Abstract
CD133 is a hallmark of primitive myeloid progenitors. We have addressed whether human cord blood cells selected for CD133 can generate dendritic cells, and Langerhans cells in particular, in conditions that promote that generation from CD34+ progenitors. Transforming growth factor-β1 (TGF-β1) and anti–TGF-β1 antibody, respectively, were added in some experiments. With TGF-β, monocytoid cells were recognized after 7 days. Immunophenotypically immature dendritic cells were present at day 14. After 4 more days, the cells expressed CD54, CD80, CD83, and CD86 and were potent stimulators in mixed lymphocyte reaction; part of the cells expressed CD1a and langerin, but not Birbeck granules. Without TGF-β, only a small fraction of cells acquired a dendritic shape and expressed the maturation-related antigens, and lymphocytes were poorly stimulated. With anti–TGF-β, the cell growth was greatly hampered, CD54 and langerin were never expressed, and lymphocytes were stimulated weakly. In conclusion, CD133+ progenitors can give rise in vitro, through definite steps, to mature, immunostimulatory dendritic cells with molecular features of Langerhans cells, although without Birbeck granules. Addition of TGF-β1 helps to stimulate cell growth and promotes the acquisition of mature immunophenotypical and functional features. Neither langerin nor Birbeck granules proved indispensable for lymphocyte stimulation.
Introduction
Dendritic cells (DCs) are the most efficient, professional antigen-presenting cells of the immune system and are key components for the induction of primary immune responses and the maintenance of immunologic tolerance.1 DCs are heterogeneous; and in humans, one can recognize immature and mature DCs and, among the former, epidermal DCs (ie, Langerhans cells [LCs] and connective tissue DCs).
Immature DCs are particularly able to uptake and process antigens in late endosomal and lysosomal compartments that are rich in major histocompatibility complex class II molecules (MHC-II).2
LCs are immature DCs of the epidermis and neighboring mucosae. Because of this position, they are critical in the induction of immune responses toward infections and skin tumors3 and are credited to play a role in allergic skin diseases.4 The defining marker of LCs is the presence of Birbeck granules (BGs), which are detected by electron microscopy.5 LCs are characterized by the expression of MHC-II, CD1a, E-cadherin, and langerin (CD207).6 The c-type lectin CD207 is localized not only at the cell surface, but also in the endosomal recycling compartment, in BG, and in other tubular and vesicular structures.7,8 CD1a and BG-positive cells, also defined as LCs, have been furthermore described in the arterial wall,9 besides skin and skin-draining lymph nodes.
Mature DCs are able to present previously uptaken antigens to lymphocytes; during maturation, the cells acquire or increase the expression of CD83 and of the costimulatory adhesion molecules CD54, CD80, and CD86; if they derive from LCs, they lose CD1a, langerin, and E-cadherin expression.10
The most commonly used marker to identify human hematopoietic stem cells is CD34, a stage-specific antigen associated with hematopoietic stem and progenitor cells. CD34 antigen density is highest on early progenitors, and the density decreases progressively as cells mature. Fully differentiated hematopoietic cells do not express CD34.11
A more recently described antigen for the identification of human hematopoietic stem cells is AC133 (CD133),12 a 5-transmembrane glycoprotein homologous to murine prominin. The antigen is phylogenetically conserved from Caenorhabditis elegans to Drosophila, zebrafish, and mammals.13 The majority of CD133+ cells coexpress CD34.14
Using CD133 as a stem cell marker, a functional hierarchy within the CD34+ cell population has been described: CD133+ cells are interpreted as the most primitive blood cells.14 Selection for CD133 can provide a source of cells for expansion and differentiation alternative to CD34,15 and CD133+ cells have been successfully tested in hematopoietic stem cell transplantation.15,16
Experiments designed to generate LCs from immature precursors in vitro have given contradictory results (Table 1). Starting from apparently similar populations of CD34+ circulating progenitors, DCs have been obtained in culture using very different mixtures and timing of administration of cytokines. In particular, LCs equipped with BG have been obtained in some instances,17,26 whereas in other instances the cells generated in vitro were said to be LCs without verification by electron microscopy,18,19 and in one report the generated cells had immunocytochemical features of LCs but were explicitly defined as devoid of BG20 ; DCs with features of LCs but lacking the expression of BG have been described also by other groups.21,22 Strunk et al,23 culturing CD34+ cells isolated from human peripheral blood, obtained 3 distinct CD1a+ populations: LCs, as evidenced by the presence of BG; CD14+ monocytes; and BG-negative cells with a dendritic morphology. They interpreted this last population as LCs that had not yet acquired BG. Although langerin is involved in the formation of BG,8 DCs expressing this marker do not necessarily display BG and langerin expression does not fully correlate with BG formation; hence, additional signals must be necessary to complete LC differentiation.21
Comparison of the culture condition and results in studies on CD34+ and CD133+ precursors
| . | Caux et al . | Caux et al . | Strunk et al . | Ito et al . | Riedl et al . | Mohamadzadeh et al . | Mollah et al . | Mollah et al . | Bartz et al . | Hubert et al . | Goussetis et al . | Current study . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | 24 | 24 | 23 | 22 | 18 | 21 | 25 | 25 | 20 | 17 | 19 | NA |
| Year | 1992 | 1992 | 1996 | 1999 | 2000 | 2001 | 2003 | 2003 | 2003 | 2005 | 2006 | 2010 |
| Seeded cells | Cord blood CD34+ | Cord blood CD34+ | Peripheral blood CD34+ | Peripheral blood CD14+ | Cord blood CD34+ | Peripheral blood CD14+ | Cord blood CD34+ | Cord blood CD34+ | Cord blood CD34+/CD133+ | Cord blood CD34+ | Cord blood CD133+ | Cord blood CD133+ |
| Medium | RPMI 1640 | RPMI 1640 | IMDM | RPMI 1640 | X-VIVO 15 | RPMI 1640 | X-VIVO 15 | X-VIVO 15 | RPMI 1640 | RPMI 1640 | StemSpan | RPMI 1640 |
| Serum | 10% FBS | 10% FBS | 10% FBS | 10% human | 10% FBS | No | No | 10% FBS | 10% FBS | 10% FBS | No | 10% FBS |
| Cytokines | ||||||||||||
| GM-CSF | + | + | + | + | + | + | + | + | + | + | + | |
| M-CSF | + | |||||||||||
| SCF | + | + | + | + | + | + | + | + | + | |||
| TPO | + | + | + | |||||||||
| Flt3L | + | + | + | + | + | + | ||||||
| IL-4 | + | + | + | + | + | |||||||
| IL-6 | + | |||||||||||
| IL-15 | + | |||||||||||
| TNF-α | + | + | + | + | + | + | + | + | + | |||
| TGF-β1 | + | + | + | + | + | + | + | |||||
| Harvested cells | ||||||||||||
| Dendritic shape | + | + | + | + | + | + | + | + | + | + | + | + |
| CD1a | + | CD1a−/CD14+ | + | + | + | + | + | +* | + | + | + | + |
| Birbeck granules | + | − | +/−† | + | Not tested | − | + | +* | − | + | Not tested | − |
| Langerin | + | − | + | Not tested | + | + | +* | + | + | Not tested | + | |
| Langerin/CD207 | Not tested | Not tested | Not tested | + | Not tested | Not tested | Not tested | Not tested | + | + | Not tested | + |
| Mixed lymphocyte reaction | Potent stimulators | Potent stimulators | Potent stimulators | Potent stimulators | Not tested | Not tested | Potent stimulators | Potent stimulators‡ | Potent stimulators§ | Not tested | Not tested | Potent stimulators |
| . | Caux et al . | Caux et al . | Strunk et al . | Ito et al . | Riedl et al . | Mohamadzadeh et al . | Mollah et al . | Mollah et al . | Bartz et al . | Hubert et al . | Goussetis et al . | Current study . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | 24 | 24 | 23 | 22 | 18 | 21 | 25 | 25 | 20 | 17 | 19 | NA |
| Year | 1992 | 1992 | 1996 | 1999 | 2000 | 2001 | 2003 | 2003 | 2003 | 2005 | 2006 | 2010 |
| Seeded cells | Cord blood CD34+ | Cord blood CD34+ | Peripheral blood CD34+ | Peripheral blood CD14+ | Cord blood CD34+ | Peripheral blood CD14+ | Cord blood CD34+ | Cord blood CD34+ | Cord blood CD34+/CD133+ | Cord blood CD34+ | Cord blood CD133+ | Cord blood CD133+ |
| Medium | RPMI 1640 | RPMI 1640 | IMDM | RPMI 1640 | X-VIVO 15 | RPMI 1640 | X-VIVO 15 | X-VIVO 15 | RPMI 1640 | RPMI 1640 | StemSpan | RPMI 1640 |
| Serum | 10% FBS | 10% FBS | 10% FBS | 10% human | 10% FBS | No | No | 10% FBS | 10% FBS | 10% FBS | No | 10% FBS |
| Cytokines | ||||||||||||
| GM-CSF | + | + | + | + | + | + | + | + | + | + | + | |
| M-CSF | + | |||||||||||
| SCF | + | + | + | + | + | + | + | + | + | |||
| TPO | + | + | + | |||||||||
| Flt3L | + | + | + | + | + | + | ||||||
| IL-4 | + | + | + | + | + | |||||||
| IL-6 | + | |||||||||||
| IL-15 | + | |||||||||||
| TNF-α | + | + | + | + | + | + | + | + | + | |||
| TGF-β1 | + | + | + | + | + | + | + | |||||
| Harvested cells | ||||||||||||
| Dendritic shape | + | + | + | + | + | + | + | + | + | + | + | + |
| CD1a | + | CD1a−/CD14+ | + | + | + | + | + | +* | + | + | + | + |
| Birbeck granules | + | − | +/−† | + | Not tested | − | + | +* | − | + | Not tested | − |
| Langerin | + | − | + | Not tested | + | + | +* | + | + | Not tested | + | |
| Langerin/CD207 | Not tested | Not tested | Not tested | + | Not tested | Not tested | Not tested | Not tested | + | + | Not tested | + |
| Mixed lymphocyte reaction | Potent stimulators | Potent stimulators | Potent stimulators | Potent stimulators | Not tested | Not tested | Potent stimulators | Potent stimulators‡ | Potent stimulators§ | Not tested | Not tested | Potent stimulators |
NA indicates not applicable; FBS, fetal bovine serum; human, human AB serum; M-CSF, macrophage colony-stimulating factor.
More than with GM-CSF.
Langerin (lag)+/Birbeck granules (BG)+ (Langerhans cells [LC]); lag−/BG− (dendritic cells other than LC); lag+/BG− (LC-like, minor population).
Higher than with GM-CSF.
In part CLA+ and weak stimulators.
Until now, little experience has been gained on the generation of DCs in general and of LCs in particular from CD133+ cells (Table 1). Although one group stated that they had generated LCs irrespectively of starting from CD34+ or from CD133+ cells, they have only shown the results for the cultures of CD34+ progenitors.20 Another group reported to have generated DCs from CD133+ precursors; the differentiated cells were recognized primarily by the shape and were in part CD1a+ and in part CD1a−; the relationships between the culture conditions and the type of cells generated were unclear, and other relevant features were not investigated (presence of BG, lymphocyte-stimulating activity).19
The differentiation of DCs from hematopoietic stem cells and from circulating monocytes can be driven in vitro by cytokine mixtures containing granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-4 (IL-4), and tumor necrosis factor-α (TNF-alpha).24 Transforming growth factor-β1 (TGF-β1) has been indicated as required for the generation of LCs in particular,20,27-30 as assessed also in serum-free culture,27 and concurs with GM-CSF and TNF-α to the maintenance of the differentiated state of LC within epidermis.31 However, Caux et al24,26 could generate CD1a+, BG-containing LCs from human cord blood CD34+ precursors incubated without that cytokine in the presence of fetal bovine serum, which suggests that serum can supply enough TGF-β for the differentiation of LCs. Endogenous TGF-β is required for the development of LCs from CD34+ hematopoietic progenitor cells, and addition of anti–TGF-β antibody inhibits the generation of LC-like DCs from CD34+ cells.32
Here we have adopted a culture protocol that had been successfully used by other authors17 to generate LCs from CD34+ progenitors (Table 1), to generate mature DCs from CD133+ hematopoietic stem cells in vitro. We aimed at: (1) verifying the possibility of differentiating DCs in general and LCs in particular from CD133+ cord blood progenitors; (2) assessing the steps during this differentiation, as a premise for manipulating these steps in future studies; and (3) verifying the influence of TGF-β on the differentiation of DCs from CD133+ precursors, given the ambiguity of existing studies on the role of this cytokine on the differentiation of LCs from CD34+ precursors.
Methods
Isolation of CD133+
Umbilical cord blood samples were obtained from donations unsuitable for banking because of too few nucleated cells (average, 0.9 × 109; range, 0.8 × 109-1 × 109), according to the Italian law and the Institution ethical rules. The donors were considered healthy on the following bases: physical examination of their mothers during pregnancy, physiologic delivery of fetus, normal aspect of newborn at clinical examination, lack of known diseases of the mother incompatible with blood and stem cell donation, and negativity of newborn blood at serologic tests for blood-borne diseases. The blood was diluted 1:2 in 0.1M phosphate-buffered saline (PBS), pH 7.4, and mononuclear cells were isolated by centrifugation on Ficoll density gradient (Lymphoprep, Nicomed Pharma), washed twice in PBS and suspended in a final volume of 300 μL of PBE buffer (ie, PBS containing 1% bovine serum albumin; Sigma-Aldrich) and 1mM ethylenediaminetetraacetic acid at pH 7.6, per 108 total cells. On blocking unspecific binding sites with human IgG (Miltenyi Biotec), the cells were labeled with colloidal superparamagnetic microbeads conjugated with mouse antihuman CD133 monoclonal antibody (MiniMACS; Miltenyi Biotec), for 30 minutes at 4°C. The cells were washed, applied to a prefilled separation column, placed into a magnetic field, and allowed to pass through it; CD133+ retained cells were eluted by removing the magnetic field and flushing the column with PBE buffer. To increase the purification rate, this fraction was loaded onto a second freshly prepared column and CD133+ cells were isolated again as described in the preceding sentence. A sample of the purified cell fraction was processed for the determination of cell number, degree of pureness, and viability. By comparison, CD34+ cells were isolated from cord blood with the same procedure as described in the preceding sentences about CD133 selection but using microbeads conjugated with mouse antihuman CD34 monoclonal antibody (Miltenyi Biotec). These cells were analyzed for CD133 expression with the same monoclonal antibodies (Miltenyi Biotec) used for the separation of CD133+ cells, not bound to microbeads, but were not subjected to culture.
Cell culture
The cells were seeded at a mean concentration of 2.0 ± 0.1 × 105 cells/mL (at least 95% vital, SD ± 7) in RPMI 1640 with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 0.1 ng/mL streptomycin (all from Sigma-Aldrich). The serum was Sigma-Aldrich catalog no. F4135; the biochemical and hormone profile is available by the producer; 3 different lots were used for the experiments, always with similar results. The cells were expanded until day 7 with GM-CSF (10 ng/mL), TNF-α (10 ng/mL), IL-4 (10 ng/mL), thrombopoietin (TPO; 10 ng/mL), fms-like tyrosine kinase-3 ligand (Flt3L; 25 ng/mL), stem cell factor (SCF; 20 ng/mL), and TGF-β (10 ng/mL). All cytokines were purchased from PeproTech. The cells were differentiated until day 18 in the same medium without SCF, TPO, and Flt3L; TGF-β concentration was raised to 20 ng/mL since day 14. Some experiments were conducted without TGF-β and some without this cytokine and with a monoclonal antibody anti–TGF-β1 (clone 9016.2, Sigma-Aldrich). This antibody neutralizes the biologic activity of TGF-β1 and TGF-β2; it was used at a final concentration of 10 ng/mL, throughout all the cultures except during mixed lymphocyte reaction. The cells cultured in the presence of anti–TGF-β were analyzed in detail only at day 18. The culture medium was changed every third day; all the cells in the flasks were replated (ie, those adhering to the bottom and those in suspension, which were a nonnegligible fraction until 7 days). We have tried to separate floating cells from adhering cells and replate them in separate flasks, but neither population could thrive, and afterward the cells were always kept in the same flask.
Electron microscopy
Cytocentrifugates were fixed in 2% formaldehyde and 2.5% glutaraldehyde in 0.1M cacodylate buffer, pH 7.4, osmicated, and embedded in epoxy resin. Sections approximately 70 nm thick were stained with lead acetate and uranyl acetate and observed in a JEM 1010 electron microscope (Jeol) at 80 kV. The numbers of cells with features of apoptosis and of necrosis were counted on electron photomicrographs, scanning at least 50 cells for each experimental condition and time point. Initial apoptosis was indicated by cell shrinkage and condensation of the nuclear and cytoplasmic matrix, and advanced apoptosis by smoothening of cell profile, increased condensation of the cytoplasmic matrix alternated with vacuoles, karyolysis, and karyorrhexis.33 Necrosis was indicated by interrupted cell membrane, cytoplasmic swelling, organelle dispersion, and homogeneous, poorly dense chromatin.33
Flow cytometry
The cells were immunolabeled for 20 minutes at 4°C. The following monoclonal antibodies were applied: HLA-DR, DP, DQ-fluorescein isothiocyanate (FITC); CD11b-phycoerythrin (PE); CD14-PE; CD16-FITC; CD34-PE or CD34-FITC; CD45-FITC (BD Biosciences PharMingen); CD80-FITC, CD83-phycerythrin-Cy5 (PC5), CD86-PE (Immunotech); CD133/2-PE (Miltenyi Biotec); purified CD207/langerin (Dendritics; IgG1, clone DCGM4/122D5) followed by goat antimouse IgG F(ab′)2 (H + L)–AlexaFluor-546 (Invitrogen); unspecific IgG1-FITC and IgG1-PE (BD Biosciences PharMingen) were used as negative controls. Dead cells were counted by flow cytometry on labeling with 7-amino-actinomicin D (BD Biosciences PharMingen). Flow cytometric acquisition was performed by collecting 104 events on a FACScalibur (BD Biosciences) or an Epics XL-System II flow cytometer (Beckman Coulter), and data were analyzed as previously described.34
Immunofluorescence
Cytospins were fixed with acetone for 5 minutes at room temperature. After blocking nonspecific binding sites with 10 ng/mL bovine serum albumin (Sigma-Aldrich) in PBS, primary monoclonal antibodies against the following antigens were applied for 90 minutes at 37°C at the indicated dilutions: MHC-II (Cymbus), 1:100; CD1a (Harlan/SeraLab), 1:50; CD207/langerin, from Novocastra Laboratories (IgG2b, clone 12D6), 1:100; CD207/langerin, from Dendritics (IgG1, clone DCGM4/122D5), 1:50; CD54 (Cymbus), 1:50. FITC-labeled, goat antimouse, polyclonal antibodies (Sigma-Aldrich), 1:50, were applied for 60 minutes at 37°C as secondary ones. Omission of primary antibodies or substitution with irrelevant ones were used as negative controls. The slides were mounted with Gel/Mount (Biomeda), observed in an Axioskop microscope equipped for epifluorescence (Carl Zeiss) and captured with an Axio Vision 4 system, consisting of a digital multichannel fluorescence module and dedicated software (Carl Zeiss).
Fluorescent labeling of mitochondria
Cell pellets were suspended in prewarmed (37°C) staining solution containing MitoTracker Green FM (50nM; Invitrogen) and incubated for 45 minutes at 37°C. The cells were then pelleted again by centrifugation, suspended in fresh, prewarmed medium, deposited on poly-D-lysine-coated slides, and analyzed by fluorescence microscopy. Digital photomicrographs were taken through Axio Vision 4 system (Carl Zeiss) at original magnification × 40, with fixed exposition time (96 seconds) to make the image dependent only on the fluorescent emission and not on the subjective perception of the observer or on averaging algorithms of the photographing system. The photomicrographs were analyzed with ImageJ software (National Institutes of Health) at the single-cell level. For each analyzed cell, the signal level (in arbitrary units; 0 = black, ie, absence of signal; 255 = white) was measured in a cell area devoid of signal at visual inspection and assumed as background; the threshold was then set at 1.5 times the background, and the surface area and mean signal intensity were measured for all areas above threshold. The background was subtracted from the mean signal intensity, and the result was multiplied for the surface area above threshold, to get the signal intensity of that cell, in arbitrary units.35
Autofluorescence analysis
Cell and tissue autofluorescence is a fluorescence emission arising from endogenous fluorophores, the main ones being NAD(P)H and oxidized flavins. Their fluorescence emission, peaked at approximately 460 nm and 530 nm, respectively, is considered an indicator of the intracellular redox state.36 The analysis of autofluorescence does not require any treatment with chemical reagent or labeling substances and may provide information on cellular energy metabolism and morphology as well,36 and may be helpful to follow the differentiation and maturation of living DCs.
In our experiments, cell autofluorescence was analyzed using an inverted epifluorescence microscope (Eclipse TE-2000-E; Nikon) equipped with an oil-immersion CF-UV Fluor 100× objective (NA 1.3), less than 365 nm excitation from a filtered (10-nm bandwidth interference filter, 365FS10-25, Andover), high-pressure mercury lamp (HBO 100W; Osram). The signal, transmitted through a dichroic mirror at 400 nm (DM400, Nikon), was detected by a Hires IV digital CCD camera (DTA) equipped with a Kodak KAF261E detector (20 μm, 512 × 512 pixels). Imaging was accomplished using a motorized filter wheel, containing up to 8 different interference filters, placed in front of the CCD detector. This allowed for multispectral, sequential acquisition in different emission bands. The choice of the filter combination was made on the basis of the main spectral bands determined by preliminary analysis of the autofluorescence spectra. Both the CCD camera and the filter wheel were controlled by a modified routine running under ViSTA software v1.0.3 (DTA). The images were directly digitalized in the CCD controller with 16-bit dynamics and transferred to the storage computer on a digital interface. The size of the field detected by the 100× objective was approximately 69 × 69 μm (spatial calibration of 0.13 μm pixel), as determined by imaging 6-μm fluorescent microspheres (Invitrogen). For each sample, 3 monochrome images were sequentially acquired in spectral bands 40 nm wide (full width at half maximum), peaked at 450, 550, and 658 nm, respectively, with integration times of approximately 6 seconds. The monochrome images were then combined in a single image using the red-green-blue technique. Fluorescence spectra were recorded through a band-pass filter (GG400-Schott, UGQ) to remove ultraviolet excitation. Fluorescence spectra, recorded with a 4 seconds integration time and as average from at least 30 different cells, were corrected for the optical system spectral response and smoothed by a gaussian convolution algorithm. After correction, spectra were normalized to their respective peaks as 100% for comparison.
Mixed lymphocyte reaction
At culture day 18, the cells were transferred into quadruplicate cultures at 1 × 104, 1 × 103, and 1 × 102 cells per 100 μL per well in 96-well round-bottom plates with 1 × 105 allogeneic CD4+ T cells (purified from peripheral blood mononuclear cells with MACS CD4 isolation kit II of Miltenyi Biotec). The culture medium was RPMI 1640 with 10% heat inactivated fetal bovine serum (without cytokines). After 5 days, cells were pulsed overnight with 3H-thymidine (1 μCi/well = 0.037 MBq/well). Thymidine incorporation was measured by standard liquid scintillation counting. Results are expressed in counts per minute and shown as mean ± SD of quadruplicate counts.
Statistics
The results are presented as mean and SD. Analysis of variance and, for the quantitative results of immunohistochemistry and for apoptosis and necrosis counts, nonparametric χ2 tests were used with 2 tails, assuming P < .05 as significant.
Results
Start of culture
On selection for CD133 of Ficoll-isolated mononuclear cells, an average of 9.7 × 105 cells (range 4.5 × 105-2.2 × 106) were obtained from each donation. As estimated by flow cytometry, the percentage of CD133-labeled cells was 95% (SD 5%) and viable cells were 95% (SD 7%). The percentage of CD133+ cells, which also expressed CD34, was approximately 98% (SD 8%) and those that also expressed CD45 was 99% (SD 8%). By contrast, in parallel experiments, the percentage of CD34+ cells, which also expressed CD133, was 75% (SD 3%; average of 10 experiments). On culture, the cells increased to a maximum after 7 days and then decreased progressively until day 18, alike with and without added TGF-β, whereas in the presence of anti–TGF-β antibody they grew less numerous than in the other conditions (Figure 1A).
Cell growth, phase-contrast microscopy, and MitoTracker mitochondrial labeling of cells. (A) Numbers of cells at seeding (0 days) and during culture. Black line indicates cultures with TGF-β; continuous gray line, cultures without TGF-β; and discontinuous pale gray line, cells cultured with anti–TGF-β. In the last condition, the cells grew significantly less than in the other ones (P < .05). The results came from 15 experiments with and without TGF-β and 2 experiments with anti–TGF-β. (B,E,G,I,K,M,O) Fluorescence microscopy on MitoTracker Green FM staining. (C,F,H,J,L,N,P) Phase-contrast microscopy. (B-C) Start of culture. (E-H) Seven-day culture with (E-F) and without (G-H) TGF-β. (I-L) Fourteen-day culture with (I-J) and without (K-L) TGF-β. (M-P) Eighteen-day culture with (M-N) and without (O-P) TGF-β. Bar represents 20 μm. (D) MitoTracker Green FM staining intensity (in arbitrary units; mean ± SD), in cells cultured with (black columns) and without TGF-β (gray columns). The differences between cultures with and without TGF-β were significant at all time points (P < .05). Slides were mounted with Gel/Mount (Biomeda); images were taken with Axio Vision 4.1 software through an AxioCam HRm camera applied to an Axioskop microscope with a 40×/0.75 NA air objective (Carl Zeiss), and were processed for print with Photoshop 6.0 for Macintosh (Adobe).
Cell growth, phase-contrast microscopy, and MitoTracker mitochondrial labeling of cells. (A) Numbers of cells at seeding (0 days) and during culture. Black line indicates cultures with TGF-β; continuous gray line, cultures without TGF-β; and discontinuous pale gray line, cells cultured with anti–TGF-β. In the last condition, the cells grew significantly less than in the other ones (P < .05). The results came from 15 experiments with and without TGF-β and 2 experiments with anti–TGF-β. (B,E,G,I,K,M,O) Fluorescence microscopy on MitoTracker Green FM staining. (C,F,H,J,L,N,P) Phase-contrast microscopy. (B-C) Start of culture. (E-H) Seven-day culture with (E-F) and without (G-H) TGF-β. (I-L) Fourteen-day culture with (I-J) and without (K-L) TGF-β. (M-P) Eighteen-day culture with (M-N) and without (O-P) TGF-β. Bar represents 20 μm. (D) MitoTracker Green FM staining intensity (in arbitrary units; mean ± SD), in cells cultured with (black columns) and without TGF-β (gray columns). The differences between cultures with and without TGF-β were significant at all time points (P < .05). Slides were mounted with Gel/Mount (Biomeda); images were taken with Axio Vision 4.1 software through an AxioCam HRm camera applied to an Axioskop microscope with a 40×/0.75 NA air objective (Carl Zeiss), and were processed for print with Photoshop 6.0 for Macintosh (Adobe).
The cells at the start of culture appeared a homogeneous population, small and round (Figures 1C, 2B). Mitochondria were few, as shown by MitoTracker staining (Figure 1B), and only a few blue (460 nm) autofluorescent structures were recognized in the cytoplasm (Figure 2A,I-J). At electron microscopy, the cytoplasm was poor in organelles except for ribosomes. The nucleus had only a few shallow indentures; the chromatin was finely granular, with a thin peripheral condensed rim and small, sparse chromocenters; and the nucleolus was large (Figure 3A-B). Chromatin texture and nucleolar size did not vary appreciably with culture (Figure 3C-H).
Autofluorescence analysis by deconvolution microscopy. (A) Small cell with few blue autofluorescent structures at the start of culture. (B) Same cell as panel A, by phase-contrast microscopy. (C) Intermediate size cell with many, paranuclear, blue autofluorescent structures after 7-day culture with TGF-β. (D) Same cell as panel C by phase-contrast microscopy: the cell is dendritic in shape. (E) Large cells with many autofluorescent structures that appear in part green, after 14-day culture with TGF-β. (F) Same cells as panel E by phase-contrast microscopy: the cells are markedly dendritic in shape. (G-H) Cell after 18-day culture with TGF-β; green autofluorescent structures are less numerous and less intensely fluorescent than after 14-day culture (compare with panel E). Bar represents 10 μm. (I-J) Spectra of autofluorescence emission after 1-day (black), 7-day (dark gray), 14-day (continuous light gray), and 18-day culture (discontinuous light gray). (J) The spectra were normalized assuming the respective highest peak as 100%. Live cells were mounted on slides with PBS; images were taken with ViSTA 1.9.3 software through a KAF261E detector (Kodak) inserted in a Hires IV camera (DTA) applied to an Eclipse TE-2000-E microscope with a 100×/1.3 NA oil objective (Nikon), and were processed for print with Photoshop 6.0 for Macintosh (Adobe).
Autofluorescence analysis by deconvolution microscopy. (A) Small cell with few blue autofluorescent structures at the start of culture. (B) Same cell as panel A, by phase-contrast microscopy. (C) Intermediate size cell with many, paranuclear, blue autofluorescent structures after 7-day culture with TGF-β. (D) Same cell as panel C by phase-contrast microscopy: the cell is dendritic in shape. (E) Large cells with many autofluorescent structures that appear in part green, after 14-day culture with TGF-β. (F) Same cells as panel E by phase-contrast microscopy: the cells are markedly dendritic in shape. (G-H) Cell after 18-day culture with TGF-β; green autofluorescent structures are less numerous and less intensely fluorescent than after 14-day culture (compare with panel E). Bar represents 10 μm. (I-J) Spectra of autofluorescence emission after 1-day (black), 7-day (dark gray), 14-day (continuous light gray), and 18-day culture (discontinuous light gray). (J) The spectra were normalized assuming the respective highest peak as 100%. Live cells were mounted on slides with PBS; images were taken with ViSTA 1.9.3 software through a KAF261E detector (Kodak) inserted in a Hires IV camera (DTA) applied to an Eclipse TE-2000-E microscope with a 100×/1.3 NA oil objective (Nikon), and were processed for print with Photoshop 6.0 for Macintosh (Adobe).
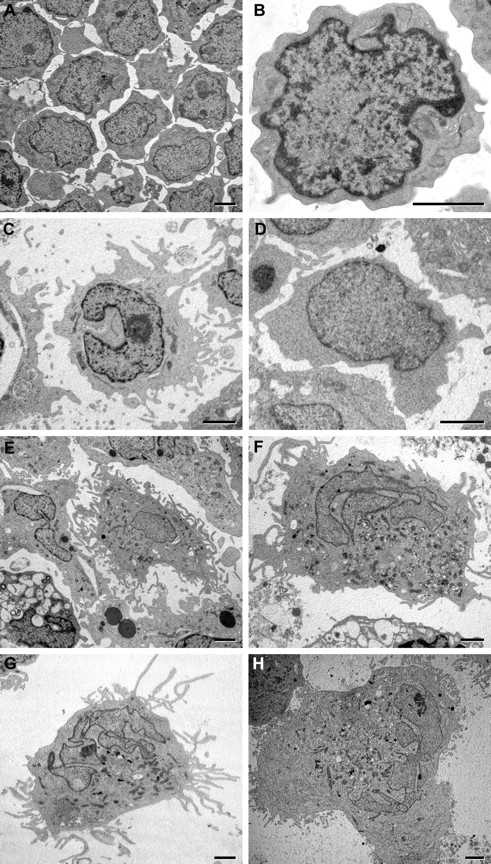
Electron microscopy. CD133+ cells at the start of culture (A-B) and on culture with (C,E,G) and without TGF-β (D,F,H) for 7 days (C-D), 14 days (E-F), and 18 days (G-H). (E) Bottom left corner: cell shows signs of apoptosis. Bars represent 5 μm. Comments on the cell structure at the various time points are detailed in the text. Images were taken with analySIS 5.0 software through a MegaView III camera applied to a JEM 1010 electron microscope at 80kV (Jeol), and were processed for print with Photoshop 6.0 for Macintosh (Adobe).
Electron microscopy. CD133+ cells at the start of culture (A-B) and on culture with (C,E,G) and without TGF-β (D,F,H) for 7 days (C-D), 14 days (E-F), and 18 days (G-H). (E) Bottom left corner: cell shows signs of apoptosis. Bars represent 5 μm. Comments on the cell structure at the various time points are detailed in the text. Images were taken with analySIS 5.0 software through a MegaView III camera applied to a JEM 1010 electron microscope at 80kV (Jeol), and were processed for print with Photoshop 6.0 for Macintosh (Adobe).
Seven-day culture
After 7-day culture with TGF-β, part of the cells adhered to the flask and some showed thin projections all around (Figures 1F, 2D). The expression of immature cell antigens was reduced, more markedly than that of CD133, whereas CD45 persisted; MHC-II came to be expressed by a majority of cells, and CD11b by a minority of cells (Figure 4). The mitochondria appeared significantly increased in number, as shown by the intensity of MitoTracker cell staining (Figure 1E). The intensity of blue autofluorescent emission did not change appreciably (Figure 2C,I-J). At electron microscopy, large cells with cytoplasmic projections of various width and length and with oval, indented nuclei became apparent (Figure 3C) together with cells retaining immature features. Here and at later time points, the organelles of the secretory pathway (rough endoplasmic reticulum and Golgi apparatus) were relatively small with respect to the amount of cytoplasm. Occasional multilaminar inclusions were found, which appeared as round or cup-shaped, membrane-bound bodies with a tightly arranged inner array of concentric electron-dense lamellae (Figure 5A-C).
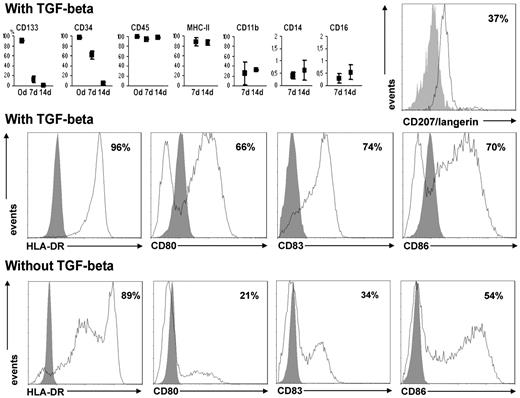
Flow cytometry. (Top row) Percentages of cells expressing several membrane antigens after 7- and 14-day culture with TGF-β (mean and range). Only CD133, CD34, and CD45 were tested at the start of culture. The differences for CD133 and CD34 were significant (P < .05). For CD14 and CD16, the scale is limited to 2% because these antigens were expressed by a strict minority of cells. Far right: Results of a representative experiment showing the expression of CD207/langerin (Dendritics) after 18-day culture with TGF-β. (Middle row) Results of a representative experiment showing the expression of MHC-II and of late differentiation markers of dendritic cells after 18-day culture with TGF-β. (Bottom row) Results of a representative experiment showing the expression of MHC-II and of late differentiation markers of dendritic cells after 18-day culture without TGF-β.
Flow cytometry. (Top row) Percentages of cells expressing several membrane antigens after 7- and 14-day culture with TGF-β (mean and range). Only CD133, CD34, and CD45 were tested at the start of culture. The differences for CD133 and CD34 were significant (P < .05). For CD14 and CD16, the scale is limited to 2% because these antigens were expressed by a strict minority of cells. Far right: Results of a representative experiment showing the expression of CD207/langerin (Dendritics) after 18-day culture with TGF-β. (Middle row) Results of a representative experiment showing the expression of MHC-II and of late differentiation markers of dendritic cells after 18-day culture with TGF-β. (Bottom row) Results of a representative experiment showing the expression of MHC-II and of late differentiation markers of dendritic cells after 18-day culture without TGF-β.
Electron microscopy details. (A-F) Details of cells cultured with TGF-β. (A-C) Variably well-developed multilaminar inclusions (14-day culture). (D) Smooth endoplasmic reticulum (18-day culture). (E) Myelin-like figures (18-day culture). (F) Tubuloreticular inclusions (14-day culture). Bars represent 100 nm. (G-H) Percentage of cells in apoptosis (G) and in necrosis (H) in cultures with TGF-β (black column) and without TGF-β (gray column); only the difference in apoptosis at 14 days was significant (P < .05; χ2 test). Images were taken with analySIS 5.0 software through a MegaView III camera applied to a JEM 1010 electron microscope at 80kV (Jeol), and were processed for print with Photoshop 6.0 for Macintosh (Adobe).
Electron microscopy details. (A-F) Details of cells cultured with TGF-β. (A-C) Variably well-developed multilaminar inclusions (14-day culture). (D) Smooth endoplasmic reticulum (18-day culture). (E) Myelin-like figures (18-day culture). (F) Tubuloreticular inclusions (14-day culture). Bars represent 100 nm. (G-H) Percentage of cells in apoptosis (G) and in necrosis (H) in cultures with TGF-β (black column) and without TGF-β (gray column); only the difference in apoptosis at 14 days was significant (P < .05; χ2 test). Images were taken with analySIS 5.0 software through a MegaView III camera applied to a JEM 1010 electron microscope at 80kV (Jeol), and were processed for print with Photoshop 6.0 for Macintosh (Adobe).
The features of the cells in culture without TGF-β resembled those in culture with this cytokine (Figures 1G-H, 3D, 6A), except that the mitochondria increased less in number than in the cultures with TGF-β (Figure 1D,G). There were cells in apoptosis or necrosis, both with and without TGF-β (Figure 5G-H).
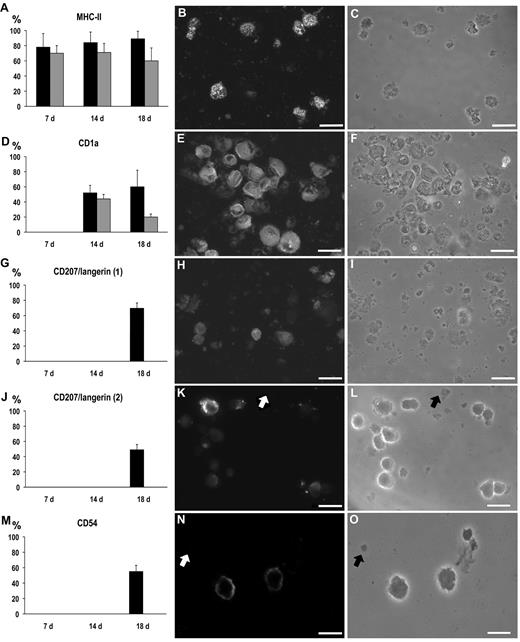
Immunofluorescent labeling. (Left panels) Percentage of labeled cells at immunocytochemistry at 7, 14, and 18 days of culture with (black columns) and without TGF-β (gray columns; mean ± SD). (Middle panels) Immunolabeled cells at fluorescence microscopy, on 18-day culture with TGF-β. (Right panels) Phase-contrast microscopy of the same fields as in the middle panels. (A-C) MHC-II: all the differences between TGF-β treated and untreated cultures were significant (P < .05). (D-F) CD1a; the difference at 18 days was significant (P < .05). (G-I) CD207/langerin (antibody from Novocastra Laboratories). (J-L) CD207/langerin (antibody from Dendritics); note that cellular debris were not stained (arrows). (M-O) CD54; note that cellular debris were not stained (arrows). Bar represents 20 μm. Slides were mounted with Gel/Mount (Biomeda), images were taken with Axio Vision 4.1 software through an AxioCam HRm camera applied to an Axioskop microscope with a 40×0.75 NA air objective (Carl Zeiss), and were processed for print with Photoshop 6.0 for Macintosh (Adobe).
Immunofluorescent labeling. (Left panels) Percentage of labeled cells at immunocytochemistry at 7, 14, and 18 days of culture with (black columns) and without TGF-β (gray columns; mean ± SD). (Middle panels) Immunolabeled cells at fluorescence microscopy, on 18-day culture with TGF-β. (Right panels) Phase-contrast microscopy of the same fields as in the middle panels. (A-C) MHC-II: all the differences between TGF-β treated and untreated cultures were significant (P < .05). (D-F) CD1a; the difference at 18 days was significant (P < .05). (G-I) CD207/langerin (antibody from Novocastra Laboratories). (J-L) CD207/langerin (antibody from Dendritics); note that cellular debris were not stained (arrows). (M-O) CD54; note that cellular debris were not stained (arrows). Bar represents 20 μm. Slides were mounted with Gel/Mount (Biomeda), images were taken with Axio Vision 4.1 software through an AxioCam HRm camera applied to an Axioskop microscope with a 40×0.75 NA air objective (Carl Zeiss), and were processed for print with Photoshop 6.0 for Macintosh (Adobe).
Fourteen-day culture
In cultures with TGF-β, all the cells adhered to the flask and the majority were large, with dendritic shape (Figures 1J, 2F). The immature cell antigens CD34 and CD133 were no more expressed, whereas MHC-II and CD11b were expressed by approximately as many cells as after 7-day culture (Figure 4). At immunocytochemistry, part of the cells were stained for CD1a (Figure 6D). The mitochondria appeared increased in number above what had been seen at 7-day culture (Figure 1D,I). The brightly blue autofluorescent structures in the cytoplasm were much more numerous than at day 7 (Figure 2E), with a much higher emission peak at 460 nm, and the spectral analysis of autofluorescence showed a new, relevant peak at approximately 580 nm (Figure 2I-J). At electron microscopy, the cells showed a variable number of long, thin cytoplasmic projections (Figure 3E); the nuclei appeared with highly irregular profile, sometimes convoluted with lobes connected by extremely thin sheets. The cytoplasm contained areas of smooth endoplasmic reticulum, many multilaminar inclusions, myelin-like figures in various amounts among cells, and occasional inclusions containing tightly packed tubules (Figure 5D-F).
Eighteen-day culture
In cultures with TGF-β, almost all cells had a dendritic shape (Figure 1N). Besides MHC-II (Figures 4, 6A-C), the majority of cells expressed the DC differentiation markers CD80, CD83, and CD86 (Figure 4), as well as the LC marker CD1a and the costimulatory molecule CD54 (Figure 6D-F,M-O). Slightly more than one-third of the cells expressed the LC marker CD207/langerin at flow cytometry (Figure 4) and even more at immunofluorescence (Figure 6G-L). The mitochondria appeared reduced in number compared with 14-day culture (Figure 1D,M). The autofluorescence peak at 580 nm was still noticeable but less intense (Figure 2G-J). At electron microscopy, the picture was similar to that after 14-day culture; BGs were never seen (Figure 3G).
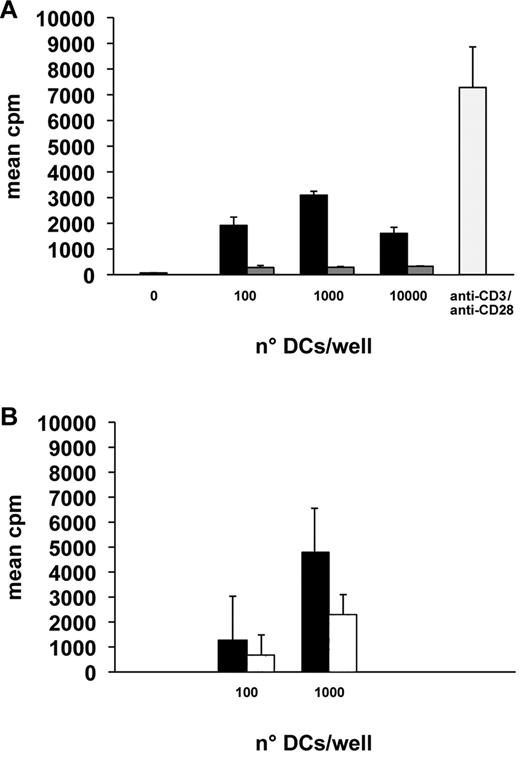
In mixed lymphocyte reaction, a powerful stimulation of CD4+ cell proliferation was seen already at a ratio of one cell per 103 lymphocytes, and the maximum was achieved at a 1:102 cell ratio (Figure 7A).
Mixed lymphocyte reaction. Results (mean ± SD of 4 independent measurements) of representative mixed lymphocyte reaction experiments with the indicated stimulatory cell numbers per 105 respondent cells. (A) Cells cultured 18 days with (black columns) and without TGF-β (gray columns). 0 indicates no stimulatory cell; and anti-CD3/anti-CD28 (pale gray column), lymphocytes stimulated only with the indicated antibodies. The differences between TGF-β treated and untreated cultures were significant for all cell ratios (P < .05). (B) Cells cultured 18 days with TGF-β (black columns) and with anti–TGF-β (white columns). The differences between TGF-β treated and untreated cultures were significant for both cell ratios (P < .05).
Mixed lymphocyte reaction. Results (mean ± SD of 4 independent measurements) of representative mixed lymphocyte reaction experiments with the indicated stimulatory cell numbers per 105 respondent cells. (A) Cells cultured 18 days with (black columns) and without TGF-β (gray columns). 0 indicates no stimulatory cell; and anti-CD3/anti-CD28 (pale gray column), lymphocytes stimulated only with the indicated antibodies. The differences between TGF-β treated and untreated cultures were significant for all cell ratios (P < .05). (B) Cells cultured 18 days with TGF-β (black columns) and with anti–TGF-β (white columns). The differences between TGF-β treated and untreated cultures were significant for both cell ratios (P < .05).
Without TGF-β (Figures 1P, 3H), the expression of MHC-II and even more of CD80, CD83, CD86, and CD1a was lower than in cultures with this cytokine (Figures 4, 6A,D), and CD207/langerin and CD54 were not expressed at all (Figure 6G,J,M). The mitochondria were less increased in number than in cultures with TGF-β (Figure 1D,O). Only a weak stimulation of CD4+ cell proliferation was seen in mixed lymphocyte reaction (Figure 7A).
In the presence of anti–TGF-β antibody, only part of the cells acquired a dendritic profile. Those with a dendritic shape were similar at electron microscopy to those from cultures without the antibody and BGs were never seen. The expression of maturation-related, stimulatory and costimulatory molecules was only 60% for HLA-DR, 33% for CD80, 61% for CD83, and 34% for CD86. Notwithstanding, in mixed reaction, these cells could stimulate lymphocyte proliferation, although less than cells cultured with TGF-β (Figure 7B).
Discussion
In this study, we could show that CD133+ cells harvested from human cord blood can be drawn to differentiate in vitro, through well-defined steps, into cells with aspect, immunophenotype, and lymphocyte-stimulating capacity of LCs but without demonstrable BGs; the process requires 18 days. The selected cells were highly homogeneous for morphology and antigen expression; they were small, with few organelles. On culture, early differentiating cells were larger, with cytoplasmic projections of various widths and lengths, a nucleus with shallow indentations, and many mitochondria. The further stage was represented by immature dendritic cells with a deeply indented nucleus and multilaminar inclusions; the latter have features of class II compartments known to develop from multivesicular bodies.37 Other, less numerous inclusions contained multitubular arrays and resembled tubuloreticular inclusions, which may be interpreted as correlated with the secretion of cytokines.38 The mitochondria reached the zenith at this step, and a new emission peak at 580 nm appeared in the cytoplasm at autofluorescence. The last stage was represented by cells morphologically similar to those just described and also expressing costimulatory molecules (CD54, CD80, CD86), CD1a, and langerin and capable of stimulating mixed leukocyte reaction; therefore, they proved to be mature, immunostimulatory DCs; at this last stage, the number of mitochondria and the 580 nm peak were reduced with respect to the previous stage.
The addition of TGF-β, besides serum, does not seem necessary for the morphologic differentiation of cells but is required for the full acquisition of mature immunophenotypic and functional features.
The present results indicate that the culture of cells selected on the basis of CD133 leads to cells similar to LCs for morphology, antigen expression, and function but is devoid of BG. Similar results had been previously shown for cultures of cells selected for CD34, but with inconstant outcomes regarding BG.17-23,25,26 Only 2 studies until now have dealt with the generation of LCs, and more in general of DCs, from cells selected for CD133.19,20 However, one of these studies dealt primarily with CD34+ cells and only considered CD133+ cells en passant, just stating (without showing) that, in control experiments with these cells, the differentiation on culture was similar to the experiments with CD34+ cells.20 The other study was limited to check for the dendritic shape of cells and the expression of stimulatory and costimulatory molecules on culture, without analyzing the expression of LC markers except CD1a, or the ability to stimulate lymphocytes.19 Our results show that LCs can be generated in vitro from CD133+ precursors through well-defined steps, that they are efficient stimulators of lymphocytes, and that they do not form BG, therefore they should be better designated as LC-like cells.
We found that several differentiated cells expressed CD207/langerin; this result was confirmed by immunocytochemistry with 2 different antibodies and by flow cytometry with one of them. Although langerin is involved in the formation of BG,7 cells expressing this molecule do not necessarily display such granules because it is also localized in other endosomal structures.6,7 BGs are considered to be involved in the trafficking of antigens, especially when the latter are linked to langerin; langerin concentrates in the cytomembrane of these structures.7 The absence of BG (and even of langerin39 ) apparently does not reduce the ability to mount an efficient immune response, either in humans40 or in rodents.39 However, such a lack is a rare exception in the wild, which suggests that BGs and langerin are useful to organisms, although it is impossible to state whether this applies equally to the microscopic inclusions (BGs) and the molecule (langerin). The present results indicate that there is more in BGs than langerin and that langerin recycling does not depend exclusively on the granules, although the requirements for the formation of these peculiar inclusions and the functional consequences of their presence remain to be defined.
In this study, the first phase of differentiation was accompanied by enrichment in cytoplasmic autofluorescent structures; the number, position, and size, compared with MitoTracker staining, and the nature of fluorochromes (mainly NADH), indicate that the structures responsible for blue fluorescence are mitochondria and should therefore be correlated with the metabolic activity.41 Therefore, the significant increase in the blue fluorescence intensity from day 1 to day 14 (Figure 2) was not the result of an accumulation of NADH in the mitochondria (as in the case of metabolic deficits), but rather to an increase in the number of mitochondria. The results of both autofluorescence and MitoTracker staining indicate that the metabolic activity of the cells reaches a zenith at a stage roughly comparable with that of “immature” DCs and decreases during the transition to “mature,” lymphocyte-stimulating DCs. It is not surprising that the energy metabolism of immature DCs is especially high because at this stage the cell is ready for, or even actually involved in, the endocytosis and partial hydrolysis of foreign molecules and in membrane recycling. In addition, green-yellow fluorescent inclusions were detected on culture, most clearly after 14 days. The nature of the corresponding fluorochromes has not yet been assessed. However, the granular aspect of the fluorescence and the concomitance with the widespread appearance of multilaminar inclusions suggest that those fluorochromes are correlated with organelles of the endocytic pathway. The appearance of an autofluorescence peak at 580 nm had been observed in previous studies for mature, not activated DCs42 and, therefore, in addition to the other features found here, can be taken as expressive of that stage of DC differentiation.43
Dying cells were identified at all time points after the start of cultures. Apoptosis and necrosis, as recognized on the basis of morphology,33 occurred as well. Electron microscopy is indeed a very accurate method for distinguishing apoptosis from necrosis in cell cultures.33 We cannot obviously exclude that necrotic cells were in part expression of necroptosis (programmed necrosis) because this process differs from necrosis for the activation but not for the execution.44 However, the lack of added TGF-β influenced only apoptosis, which indicates that only this process was regulated by this cytokine, as previously suggested.45 The increase in frequency of apoptosis on 14 days of culture without TGF-β suggests that this is a critical moment for the culture, with a special need for this growth factor.
In the absence of TGF-β, only a few cells reached a dendritic shape, and the expression of maturation-related antigens was restricted to either a small fraction of cells (CD80, CD83, CD86) or absent (langerin, CD54). These cells were poor stimulators of mixed leukocyte reaction, a finding presumably related to the defective immunophenotype. These results suggest that the differentiation of CD133+ precursors to DCs is best promoted by a relatively high concentration of TGF-β, at variance with what had been reported for CD34+ precursors.46
The present results reinforce the notion that TGF-β plays a role in DC differentiation and is mandatory for the differentiation of LCs30 ; its action is mediated by several transcription factors, including PU.1, Id2,47 and RunX3.48 Mice knockout for TGF-β49 or for RunX3 totally lack LCs,48 although they are equipped with connective tissue DCs. Furthermore, TGF-β has been reported to protect DC precursors from apoptosis.45 LC precursors secrete TGF-β as an autocrine stimulus,30 which may explain the limited differentiation of DCs found here in the absence of added TGF-β (where serum may be a further source of the cytokine) and the marked hampering of morphologic and immunophenotypic differentiation in the cultures where TGF-β was actively inhibited. The interference of TGF-β with the differentiation of DCs is especially relevant for the morphologic and immunologic phenotype because the cells could become functionally competent to stimulate lymphocytes even in the presence of anti–TGF-β antibody, although less than on culture with the cytokine.
The results of this study indicate that virtually all CD133+ cells in the human cord blood express CD34, although the reverse is not true. The CD133+ cell population contains a higher fraction of highly immature progenitors and of myeloid colony-forming cells than the population selected for the expression of CD34,50 and it is reasonable to speculate that this depends on the CD133+/CD34− cell fraction. In addition, the results indicate that at least some features of differentiated cells vary depending on the selection criterion of the seeded cells and, in particular, that the differentiation of CD133+ precursors to LCs cannot be completed with the appearance of BGs in the same culture conditions, which had proved sufficient for CD34+ precursors to reach that degree of differentiation.18 We cannot yet propose an explanation for this difference, which, however, prompts to propose a role in this respect for the CD133−/CD34+ cells seeded in cultures on selection for CD34, which correspond to approximately one-fourth of the selected cells as shown here by parallel experiments. The differences between this study and that of Hubert et al17 include the testing of differentiated cells for the ability to stimulate lymphocyte proliferation. Indeed, several studies have shown that DCs differentiated from CD34+ precursors, independent of the culture conditions, are immunostimulatory cells. On the contrary, such proof had not yet been explicitly provided for cells selected by CD133 expression. This study has not addressed the ability of differentiated cells to infiltrate an in vitro reconstructed epithelium, which instead has been investigated by Hubert et al.17 Bartz et al20 have also studied cultures of CD133+ cells, but the results for these cultures were only briefly described compared with cultures of CD34+ cells; therefore, the immunostimulatory ability was clearly shown only for those derived from CD34+ cells.
The use of CD133+ cells to start cultures has shown some advantages: highly reproducible results, well-defined intermediate steps of differentiation, generation of cells with good lymphocyte-stimulating capacity. However, it also has drawbacks, in particular the relatively small number of seeded and of harvested cells and the incapability to generate full-blown LCs. Indeed, the major difference from the cultures of CD34+ cells has been the lack of formation of BGs in the differentiated cells, despite the expression of CD1a and langerin, which are molecular markers of LCs.
Indeed, the question “what is a Langerhans cell” (ie, which are all the peculiarities that distinguish this from other DC types and consequently which is the advantage to have this cell type within epithelia) is still open to discussion.3 A comparison of the findings among LCs (with BG) derived from CD34+ progenitors, LC-like cells with langerin but without BGs derived from CD133+ progenitors as achieved in the present study, and DCs derived from CD34+ progenitors in the absence of added TGF-β might help to address this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof M. Serio for precious encouragement and support; the anonymous referees for constructive criticism leading to improvement of the manuscript; Mr P. Guasti, Ms L. Calosi, Mr D. Guasti, and Mr S. Catarinicchia for skillful technical help; and Ms R. Scantimburgo for secretarial help.
This work was supported by the Italian Ministry of Education, University and Research (Project 2007LTAJMA_003), the University of Florence, Tuscany Region (project TRESOR), and Foemina Foundation.
Authorship
Contribution: M.I.B. and L.P. performed the experiments, generated microscopic, flow cytometric, and immunocytochemical results, and took primary responsibility in writing the manuscript; L.D. contributed to plan experiments, performed electron microscopic observations, and discussed the results; S.U., R.S., and A.B. provided the cord blood samples, gave advice and help for the analysis of freshly isolated cells, and discussed the manuscript; G.R., M.M., and V.B. conducted autofluorescence analyses and interpreted the corresponding results; A.A. and C.B. gave advice and help for flow cytometry of cultured cells and performed mixed lymphocyte reaction; and P.R. designed the project, supervised the experiments, participated in the interpretation of data, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maria Ida Bonetti, University of Florence, Department of Anatomy, Histology and Forensic Medicine, Section E. Allara, Viale Pieraccini 6, 50139 Firenze, Italy; e-mail: marida.bonetti@libero.it.
References
Author notes
M.I.B. and L.P. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal