 minutes with 20μM [3H]AMP in the presence of 20μM APCP, 250μM ATP, and 100μM Ap5A (as indicated on lanes 2-5), separated by TLC and developed by autoradiography. In another experiment, vascular and lymphatic cells were incubated for 25 minutes with 20μM [3H]AMP (lanes 6, 7). Blanks (Bl) show the radiochemical purity of [3H]AMP (lanes 1, 8), and arrows indicate the positions of nucleotide and adenosine standards. (B) Activities of ATPase, ADPase, ecto-5′-nucleotidase (5′-NT), and adenylate kinase were determined in vascular and lymphatic endothelial cells by using saturating concentrations of 3H-labeled nucleotide (mean ± SEM; n = 5). *P < .05. (C) Expression of CD73 on podoplanin-positive (lymphatic, left) and -negative (blood vessel, right) endothelial cells analyzed by FACS.
Nucleotide metabolism is different in vascular and lymphatic endothelial cells. (A) Vascular human dermal microvascular endothelial cells (HDMEC) were incubated 45 minutes with 20μM [3H]AMP in the presence of 20μM APCP, 250μM ATP, and 100μM Ap5A (as indicated on lanes 2-5), separated by TLC and developed by autoradiography. In another experiment, vascular and lymphatic cells were incubated for 25 minutes with 20μM [3H]AMP (lanes 6, 7). Blanks (Bl) show the radiochemical purity of [3H]AMP (lanes 1, 8), and arrows indicate the positions of nucleotide and adenosine standards. (B) Activities of ATPase, ADPase, ecto-5′-nucleotidase (5′-NT), and adenylate kinase were determined in vascular and lymphatic endothelial cells by using saturating concentrations of 3H-labeled nucleotide (mean ± SEM; n = 5). *P < .05. (C) Expression of CD73 on podoplanin-positive (lymphatic, left) and -negative (blood vessel, right) endothelial cells analyzed by FACS.
Specific activities were then assayed by using saturating concentrations of 3H-labeled and nonlabeled substrates. In comparison with vascular endothelium, lymphatic endothelial cells display significantly decreased NTPDase and enhanced ecto-5′-nucleotidase activity, as ascertained by measurement of the rates of [3H]ATP, [3H]ADP, and [3H]AMP hydrolyses (Figure 3B). Additional measurement of the extent of ATP-induced [3H]AMP phosphorylation into [3H]ADP/ATP revealed high and fairly comparable adenylate kinase activity in lymphatic and vascular endothelial cells. These enzymatic data were further substantiated by flow cytometric analysis using anti-CD73 mAbs. Although practically all podoplanin-positive cells (lymphatic endothelial cells) and negative cells (blood vessel endothelial cells) were > 95% CD73 positive, the lymphatic endothelial cells had significantly higher expression level when measured as mean fluorescence intensity (106 ± 11.4 vs 68 ± 8.2; n = 5; Figure 3C).
Lymphocyte CD73 but not CD73 on lymphatic endothelium participates in lymphocyte migration via the afferent lymphatic vessels in mice
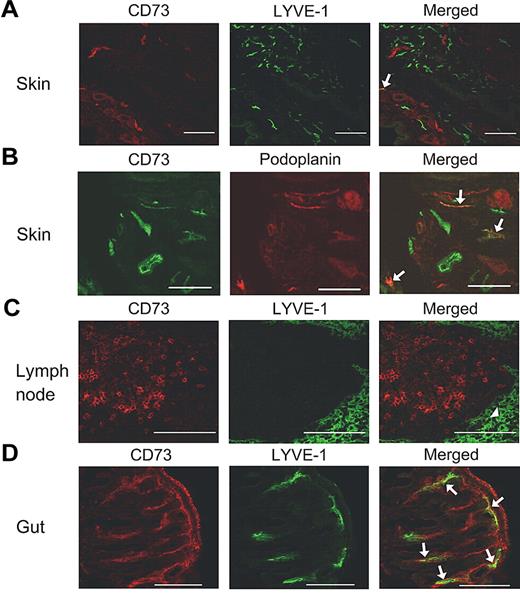
We then wanted to study the in vivo significance of CD73 in lymphocyte migration via the afferent lymphatics. First, we studied the expression of CD73 in lymphatic beds in mice. Unlike in humans, only occasional afferent lymphatic vessels were CD73 positive in the skin and only 13% of the LYVE-1–positive lymphatics (62 counted) also expressed CD73. CD73 and podoplanin were coexpressed more often, as 30% of afferent lymphatics had both markers (an example is shown in Figure 4). In addition, in mice, the lymphatic sinuses were devoid of CD73.
Expression of CD73 in mouse tissues. Double stainings are shown for skin (A-B), lymph node (C), and gut (D) using anti-CD73, anti–LYVE-1, and anti-podoplanin antibodies as indicated. Arrows point to the double-positive vessels and an arrowhead points to the lymphatic sinus. Scale bar, 100 μm.
Expression of CD73 in mouse tissues. Double stainings are shown for skin (A-B), lymph node (C), and gut (D) using anti-CD73, anti–LYVE-1, and anti-podoplanin antibodies as indicated. Arrows point to the double-positive vessels and an arrowhead points to the lymphatic sinus. Scale bar, 100 μm.
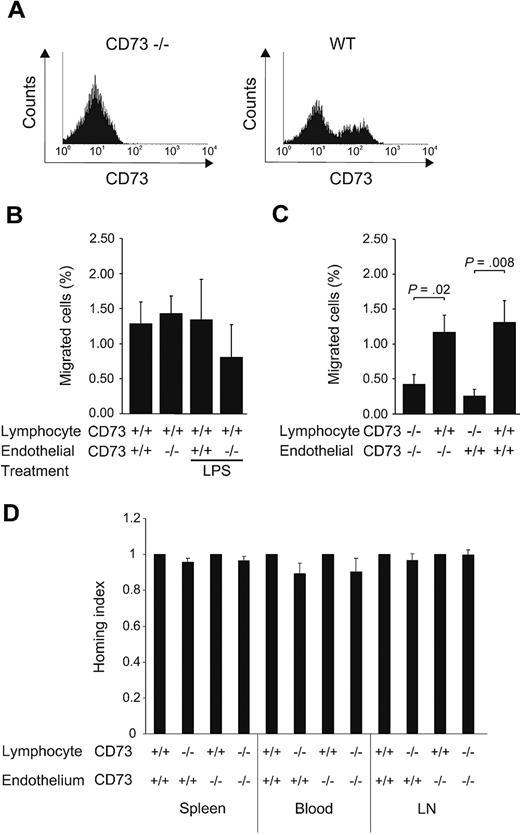
To study the in vivo significance of CD73 on lymphocyte migration via the afferent lymphatics into the draining lymph nodes, CFSE-labeled lymphocytes from wt or CD73-deficient mice were injected subcutaneously into the skin of both normal and CD73-deficient mice and the number of migrated lymphocytes was analyzed in the draining lymph nodes 12 hours later. This time point gives enough time for lymphocytes to migrate into the nodes and at this time point the homed cells have not yet left the node.19,20 The injected wt lymphocytes contained ∼ 40% CD73-positive lymphocytes (Figure 5A). The results of these experiments showed that CD73-deficient lymphatics were able to mediate the trafficking as well as wt lymphatics in normal conditions (Figure 5B).
Lymphocyte CD73 instead of endothelial CD73 is important in migration of lymphocytes via afferent lymphatics. (A) Expression of CD73 on lymphocytes used for the transfer experiments. (B) Migration of subcutaneously injected wt lymphocytes (n = 10) from the skin into the draining lymph nodes of CD73-deficient and wt mice under normal conditions and after LPS challenge. (C) Migration of lymphocytes isolated from CD73-deficient (n = 5) and wt mice (n = 5) to the draining lymph nodes of CD73-deficient and wt recipients. Minimum of 5 mice were used as recipients in each combination. (D) Lymphocyte migration via the blood vessels. CFSE-labeled wt lymphocytes and TRITC-labeled CD73-deficient lymphocytes were injected into the tail vein of CD73-deficient (n = 7) and age-matched wt recipient (n = 7). Homing of the labeled lymphocytes to peripheral and mesenteric lymph nodes (LN), spleen, and blood was analyzed by flow cytometry. The results are presented as homing indexes (mean ± SEM).
Lymphocyte CD73 instead of endothelial CD73 is important in migration of lymphocytes via afferent lymphatics. (A) Expression of CD73 on lymphocytes used for the transfer experiments. (B) Migration of subcutaneously injected wt lymphocytes (n = 10) from the skin into the draining lymph nodes of CD73-deficient and wt mice under normal conditions and after LPS challenge. (C) Migration of lymphocytes isolated from CD73-deficient (n = 5) and wt mice (n = 5) to the draining lymph nodes of CD73-deficient and wt recipients. Minimum of 5 mice were used as recipients in each combination. (D) Lymphocyte migration via the blood vessels. CFSE-labeled wt lymphocytes and TRITC-labeled CD73-deficient lymphocytes were injected into the tail vein of CD73-deficient (n = 7) and age-matched wt recipient (n = 7). Homing of the labeled lymphocytes to peripheral and mesenteric lymph nodes (LN), spleen, and blood was analyzed by flow cytometry. The results are presented as homing indexes (mean ± SEM).
As it has been reported that an LPS challenge results in increased homing via HEV of CD73-deficient mice,12 we challenged the mice with subcutaneous LPS injection subsequent to the subcutaneous lymphocyte transfer. LPS injection resulted in a more prominent local inflammation (observed as redness and swelling) in CD73–deficient than in control mice. However, LPS injection did not increase the number of CD73-positive lymphatics or the frequency of CD73/LYVE-1 double-positive lymphatics (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In line with this, lymphatic endothelium of the deficient mice supported the migration of lymphocytes as efficiently as that of wt mice (Figure 5B). In contrast, when CFSE-labeled lymphocytes from CD73-deficient mice were injected subcutaneously into the footpads of both wt and CD73-deficient mice, the lack of CD73 on lymphocytes led to their significantly decreased migration via the afferent lymphatics (Figure 5C). Thus, the lymphocyte CD73 is important for their proper migration to the draining lymph nodes via afferent lymphatics.
We have earlier demonstrated that engagement of lymphocyte CD73 causes clustering of LFA-1 and enhanced lymphocyte interaction with TNF-α–treated HUVECs.21 To test whether this phenomenon is involved in lymphocyte migration via the afferent lymphatics, we treated lymphocytes from both CD73-deficient and wt mice with a function-blocking LFA-1 antibody and a control antibody before the migration assays. The anti–LFA-1 antibody treatment did not reduce migration of either CD73-positive or -negative lymphocytes into the draining lymph nodes indicating that LFA-1 is not involved in this process (supplemental Figure 2).
We also analyzed trafficking of intravenously injected wt and CD73-deficient lymphocytes via wt and CD73-deficient HEVs in 4 hours homing assays to see, whether lymphocyte or endothelial CD73 plays a role in lymphocyte entrance to lymphoid organs via the blood vessels in noninflamed conditions. The results of these experiments unambiguously showed that lymphocyte trafficking via HEVs was the same regardless of the CD73 expression status on lymphocytes or HEVs in normal conditions (Figure 5D).
Dendritic cell trafficking via the lymphatics is not dependent on CD73
As afferent lymphatics are the central route for skin dendritic cells to enter the draining lymph nodes, we also tested whether their traffic involves CD73. The migration of dendritic cells, which are CD73 negative was comparable as equal number of migrated dendritic cells (CD11c and FITC double positive) were found in auricular skin draining lymph nodes of wt and CD73-deficient mice after FITC painting (1.50% ± 0.13% and 1.84% ± 0.43%, respectively, n = 3 + 3). In addition, similar numbers of dendritic cells emigrated from ears of CD73-deficient and wt mice during skin explant culture (5943 ± 1791 DC/ear and 5346 ± 737 DC/ear, respectively, n = 6 + 6). Thus, results of both FITC-painting and skin explant assays exclude the contribution of endothelial CD73 in trafficking of dendritic cells via afferent lymphatics.
Discussion
Major findings of this work were (1) heterogeneous expression pattern of CD73 among the lymphatics, and (2) the role of lymphocyte CD73 instead of lymphatic endothelial CD73 in lymphocyte trafficking via the afferent lymphatics. Both these findings are novel and will increase our understanding of the role of CD73 as a homing-associated molecule in different vascular beds and on lymphocytes.
Many leukocyte subtypes migrate via the afferent lymphatics into the draining lymph nodes but only lymphocytes are able to leave the nodes via the efferent lymphatics.22-24 So far, no molecular basis of this phenomenon has been found as none of the currently known markers used to identify lymphatics discriminate between afferent and efferent lymphatics. Therefore, selective expression of CD73 on afferent but not on efferent lymphatics makes CD73 unique among the lymphatic markers. Based on this expression pattern, it may be envisioned that although CD73 on afferent lymphatics does not seem to directly contribute to the leukocyte trafficking its existence on afferent lymphatics makes the adenosine milieu different in afferent and efferent lymphatics and may indirectly contribute to the cell migration in certain conditions in these different arms of lymphatics.
Adenosine is highly anti-inflammatory and it acts both on leukocyte and endothelial sites.25 Activation of adenosine receptor A2A and A2B inhibits L-selectin shedding and reduces up-regulation of β2 integrins and leads to diminished adhesion of leukocytes to microvascular endothelium. Moreover, adenosine decreases cytokine release from vasculature and leukocytes, and thus limits leukocyte extravasation and immune reactions.9,10,26-28 These mechanisms are not obviously contributing to the normal lymphocyte migration from the periphery to the draining lymph nodes as lack of CD73 from lymphatic endothelium did not alter lymphocyte trafficking via the afferent lymphatics. In this context it is worth to note that only a small minority of the afferent lymphatics in mouse skin is CD73 positive. This is in a clear contrast to the situation in humans, in which the majority of the afferent lymphatics express CD73. Therefore, we challenged the skin with LPS and studied, whether the role of CD73 becomes significant in the leukocyte trafficking under this inflammatory challenge. Interestingly, LPS injection led to a more pronounced macroscopic inflammation in the CD73-deficient than in wt mice consistent with the reported blood vessel leakiness in the CD73-deficient mice at sites of inflammation.12,29,30 However, despite inflammation the migration-supporting function of lymphatic endothelium does not seem to be dependent on CD73 as it mediated lymphocyte trafficking similarly as wt lymphatics in this condition. Thus, the reported LPS-induced increase in the size of the draining lymph nodes of CD73-deficient mice in comparison to the wt mice is mainly because of the endothelial leakage and subsequent increase of lymphocyte influx via HEV—the main route for lymphocyte entrance into the nodes.12
CD73 on blood vasculature is traditionally linked to permeability and later confirmed by the findings with the CD73-deficient mice.15,31,32 Although lymphatic endothelium has higher CD73 expression than blood vessel endothelium, it may not be connected to the endothelial barrier function as lymphatic vessels are discontinuous and do not have tight junctions in the similar fashion as blood endothelium, thus being inherently leaky. Surprisingly, we did not find a role for endothelial CD73 on lymphatics in supporting trafficking and migration of leukocytes. In contrast, however, CD73 expressed on lymphocytes seems to be fundamentally involved in lymphocyte trafficking via the afferent lymphatics. This is an excellent example of a situation, where expression of a molecule per se cannot be directly connected to the function. In the case of CD73, the cell type with its molecular “make-up” is decisive of the function and behavior of the molecule.
Although lymphocyte migration via afferent lymphatics into the draining lymph nodes is a fundamental element in a proper immune response, the only molecule on the lymphocyte surface known to be required for trafficking via this route is CCR7.20 CCR7 is a chemokine receptor binding to CCL21 chemokine presented on the surface of lymphatics.33 Thus, identification of the involvement of CD73 in this process brings new dimensions to the lymphocyte migration within afferent lymphatics and suggests that cell trafficking via the lymphatics is also a complex phenomenon involving structurally different molecular families as is the case in lymphocyte extravasation from the blood stream into the tissues.
We have earlier shown that engagement of lymphocyte CD73 by an antibody mimicking the ligand binding causes integrin (lymphocyte function associated antigen, LFA-1) clustering and increased adherence to the TNF-α–treated HUVECs.21 Our current finding of a marked decrease in trafficking of CD73-deficient lymphocytes via the afferent lymphatics is in concordance with a hypothesis that a comparable mechanism may be functional in lymphocyte trafficking via the afferent lymphatics. As a matter of fact expression of a ligand of LFA-1—ICAM-1—has been reported on lymphatic endothelium,34 making this scenario possible. To test this possibility we analyzed, whether blocking of LFA-1 either on CD73-positive or -negative lymphocytes affects the trafficking of lymphocytes via the afferent lymphatics. These experiments did not demonstrate any role for LFA-1 in this process. Thus, lymphocyte homing to peripheral lymph nodes (PLNs) via HEVs is LFA dependent,35 whereas lymphocyte entrance to PLNs from the skin via the lymphatics is apparently LFA-1 independent. In fact, the trafficking of lymphocytes via the afferent lymphatics resembles migration of dendritic cells through these vessels in the sense that they do not need any integrins for this process either.36 The lack of LFA-1 contribution in lymphocyte migration via the afferent lymphatic vessels into the draining lymph nodes further emphasizes the difference in lymphocyte trafficking via the lymphatics and blood vasculature.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Linda Thompson for CD73 knockout mice, Dontscho Kerjaschki for anti-podoplanin antibody, Heikki Huhtinen for sample collection, Sari Mäki and Mari Parsama for technical help, and Anne Sovikoski-Georgieva for secretarial help.
This work was supported by the Finnish Academy (S.J., M.S.), the Finnish Cancer Union (S.J.), the Sigrid Juselius Foundation (S.J., M.S.), and the Arvo and Inkeri Suominen Foundation (S. J.).
Authorship
Contribution: A.Å., M.K., G.G.Y., P.S., and J.N. performed research and analyzed and interpreted the data; and M.S. and S.J. designed the work and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Sirpa Jalkanen, MediCity Research Laboratory, University of Turku, Tykistökatu 6 A, FIN-20520 Turku, Finland; e-mail: sirpa.jalkanen@utu.fi.
References
Author notes
A.Å., M.K., and G.G.Y. contributed equally to this study.

![Figure 3. Nucleotide metabolism is different in vascular and lymphatic endothelial cells. (A) Vascular human dermal microvascular endothelial cells (HDMEC) were incubated 45 minutes with 20μM [3H]AMP in the presence of 20μM APCP, 250μM ATP, and 100μM Ap5A (as indicated on lanes 2-5), separated by TLC and developed by autoradiography. In another experiment, vascular and lymphatic cells were incubated for 25 minutes with 20μM [3H]AMP (lanes 6, 7). Blanks (Bl) show the radiochemical purity of [3H]AMP (lanes 1, 8), and arrows indicate the positions of nucleotide and adenosine standards. (B) Activities of ATPase, ADPase, ecto-5′-nucleotidase (5′-NT), and adenylate kinase were determined in vascular and lymphatic endothelial cells by using saturating concentrations of 3H-labeled nucleotide (mean ± SEM; n = 5). *P < .05. (C) Expression of CD73 on podoplanin-positive (lymphatic, left) and -negative (blood vessel, right) endothelial cells analyzed by FACS.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/16/10.1182_blood-2010-11-321646/4/m_zh89991169620003.jpeg?Expires=1769105865&Signature=s8XFPIAK8eL~zSwZV3GqscEFID54eDgyC6nx0OOlVg-0Xaxt8bvnAuLl64jm~pSHBFZrE4RS2I8Q76K~5D1tu1Gc9MjZ1d4K0sFhYa1M7f737BK-dsQhJAJTckpL7itXXmCAw4S5vb8hKtFe7rNvrm82s0E0TUZ9xWl8CDTLhmuAmEkqCMVUTmaB5hviNqOTuvHfVxYez3m2vF1N4JSfLimK~-DLiBw7RPqjh8Js0gq~iC4NEMI~ejRy2k07T9SCJQIN7NGy1qHGVNiWlsR23f2bzHEJJ-FsBBYzdBXvemjI3ye4-4D5~eyuf6-lAzy63jkfImY0HU2REz7ZLrxomQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal