Abstract
Here, we demonstrate a novel, direct-acting, and synergistic role for 3 hematopoietic stem cell cytokines: stem cell factor, interleukin-3, and stromal derived factor-1α, in controlling human endothelial cell (EC) tube morphogenesis, sprouting, and pericyte-induced tube maturation under defined serum-free conditions in 3-dimensional matrices. Angiogenic cytokines such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) alone or VEGF/FGF combinations do not support these responses. In contrast, VEGF and FGF prime EC responses to hematopoietic cytokines via up-regulation of c-Kit, IL-3Rα, and C-X-C chemokine receptor type 4 from either human ECs or embryonic quail vessel explants. In support of these findings, EC Runx1 is demonstrated to be critical in coordinating vascular morphogenic responses by controlling hematopoietic cytokine receptor expression. Combined blockade of hematopoietic cytokines or their receptors in vivo leads to blockade of developmental vascularization in quail embryos manifested by vascular hemorrhage and disrupted vascular remodeling events in multiple tissue beds. This work demonstrates a unique role for hematopoietic stem cell cytokines in vascular tube morphogenesis and sprouting and further demonstrates a novel upstream priming role for VEGF and FGF to facilitate the action of promorphogenic hematopoietic cytokines.
Introduction
Work over many years has revealed a dual requirement for cytokines and extracellular matrices in regulating vascular tube morphogenesis and maturation.1-4 The identity of cytokines that are necessary to stimulate particular steps in the process (ie, tubulogenesis, sprouting, vessel maturation) is complicated by the use of models both in vivo and in vitro with ill-defined components (ie, serum, multiple cell types) that have precluded the identification of individual requirements and steps necessary for these processes. For example, vascular endothelial growth factor (VEGF) has been reported to control endothelial cell (EC) tubulogenesis and sprouting events by controlling tip-cell behavior.1,3,5,6 However, dependence on other critical factors that may operate in conjunction with VEGF has not been adequately explored because of the complexities inherent in the experimental models used. In past studies, our laboratory developed the first serum-free, defined systems of human EC tube morphogenesis in 3-dimensional (3D) matrices.7,8 ECs were shown to require phorbol esters to survive, undergo tubular morphogenesis, or sprout.7-9
In separate work, hematopoietic cytokines were identified that control the proliferation and maintenance of hematopoietic stem cells as well as differentiated hematopoietic cells of all lineages.10 Of great interest is the finding that ECs are developmental precursors for hematopoietic stem cells.11-19 This critical finding also suggests that there may be functional overlaps in cytokines that affect vascularization and hematopoiesis. In addition, it appears clear that ECs affect hematopoietic cell behavior and vice-versa.17,20-23 For example, mobilized mononuclear stem cells have been shown to modulate angiogenic responses.22,23
A long-term goal has been to identify factors that substitute for phorbol ester addition under serum-free defined conditions to stimulate human EC tube morphogenesis in a 3D matrix environment and that do not require the support of other cell types (eg, pericytes, vascular smooth muscle cells, or fibroblasts). In vitro models are particularly useful for the identification of specific factor requirements and signaling cascades that control individual vascular morphogenic steps (eg, lumen formation) and were also used extensively to identify factors that controlled different steps in hematopoiesis. Here, by using defined serum-free conditions, we demonstrate that hematopoietic stem cell cytokines directly control vascular tube morphogenesis from either human ECs or quail vessel explants, whereas VEGF and fibroblast growth factor (FGF) act by priming EC responses to these promorphogenic hematopoietic factors via marked up-regulation of hematopoietic cytokine receptors. Furthermore, we demonstrate that combined blockade of hematopoietic cytokines or their receptors interferes with quail vascular development, leading to vascular hemorrhage phenotypes and marked vascular remodeling defects. This work demonstrates sequential and separable priming and promorphogenic steps, controlled by distinct cytokines, through direct action on ECs to regulate vascular tube morphogenesis and sprouting.
Methods
Vasculogenic and angiogenic 3D collagen assays
ECs were either suspended alone or with pericytes in 2.5 mg/mL collagen type I matrices, and assays were performed as described8,24 except that the culture media only contained reduced serum supplement (RSII), ascorbic acid, and FGF-2 at 40 ng/mL. Stem cell factor (SCF), interleukin-3 (IL-3), and stromal-derived factor-1α (SDF-1α) were each added at 200 ng/mL into collagen type I matrices. Cultures were allowed to assemble over time and fixed in 3% glutaraldehyde before staining with 0.1% toluidine blue in 30% methanol for nonfluorescent visualization.
For angiogenesis models, 2.5 mg/mL collagen type I gels were prepared, including SCF, SDF-1α, IL-3, and VEGF-A in the gel at 200 ng/mL and ECs seeded as a confluent monolayer on the surface of the gel. Assays were fixed at predetermined time points as described previously for data analysis.
Cell culture
Human umbilical vein ECs were purchased from Lonza and used from passages 2 to 5. Bovine retinal pericytes were isolated and cultured in Dulbecco modified Eagle medium/10% fetal bovine serum and used from passages 2 to 10. Both cell types were cultured on gelatin-coated flasks. Bovine retinal pericytes cell lines expressing enhanced green fluorescent protein (GFP) and human umbilical vein EC lines expressing monomeric red fluorescent protein were prepared by use of the methods as previously described.8
Vitelline vein explant cultures
Quail embryos were allowed to develop until day 6, at which time the vitelline vein was explanted. Typical explants were 1 cm in length, with a typical vessel 200 μm in diameter. Sterile vessels were washed in clear culture media (M199; GIBCO) and individually canulated onto 200-μm glass pipettes. Vessels were secured to the cannula and the mesenchymal tissue surrounding the vessel trimmed. Culture media were then flushed through the vessel to evacuate the blood. Each vessel explant was then cut into 0.5-mm segments, with approximately 5-7 segments generated per explant. Vessel segments were split into equal numbers and placed into culture media containing RSII for control conditions or culture media containing RSII, 40 ng/mL VEGF-A, and 40 ng/mL FGF-2 for primed vessel conditions. After priming for 16 hours, vessel segments were placed into 2.5 mg/mL 3D collagen matrices containing 200 ng/mL SCF, IL-3, and SDF-1α or 200 ng/mL VEGF-A. Cultures were allowed to assemble over 5 days, at which time cultures were fixed in 2% paraformaldehyde and immunostained for the quail EC-specific marker QH1. The vessel area of QH1-positive tube structures was measured, with data being reported as the average area ± SD.
Injection of quail eggs
Quail eggs were housed at 37°C to initiate development. Injection of drugs was performed at day 3 or day 1 of development through a small hole created at the apex of the egg and incubated at 37°C for 3 days (until embryonic day 6 or day 4). All drugs (AMD3100, imatinib, ISCK03) were first tested in vitro to determine their range of efficacy, at which time a dose of 100nM for each drug was chosen. Secondary studies designed in parallel to those included here but used an inhibitor dose of 31.6nM also were performed. Similar effects were noted for the combination of AMD3100 and imatinib or AMD3100 and ISCK03; however, the effects of each drug individually were less severe. Blocking antibodies were injected in vivo at 20 μg/mL by use of the same procedure as denoted previously. Embryos and chorioallantoic membrane (CAM) tissue were collected after the treatment with inhibitors or blocking antibodies and fixed in 2% paraformaldehyde for further analysis. Whole-mount embryo images were obtained by the use of a Leica MZ95 dissecting microscope with a Plan 1.0× lens.
Immunostaining of cultures
Cultures were fixed in 2% paraformaldehyde for at least 1 hour before the addition of blocking solution (phosphate-buffered saline containing 1% bovine serum albumin and Triton X-100). Gels were incubated with primary antibodies overnight at 4°C and then were washed in phosphate-buffered saline and incubated for 2 hours with secondary antibodies. Final washes were performed over several hours, and the cultures were examined with the use of immunofluorescence microscopy.
Microscopy, imaging, and data analysis
Time-lapse videomicroscopy and fluorescence imaging was performed with a fluorescence-inverted microscope (Eclipse TE2000-E; Nikon) and the analysis software MetaMorph (Molecular Devices). We performed data analysis by tracing the tube area of individual high-powered fields of culture assays by using MetaMorph software and Microsoft Excel to perform statistical analysis.
Statistical analysis
Statistical analysis of selected EC vasculogenic and lumen formation data were performed with the use of Microsoft Excel. Analysis of variance was used to compare means of 2 or more groups. Statistical significance was set at minimum, with P < .05. Student t tests were used when analyzing 2 groups within a single experiment (with a minimum n = 5).
Results
Hematopoietic stem cell cytokines control vascular tube morphogenesis under defined serum-free conditions
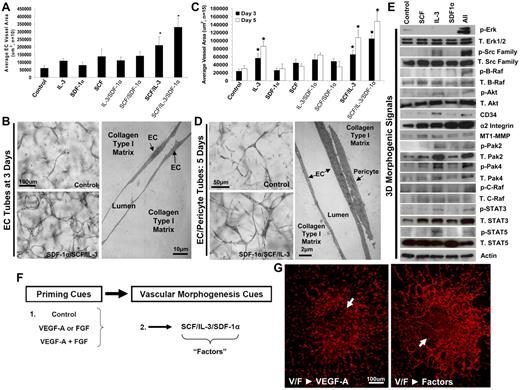
To identify cytokine requirements for human vascular tube morphogenesis in serum-free–defined medium (containing insulin, transferrin, oleic acid, albumin, and FGF-2) and 3D collagen matrices, we used a high-throughput approach by using 96-well plates, where hundreds of combinations of cytokines were screened (supplemental Table 1 and Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). FGF-2 was included as a component of the basal serum-free media during the tube morphogenesis assay because of its fundamental importance in EC survival, arteriogenesis, and epithelial branching morphogenesis.5,25,26 A surprising result was that combinations of proangiogenic factors, such as VEGF and FGF, VEGF and hepatocyte growth factor, or VEGF and angiopoietins, failed to support vascular tube morphogenesis under these conditions. However, a combination of 3 hematopoietic stem cell cytokines, SCF, IL-3, and SDF-1α, allowed for marked EC tube morphogenesis and assembly (Figure 1A-B, supplemental Figures 1-2). These cytokines are known to be synergistic for hematopoietic stem cell proliferation,27-33 a cell population that derives from EC precursors during vascular and hematopoietic development.11,12,14-19,33-35 The individual cytokines do not support vascular morphogenesis, but, once combined, they are able to exert their influence (Figure 1A). Time-lapse microscopy over 72 hours shows the influence of the 3 hematopoietic factors during these events (supplemental Video 1).
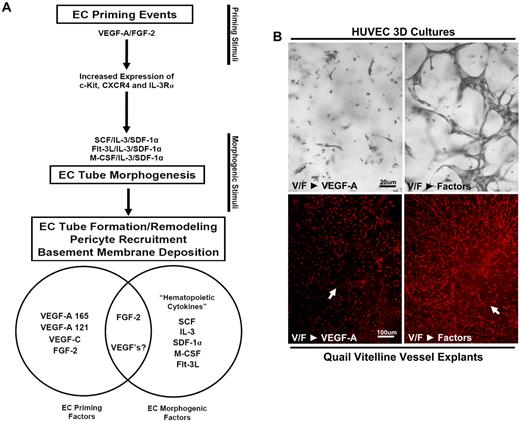
SCF, IL-3 and SDF-1α synergistically act to promote EC tube morphogenesis in the presence or absence of pericytes and function downstream of VEGF-A/FGF-2 EC priming events. (A) SCF, IL-3, and SDF-1α were each provided as morphogenic stimuli for ECs individually or in different combinations and compared with control conditions in 3D collagen matrices. Total EC tube area was quantitated for each condition after 72 hours of culture (n ≥ 10; P ≤ .01). (B) Representative images of control versus SCF/IL-3/SDF-1α–stimulated cultures are shown (Bar equals 100 μm, left) as well as an electron micrograph of EC tube assembly (10 μm, right; n ≥ 10; P ≤ .01). (C) EC-pericyte morphogenic coculture assays were established in the presence of the hematopoietic cytokines individually or in different combinations and compared with control conditions in 3D collagen matrices. EC tube areas were quantified after both 72 and 120 hours. (D) Representative images of the cocultures under control versus SCF/IL-3/SDF-1α–stimulated conditions are shown (bar equals 50 μm), along with an electron micrograph of these cultures demonstrating the relationship between the ECs and pericytes (bar equals 2 μm). (E) EC-only morphogenic assays were established in the presence of each hematopoietic cytokine alone or the 3 factors together (All) versus controls. Cell lysates were collected at day 3 and Western blot analysis performed to assess kinase signaling and protein expression changes that correlate with the ability of hematopoietic cytokines to stimulate tube morphogenesis, and determine synergistically acting promorphogenic signaling pathways. (F) A schematic depicting a 2-step process of EC priming versus morphogenic cues. (G) E6 Quail vitelline vein explants primed with VEGF-A/FGF-2 and placed into morphogenic assays containing either VEGF-A or the combined hematopoietic cytokines. *Significance over control. Fixed cultures were immunostained with QH-1 antibodies. Arrows indicate the vessel explant border (n ≥ 10; P ≤ .01).
SCF, IL-3 and SDF-1α synergistically act to promote EC tube morphogenesis in the presence or absence of pericytes and function downstream of VEGF-A/FGF-2 EC priming events. (A) SCF, IL-3, and SDF-1α were each provided as morphogenic stimuli for ECs individually or in different combinations and compared with control conditions in 3D collagen matrices. Total EC tube area was quantitated for each condition after 72 hours of culture (n ≥ 10; P ≤ .01). (B) Representative images of control versus SCF/IL-3/SDF-1α–stimulated cultures are shown (Bar equals 100 μm, left) as well as an electron micrograph of EC tube assembly (10 μm, right; n ≥ 10; P ≤ .01). (C) EC-pericyte morphogenic coculture assays were established in the presence of the hematopoietic cytokines individually or in different combinations and compared with control conditions in 3D collagen matrices. EC tube areas were quantified after both 72 and 120 hours. (D) Representative images of the cocultures under control versus SCF/IL-3/SDF-1α–stimulated conditions are shown (bar equals 50 μm), along with an electron micrograph of these cultures demonstrating the relationship between the ECs and pericytes (bar equals 2 μm). (E) EC-only morphogenic assays were established in the presence of each hematopoietic cytokine alone or the 3 factors together (All) versus controls. Cell lysates were collected at day 3 and Western blot analysis performed to assess kinase signaling and protein expression changes that correlate with the ability of hematopoietic cytokines to stimulate tube morphogenesis, and determine synergistically acting promorphogenic signaling pathways. (F) A schematic depicting a 2-step process of EC priming versus morphogenic cues. (G) E6 Quail vitelline vein explants primed with VEGF-A/FGF-2 and placed into morphogenic assays containing either VEGF-A or the combined hematopoietic cytokines. *Significance over control. Fixed cultures were immunostained with QH-1 antibodies. Arrows indicate the vessel explant border (n ≥ 10; P ≤ .01).
To assess how these hematopoietic factors act with respect to kinase-signaling pathways, the experiment was performed without cytokines, with each cytokine individually, and all 3 combined (Figure 1E). Synergistic activation of 3 downstream kinases was noted, including Src, B-Raf, and extracellular signal-regulated kinase 1/2, after treatment with the 3 hematopoietic cytokines but not without the cytokines or when they were added individually (Figure 1E). Interestingly, we have previously reported that activation of these 3 kinases controls EC lumen formation and tubulogenesis when phorbol ester is added in the system,36 and here we observed similar results downstream of hematopoietic cytokine action during EC morphogenesis in the absence of phorbol ester. Furthermore, we observed increased levels of activated Pak-4, Pak-2, signal transducers and activators of transcription-3 (Stat-3), Stat-5, and Akt with the 3 hematopoietic factors compared with control, and in several cases, this appeared to be because of increased total levels of protein (ie, Pak-2, Stat-3, and Akt).
Hematopoietic stem cell cytokines facilitate EC-pericyte tube coassembly and vascular basement membrane formation
Of importance, the addition of the combination of SCF, IL-3, and SDF-1α also allows for pericyte recruitment to forming EC-lined tubes to promote vessel maturation and stabilization (Figure 1C,D; supplemental Videos 2-6). Time-lapse microscopy reveals EC-pericyte tube coassembly under light microscopy (supplemental Video 2) and separately under fluorescence microscopy, where ECs are labeled with monomeric red fluorescent protein and pericytes are labeled with GFP in response to these 3 factors (supplemental Videos 3-6). In each case, pericytes are observed to recruit to developing EC-lined tubes from either 0-72 hours or 72-144 hours of culture and can be shown to migrate along the abluminal EC surface, providing maturation and stabilization signals. Pericyte recruitment to EC tubes stimulates vascular basement membrane matrix assembly24 in this system as detected by the use of anti–collagen type IV or laminin antibodies (Figure 2B-D).
SCF can be replaced by either Flt-3L or M-CSF to act in conjunction with IL-3 and SDF-1α to stimulate EC tube formation and hematopoietic cytokines facilitate pericyte recruitment to EC tubes and vascular basement membrane matrix assembly in 3D collagen matrices. (A) M-CSF and Flt3L were added to replace SCF and were compared with SCF/IL-3/SDF-1α by adding them to IL-3/SDF-1α. In addition, ECs were either primed with FGF alone, VEGF-A/FGF-2, or control and then cultured for 72 hours. After fixation, total EC tube area was determined (n ≥ 10; P ≤ .01). *Denotes significance over control ECs; +denotes significance over the FGF-2–only primed ECs. (B) ECs were left untreated or were primed with VEGF-A/FGF-2 and then incorporated with GFP pericytes and hematopoietic cytokines (SCF/IL-3/SDF-1α) into 3D collagen matrices. Assays were allowed to develop over 5 days and average EC vessel area measured (n ≥ 10; P ≤ .01). *Denotes significance over control. (C,D) Basement membrane matrix deposition was assessed in the presence or absence of EC priming with VEGF-A/FGF-2. Cultures were fixed and stained with antibodies to laminin, collagen type IV, and CD31. Fluorescent images of this staining were acquired, quantitated (C), or overlaid with images of GFP pericytes (D). Bar equals 20 μm.
SCF can be replaced by either Flt-3L or M-CSF to act in conjunction with IL-3 and SDF-1α to stimulate EC tube formation and hematopoietic cytokines facilitate pericyte recruitment to EC tubes and vascular basement membrane matrix assembly in 3D collagen matrices. (A) M-CSF and Flt3L were added to replace SCF and were compared with SCF/IL-3/SDF-1α by adding them to IL-3/SDF-1α. In addition, ECs were either primed with FGF alone, VEGF-A/FGF-2, or control and then cultured for 72 hours. After fixation, total EC tube area was determined (n ≥ 10; P ≤ .01). *Denotes significance over control ECs; +denotes significance over the FGF-2–only primed ECs. (B) ECs were left untreated or were primed with VEGF-A/FGF-2 and then incorporated with GFP pericytes and hematopoietic cytokines (SCF/IL-3/SDF-1α) into 3D collagen matrices. Assays were allowed to develop over 5 days and average EC vessel area measured (n ≥ 10; P ≤ .01). *Denotes significance over control. (C,D) Basement membrane matrix deposition was assessed in the presence or absence of EC priming with VEGF-A/FGF-2. Cultures were fixed and stained with antibodies to laminin, collagen type IV, and CD31. Fluorescent images of this staining were acquired, quantitated (C), or overlaid with images of GFP pericytes (D). Bar equals 20 μm.
Furthermore, we recently reported that EC-derived platelet-derived growth factor-BB and heparin-binding epidermal-like growth factor play a major role in controlling pericyte recruitment to these developing EC-lined tubes.37 Thus, the promorphogenic hematopoietic SCFs allow for both EC tubulogenesis and pericyte recruitment to EC tubes in 3D matrices, a required developmental step that regulates vascular remodeling, maturation, and stabilization.24 Of importance, these experiments were performed under serum-free conditions with human ECs, the first time that this has been experimentally accomplished. These findings have implications for tissue engineering in which construction of EC tube networks with associated pericytes will be necessary to create tissues with an associated microvasculature.
An endogenous role of VEGF can be excluded in the EC-only model through the use of neutralizing antibodies to either VEGF-A ligand or vascular endothelial growth factor receptor-2 (VEGFR2), which show no blocking influence (supplemental Figure 2C-D). In contrast, neutralizing antibodies to SCF, IL-3, and SDF-1α and c-Kit lead to blocked morphogenesis (supplemental Figure 2C-E). SiRNA suppression of c-Kit in ECs also blocked vascular morphogenesis (supplemental Figure 3A). We also observed dose-dependent inhibition of EC tube formation by using chemical inhibitors of either C-X-C chemokine receptor type 4 (CXCR4; AMD3100) or c-Kit (imatinib; supplemental Figure 3B,C). The addition of VEGF-A to the morphogenic system on its own fails to stimulate morphogenesis, and its addition with the hematopoietic factors does not enhance their influence during the morphogenic process (supplemental Figure 2A-B).
However, we do observe that it is possible to substitute Flt-3 ligand or M-CSF for SCF in combination with IL-3 and SDF-1α to support tubulogenesis, an important observation because no other combinations allow these events to occur (Figure 2A, supplemental Table 1). Our screens thus far have not identified growth factors or cytokines that can substitute for either IL-3 or SDF-1α in this system (supplemental Table 1). These findings suggest that only very specific combinations of hematopoietic stem cell cytokines can support vascular tube morphogenesis of human ECs under defined serum-free conditions in 3D collagen matrices (Figure 1, supplemental Table 1).
VEGF and FGF prime EC responses to promorphogenic hematopoietic cytokines through cytokine receptor up-regulation
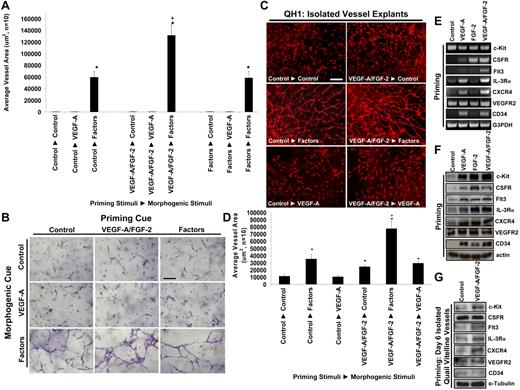
To assess how known proangiogenic cytokines such as VEGF-A and FGF-2 might influence these results, we addressed the possibility that they might act before the effects of hematopoietic cytokines as a distinct step during vascular morphogenesis and tested whether these factors could prime EC responses and, thus, precede the action of promorphogenic hematopoietic cytokines. By using our high-throughput screening technologies and introducing growth factors and cytokines during either EC priming or morphogenesis, we demonstrate a novel 2-step process of EC tubulogenesis promoted through VEGF-A/FGF-2 EC priming events followed by hematopoietic stem cell cytokine actions controlling vascular tube morphogenesis (Figure 1F-G; Figures 3A-B; supplemental Figure 4).
VEGF-A and FGF-2 prime EC tube morphogenic responses to SCF, IL-3, and SDF-1α by up-regulating hematopoietic cytokine receptors. (A,B) ECs were primed for 16 hours with either control, VEGF/FGF, or SCF/IL-3/SDF-1α treatments and then were suspended in 3D collagen matrices with either control, VEGF, or SCF/IL-3/SDF-1α additions. Priming cues are listed first, followed by the morphogenic stimuli. Cultures were fixed after 72 hours, photographed, and quantitated for total EC tube area (n ≥ 10; P ≤ .01). Bar equals 100 μm. (C) Quail vitelline vessels were isolated and the vessels primed overnight with VEGF-A/FGF-2 or control conditions. Explants were placed into collagen gels and were treated with either VEGF-A, hematopoietic cytokines, or control conditions. After 5 days, the vessels were stained with QH-1 to visualize quail ECs and representative images are shown. Bar equals 75 μm. (D) Quantification of EC tube formation from vitelline vessel explants is shown (n ≥ 6; P ≤ .01). (E) Reverse transcription polymerase chain reaction analysis of EC mRNA primed with VEGF-A, FGF-2, or the combination of VEGF-A/FGF-2 versus control treated cells for 16 hours. (F) Western blot analysis of ECs primed with VEGF-A, FGF-2, or the combination of VEGF-A/FGF-2 versus control cells for 16 hours. (G) Western blot analysis of embryonic day 6 quail vitelline vessel explants primed with VEGF-A/FGF-2 versus nonprimed control vessels for 16 hours. *Denotes significance over control-control condition; +denotes significance over the control-factors condition.
VEGF-A and FGF-2 prime EC tube morphogenic responses to SCF, IL-3, and SDF-1α by up-regulating hematopoietic cytokine receptors. (A,B) ECs were primed for 16 hours with either control, VEGF/FGF, or SCF/IL-3/SDF-1α treatments and then were suspended in 3D collagen matrices with either control, VEGF, or SCF/IL-3/SDF-1α additions. Priming cues are listed first, followed by the morphogenic stimuli. Cultures were fixed after 72 hours, photographed, and quantitated for total EC tube area (n ≥ 10; P ≤ .01). Bar equals 100 μm. (C) Quail vitelline vessels were isolated and the vessels primed overnight with VEGF-A/FGF-2 or control conditions. Explants were placed into collagen gels and were treated with either VEGF-A, hematopoietic cytokines, or control conditions. After 5 days, the vessels were stained with QH-1 to visualize quail ECs and representative images are shown. Bar equals 75 μm. (D) Quantification of EC tube formation from vitelline vessel explants is shown (n ≥ 6; P ≤ .01). (E) Reverse transcription polymerase chain reaction analysis of EC mRNA primed with VEGF-A, FGF-2, or the combination of VEGF-A/FGF-2 versus control treated cells for 16 hours. (F) Western blot analysis of ECs primed with VEGF-A, FGF-2, or the combination of VEGF-A/FGF-2 versus control cells for 16 hours. (G) Western blot analysis of embryonic day 6 quail vitelline vessel explants primed with VEGF-A/FGF-2 versus nonprimed control vessels for 16 hours. *Denotes significance over control-control condition; +denotes significance over the control-factors condition.
To address whether an ex vivo tissue could respond to these hematopoietic factor combinations, we explanted the embryonic day 6 (E6) quail vitelline vein and show that EC sprouting and tube morphogenesis occurs in response to SCF, IL-3, and SDF-1α but not VEGF (Figure 1G; Figure 3C-D; supplemental Figure 4) in 3D collagen matrices. These data demonstrate that developing embryonic vessels similarly respond, like human ECs, to hematopoietic stem cell cytokines and undergo tubulogenesis in 3D matrices. Furthermore, VEGF and FGF prime ECs to respond to hematopoietic cytokines in assays with either quail vitelline vessels or with human ECs in 3D matrices (Figure 1G, Figure 3, supplemental Figure 4). In both cases, there is a marked stimulatory response when VEGF and FGF are used together to prime ECs before exposure to hematopoietic cytokines (ie, factors) in 3D matrices. Reversing this treatment sequence by priming ECs with hematopoietic factors first followed by VEGF as a morphogenic cue fails to stimulate tubular morphogenesis (Figure 3A-B; supplemental Videos 7-16). In addition, VEGF and FGF priming of ECs also enhances EC-pericyte tube coassembly and vascular basement membrane assembly, resulting in increased EC tube network area and increased laminin deposition during basement membrane matrix assembly (Figure 2B-D).
In all cases, when the hematopoietic factors were added during the morphogenic step, tube formation was stimulated, whereas addition of VEGF during this step did not support tubulogenesis (Figures 1,3; supplemental Figures 2,4; supplemental Videos 7-16). Of great interest is that either macrophage colony-stimulating factor (M-CSF) or Flt3 ligand (Flt3L), when used as substitutes for SCF (and along with IL-3 and SDF-1α) show identical activity compared with SCF, including tube morphogenic responses after VEGF/FGF priming (Figure 2A). Thus, our data suggest that various combinations of hematopoietic cytokines are the primary stimulators of vascular tube morphogenesis, whereas VEGF's primary action appears to be upstream as a preparatory stimulus to facilitate the action of these promorphogenic cytokines. Although these results may be viewed as surprising, it should be noted that such an analysis has never been performed with the use of human ECs and serum-free–defined conditions in 3D extracellular matrices. In previous work with respect to analyses of VEGF morphogenic functions, such questions have been addressed in animals or in the use of in vitro systems with multiple cell types and serum present. Thus, other cytokines were clearly present and could have been performing just as we describe for the hematopoietic cytokines in this report.
VEGF and FGF prime ECs for morphogenic responses by inducing hematopoietic cytokine receptor expression
The mechanism by which VEGF and FGF prime EC responses to hematopoietic cytokines was investigated by the use of human ECs or isolated quail vitelline vessels (Figure 3E-G). In each case, isolated ECs or quail vessels were pretreated with no factor, VEGF alone, FGF alone, or both for 16 hours before the preparation of mRNA or lysates for Western blot analysis. As shown in Figure 3E-G, VEGF and FGF induced expression of multiple hematopoietic factor receptors (including c-Kit, IL-3Rα, and CXCR4), mRNA (Figure 3E), and protein (Figure 3F,G) levels without inducing VEGFR2 from human ECs or quail vitelline vessels. In addition, the M-CSF receptor, CSFR1, as well as Flt3L receptor, Flt3, were both induced by VEGF and FGF priming compared with controls. Interestingly, CD34, a receptor associated with vascular progenitor cells, also was induced during the priming process with either VEGF or FGF. Thus, it appears that VEGF and FGF act as priming factors because of their ability to induce multiple hematopoietic stem cell cytokine receptors in ECs.
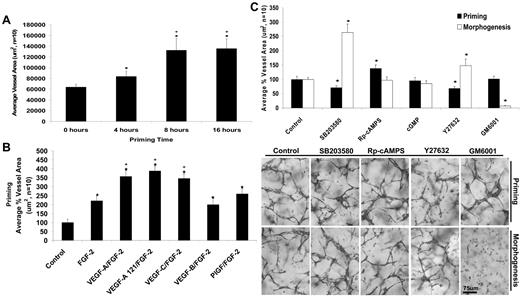
This finding is consistent with the conclusion that VEGF and FGF act upstream of hematopoietic stem cell cytokines and prepare ECs for promorphogenic cues. The maximal priming process occurs 8 hours (begins by 4 hours) after VEGF and FGF treatment and appears identical to that observed at 16 hours of treatment (Figure 4A). To assess whether different VEGF isoforms1 could prime ECs, we tested the VEGF-A 121 isoform, VEGF-C, VEGF-B, and placental growth factor and compared them with the VEGF-A 165 isoform. VEGF-A 165 and VEGF-A 121 showed equal potency to prime EC responses, as did VEGF-C, suggesting the involvement of VEGFR2 in this response (Figure 4B). In contrast, the VEGFR1 ligands, VEGF-B and placental growth factor, did not prime EC morphogenic responses to hematopoietic cytokines.
VEGF-A/FGF-2 priming events are functionally separable and show distinct signaling requirements for hematopoietic cytokine-induced EC tube morphogenesis in 3D collagen matrices. (A) ECs were primed with the combination of VEGF-A/FGF-2 for the indicated times to identify the timing requirements for the priming event. After priming, ECs were placed in culture with the hematopoietic factors and fixed after 72 hours (n ≥ 10; P ≤ .01). *Denotes significance over the 0-hour time point; +significance over the 4-hour time point. (B) The indicated VEGF isoforms were tested for their ability to prime ECs for 16 hours. ECs were then placed in culture with the hematopoietic factors and fixed after 72 hours (n ≥ 10; P ≤ .01). *Denotes significance over control; +denotes significance over the FGF-2 alone condition. (C) Inhibitors of p38 MAP kinase (SB203580, 10μM), protein kinase A (Rp-cAMPS, 10μM), protein kinase G (cGMPS, 10μM), Rho kinase (Y27632, 10μM) and MMPs (GM6001, 5μM) were added during the priming step or separately during the tube morphogenesis step to assess whether signaling differences exist between the 2 steps. Cultures were fixed after 72 hours, and total tube area was quantitated (n ≥ 10; P ≤ .01). *Denotes significance from control. Representative images of cultures treated with the indicated inhibitors during the priming versus morphogenesis are shown. Bar equals 100 μm.
VEGF-A/FGF-2 priming events are functionally separable and show distinct signaling requirements for hematopoietic cytokine-induced EC tube morphogenesis in 3D collagen matrices. (A) ECs were primed with the combination of VEGF-A/FGF-2 for the indicated times to identify the timing requirements for the priming event. After priming, ECs were placed in culture with the hematopoietic factors and fixed after 72 hours (n ≥ 10; P ≤ .01). *Denotes significance over the 0-hour time point; +significance over the 4-hour time point. (B) The indicated VEGF isoforms were tested for their ability to prime ECs for 16 hours. ECs were then placed in culture with the hematopoietic factors and fixed after 72 hours (n ≥ 10; P ≤ .01). *Denotes significance over control; +denotes significance over the FGF-2 alone condition. (C) Inhibitors of p38 MAP kinase (SB203580, 10μM), protein kinase A (Rp-cAMPS, 10μM), protein kinase G (cGMPS, 10μM), Rho kinase (Y27632, 10μM) and MMPs (GM6001, 5μM) were added during the priming step or separately during the tube morphogenesis step to assess whether signaling differences exist between the 2 steps. Cultures were fixed after 72 hours, and total tube area was quantitated (n ≥ 10; P ≤ .01). *Denotes significance from control. Representative images of cultures treated with the indicated inhibitors during the priming versus morphogenesis are shown. Bar equals 100 μm.
Distinct signal transduction requirements demonstrate that the priming and morphogenic steps are functionally separable
To assess whether we could provide evidence that signal transduction differences exist that distinguish the priming versus morphogenic steps, we added known chemical inhibitors of p38 mitogen-activated protein (MAP) kinase, cyclic adenosine monophosphate (cAMP)-dependent protein kinase, cyclic guanosine monophosphate (cGMP)-dependent protein kinase, Rho kinase, and matrix metalloproteinases (MMPs) either during the priming or separately during the morphogenic step (Figure 4C). Blockade of p38 MAP kinase or Rho kinase interfered with the priming step, but interestingly, stimulated the morphogenic step compared with control, suggesting that priming and morphogenesis are functionally separable (Figure 4C). In addition, blockade of cAMP-dependent kinase stimulated the priming step but had no effect on the morphogenic step, whereas blockade of MMPs had no influence on the priming step but completely inhibited the morphogenic step (Figure 4C). We have previously reported that MT1-MMP controls vascular tube formation, and this process is inhibited by GM6001.5 This experiment clearly demonstrates that VEGF and FGF-dependent priming is a distinct step from the hematopoietic stem cell cytokine-dependent morphogenic step.
Hematopoietic cytokines control EC sprouting in 3D collagen matrices whereas VEGF and FGF prime these responses
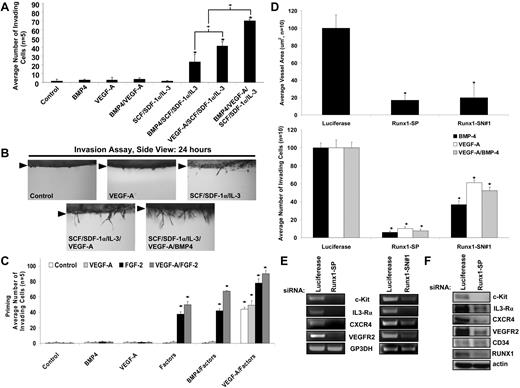
The previous experiments addressed the role of hematopoietic stem cell cytokines during vasculogenic tube assembly. To address whether these factors affect EC sprouting, we performed experiments with hematopoietic cytokines, VEGF-A, and bone morphogenetic protein-4 (BMP-4; Figure 5A-B). The individual factors, BMP-4, VEGF-A, or the hematopoietic factors alone, were unable to induce EC sprouting under these defined conditions. Only when the hematopoietic factors were combined with VEGF or BMP-4 was sprouting observed (Figure 5A-B). Thus, despite previous work describing that VEGF-A directly controls EC sprouting, our findings suggest that it does not have this ability on its own under defined conditions and in 3D collagen matrices. In contrast, it requires the presence of hematopoietic factors to regulate EC sprouting in 3D collagen matrices (Figure 5A-B).
Hematopoietic cytokines stimulate EC angiogenic sprouting in conjunction with VEGF-A or BMP-4, and the EC transcription factor, Runx1, controls hematopoietic cytokine receptor expression that is necessary for EC morphogenic responses in 3D collagen matrices. (A,B) The indicated factors were added to the collagen matrix and ECs were seeded on the collagen gel surface and EC sprouting was quantitated (A) after 24 hours (n ≥ 10; P ≤ .01) and photographed (B) from the side. Arrowheads indicate the monolayer surface. (C) ECs were primed with VEGF-A, FGF-2, VEGF-A/FGF-2, or control and then were seeded on the surface of gels that contained the indicated individual or combined factors (n ≥ 10; P ≤ .01). *Denotes significance over control. (D) SiRNA suppression of the transcription factor Runx1 in ECs with the use of 2 independent siRNAs (Smartpool-SP or Single-SN) directed to Runx1 was tested during EC tube formation (top) or EC sprouting (bottom; n ≥ 10; P ≤ .01). *Denotes significant blockade from Luciferase controls. (E) Reverse transcription polymerase chain reaction analysis was performed on ECs treated with siRNAs to Runx1 versus luciferase controls to determine mRNA expression for each of the hematopoietic cytokine receptors as well as VEGFR2 and controls. (F) Western blot analysis was performed to determine protein expression for each of the hematopoietic cytokine receptors as well as VEGFR2 after siRNA suppression of Runx1 in ECs.
Hematopoietic cytokines stimulate EC angiogenic sprouting in conjunction with VEGF-A or BMP-4, and the EC transcription factor, Runx1, controls hematopoietic cytokine receptor expression that is necessary for EC morphogenic responses in 3D collagen matrices. (A,B) The indicated factors were added to the collagen matrix and ECs were seeded on the collagen gel surface and EC sprouting was quantitated (A) after 24 hours (n ≥ 10; P ≤ .01) and photographed (B) from the side. Arrowheads indicate the monolayer surface. (C) ECs were primed with VEGF-A, FGF-2, VEGF-A/FGF-2, or control and then were seeded on the surface of gels that contained the indicated individual or combined factors (n ≥ 10; P ≤ .01). *Denotes significance over control. (D) SiRNA suppression of the transcription factor Runx1 in ECs with the use of 2 independent siRNAs (Smartpool-SP or Single-SN) directed to Runx1 was tested during EC tube formation (top) or EC sprouting (bottom; n ≥ 10; P ≤ .01). *Denotes significant blockade from Luciferase controls. (E) Reverse transcription polymerase chain reaction analysis was performed on ECs treated with siRNAs to Runx1 versus luciferase controls to determine mRNA expression for each of the hematopoietic cytokine receptors as well as VEGFR2 and controls. (F) Western blot analysis was performed to determine protein expression for each of the hematopoietic cytokine receptors as well as VEGFR2 after siRNA suppression of Runx1 in ECs.
To assess whether VEGF and FGF priming affects EC sprouting responses, we treated ECs with VEGF, FGF, or the combination of VEGF and FGF. Priming with FGF and the combination of VEGF/FGF led to EC sprouting in response to hematopoietic cytokines, a phenotype not observed without priming (Figure 5A-C) or when VEGF or BMP-4 were added during the sprouting assay instead of hematopoietic cytokines (Figure 5C). A further increase in sprouting was observed in the priming experiment when the combination of hematopoietic factors and VEGF were used as a stimulus for sprouting (Figure 5C). In addition, sprouting was observed from E6 embryonic vitelline explants in response to hematopoietic factors and, furthermore, VEGF/FGF priming of these vessels increased the sprouting responses observed from these vessels (Figures 1,3,7; supplemental Figure 4C). Thus, different types of ECs respond to the sequential application of priming and morphogenic cytokines for both tube morphogenesis and sprouting responses and reveal a fundamental role for hematopoietic factors in controlling the morphogenic responsiveness of ECs (Figures 1,Figure 2,Figure 3,Figure 4–5).
EC Runx1 controls hematopoietic cytokine receptor expression and regulates EC tube morphogenesis and sprouting responses
Specific transcription factors are known to control both vascular and hematopoietic development.12,18,38-40 Runx1 mouse knockouts and morpholino knockdown of Runx1 in zebrafish lead to a loss of definitive hematopoiesis and also show substantial defects in vascular development, including hemorrhage.12,17,18,39 To address a potential role for Runx1 during these processes, we performed siRNA suppression studies by using human ECs. SiRNA suppression of Runx1 in ECs with 2 independent siRNAs led to marked blockade of tube morphogenesis in response to SCF, IL-3, and SDF-1α and also completely interfered with EC sprouting in response to VEGF, BMP-4, or the combination of VEGF and BMP-4 (in each case in the presence of the hematopoietic factors; Figure 5D). Runx1 appears to control EC morphogenesis by regulating the expression of critical morphogenic and priming signaling receptors, including c-Kit, IL-3Rα, CXCR4, and VEGFR2, both at the mRNA and protein level (Figure 5E-F). Thus, a critical transcriptional regulator of vascular and hematopoietic development, Runx1, plays a fundamental role in controlling the expression of hematopoietic cytokine receptors that control vascular tube morphogenesis in 3D extracellular matrices.
Hematopoietic cytokines and their receptors control developmental vascularization events
To address the role of these hematopoietic cytokines and their receptors in vivo during quail vascular development, we performed experiments to assess whether c-Kit, IL-3Rα, and CXCR4 could be detected on embryonic quail ECs with the use of immunofluorescence microscopy. Each receptor could be demonstrated to be expressed by E6 quail ECs along with circulating hematopoietic cells, confirming previous reports (Figure 6A).41-44 QH1 staining was shown as a marker of quail ECs. Their presence was also confirmed by Western blot from E6 quail vitelline vessels (Figure 6B). To assess whether functional blockade of these receptors affected vascular development, chemical inhibitors of c-Kit (imatinib or ISCK03) and CXCR4 (AMD3100) were administered either singly or in combination at day 3 of development, as were cytokine blocking antibodies directed to SCF and IL-3 (in separate experiments). Quail embryos and choriallantoic membranes were examined at day 6 in both sets of experiments. These chemical inhibitors and neutralizing antibodies displayed blocking effects on ECs in 3D collagen assays (supplemental Figure 2E, supplemental Figure 3B-C).
Role for hematopoietic stem cell cytokines and their receptors during quail developmental vascularization events. (A) Quail CAM tissue was isolated at day 6 of development, fixed, and double stained for the quail EC-specific marker, QH1, versus c-Kit, IL-3Rα, and CXCR4. Arrows indicate vessel wall borders; arrowheads indicate circulating blood cells. (B) Western blot analysis of embryonic 6-day quail vitelline vessels reveals the presence of hematopoietic cytokine receptors in the vasculature. (C) The chemical inhibitors AMD3100, imatinib, and ISCK03 were injected into quail eggs at day 3 of development (100nM) individually or in combination as well as blocking antibodies to SCF and IL-3 (20 μg/mL) versus controls. Embryos were allowed to develop until day 6. Embryo images reveal cranial and abdominal hemorrhage phenotypes (arrows) in embryos treated with hematopoietic cytokine or receptor antagonists, but not in controls. (D) Histologic analysis of developing quail tissue reveals marked hemorrhage in treated embryos. (E) Data are presented showing the frequency and location of hemorrhage visualized in treated versus control embryos. In addition, survival data as well as tissue mass are indicated (n ≥ 7; P ≤ .01). (F) Quail CAM tissue was collected from control versus imatinib/AMD3100 or α-IL-3/SCF–treated embryos at day 6 of development and immunostained for the quail EC-specific marker, QH1. The number of vessel branch points is quantified (top), as well as the area of nonvascularized tissue space (bottom; n ≥ 10; P ≤ .01). *Significance from control. (G) Left panels are representative sections from control versus α-IL-3/SCF–treated embryos at 6 days of development, where reduced sprouting is observed in forebrain parenchyma. Bar equals 100 μm. Representative QH1 stained images from 6-day quail CAM are shown from the indicated conditions showing marked vascular remodeling defects in the imatinib/AMD3100 or α-IL-3/SCF–treated embryos compared with control. Bar equals 100 μm.
Role for hematopoietic stem cell cytokines and their receptors during quail developmental vascularization events. (A) Quail CAM tissue was isolated at day 6 of development, fixed, and double stained for the quail EC-specific marker, QH1, versus c-Kit, IL-3Rα, and CXCR4. Arrows indicate vessel wall borders; arrowheads indicate circulating blood cells. (B) Western blot analysis of embryonic 6-day quail vitelline vessels reveals the presence of hematopoietic cytokine receptors in the vasculature. (C) The chemical inhibitors AMD3100, imatinib, and ISCK03 were injected into quail eggs at day 3 of development (100nM) individually or in combination as well as blocking antibodies to SCF and IL-3 (20 μg/mL) versus controls. Embryos were allowed to develop until day 6. Embryo images reveal cranial and abdominal hemorrhage phenotypes (arrows) in embryos treated with hematopoietic cytokine or receptor antagonists, but not in controls. (D) Histologic analysis of developing quail tissue reveals marked hemorrhage in treated embryos. (E) Data are presented showing the frequency and location of hemorrhage visualized in treated versus control embryos. In addition, survival data as well as tissue mass are indicated (n ≥ 7; P ≤ .01). (F) Quail CAM tissue was collected from control versus imatinib/AMD3100 or α-IL-3/SCF–treated embryos at day 6 of development and immunostained for the quail EC-specific marker, QH1. The number of vessel branch points is quantified (top), as well as the area of nonvascularized tissue space (bottom; n ≥ 10; P ≤ .01). *Significance from control. (G) Left panels are representative sections from control versus α-IL-3/SCF–treated embryos at 6 days of development, where reduced sprouting is observed in forebrain parenchyma. Bar equals 100 μm. Representative QH1 stained images from 6-day quail CAM are shown from the indicated conditions showing marked vascular remodeling defects in the imatinib/AMD3100 or α-IL-3/SCF–treated embryos compared with control. Bar equals 100 μm.
The administration of either combination of chemical inhibitors or blocking antibodies caused a high frequency of both brain and abdominal hemorrhage phenotypes in developing quail embryos (Figure 6C-E, supplemental Figure 5). Similar hemorrhage phenotypes have been reported in Runx1 knockout mice and zebrafish, including those in which Runx1 has been selectively knocked in ECs.17,39,45 The hemorrhage phenotype is manifest by evident hemorrhage in histologic sections with marked vessel dilation, particularly in periventricular meningeal tissue as well as decreased sprouting and vessel formation in underlying brain parenchyma (Figure 6D-G, supplemental Figure 5). We also observed marked vessel dilation in the 6-day quail CAM, implicating a defect in vascular remodeling (Figure 6F-G) such that larger vessels do not appear to remodel into the smaller capillary networks observed in the control (supplemental Figure 5).
In addition, the apparent rupture of these dilated vessels occurs in a variety of tissue locations within the treated embryos (supplemental Figure 5). These phenotypes are observed with receptor blocking agents as well as blocking antibodies directed to the hematopoietic cytokines themselves. We also observed the same results but with more severe consequences when we administered the same combinations of inhibitors at day 1 of development and examined embryos at day 4 (supplemental Figure 5). In both cases, overall embryo weights were reduced when these blocking agents were combined, a further indicator of vascular perfusion defects (Figure 6E, supplemental Figure 5). Of importance, the vascular hemorrhage phenotype is most prominently observed in vivo when more than one receptor or ligand is blocked, a phenotype consistent with our findings that these hematopoietic cytokines act as synergistic factors controlling vascular morphogenic events in vitro and in vivo.
Discussion
This work is focused on a central question in vascular biology, namely, what growth factors control vascular tube morphogenesis and sprouting in 3D matrix environments. The answer to this question has fundamental implications for our basic understanding of how blood vessels form but is also critical toward the development of appropriate vascular therapeutics to affect diseases such as cancer, diabetes, and macular degeneration, where the vasculature plays a major pathogenetic role. For this question to be properly addressed, defined systems are necessary to identify specific growth factor requirements for different steps in vascular morphogenesis. In our view, the systems used previously are too complex to resolve such questions in that they represent in vivo models with multiple cell types coupled with the inherent complexity of intact organisms or in vitro models that use serum and/or multiple cell types.
Here, by using a high-throughput screening approach under serum-free–defined conditions, we demonstrate that hematopoietic stem cell cytokines are required for human vascular tube morphogenesis and maturation, including pericyte recruitment with concomitant vascular basement membrane assembly. This conclusion was also confirmed by the use of a distinct source of ECs from embryonic quail vessels, which similarly sprout and form tubes in response to hematopoietic cytokines. In contrast, known proangiogenic factors such as VEGF, FGF, and angiopoietins failed to support such morphogenic events. In no case under our defined conditions with human ECs did VEGF and FGF on their own or in combination stimulate tube morphogenesis or sprouting in 3D matrices when added during the morphogenic assays. However, the addition of VEGF, FGF, and VEGF/FGF before morphogenesis as an EC priming step led to marked enhancement of human or embryonic quail EC tube morphogenesis and sprouting in response to combinations of hematopoietic stem cell cytokines, SCF, IL-3, and SDF-1α. Thus, VEGF and FGF affect EC tube morphogenesis and sprouting but do so primarily through upstream action via up-regulation of hematopoietic cytokine receptors, including c-Kit, IL-3Rα, and CXCR4, which then allows the downstream action of hematopoietic cytokines to control vascular morphogenesis (Figure 7).
A 2-step model of vascular tube morphogenesis and sprouting: VEGF and FGF prime ECs to respond to hematopoietic stem cell cytokines to form tubes and sprout in 3D matrices. (A) A schematic is shown depicting the 2-step process of EC priming followed by tube morphogenesis and associated downstream effects connected to each step, including up-regulation of the hematopoietic cytokine receptors after VEGF-A/FGF-2 priming events, hematopoietic cytokine-induced morphogenesis, and vessel stabilization through the recruitment of pericytes and basement membrane matrix deposition. A Venn diagram compartmentalizes each growth factor on the basis of its primary function as either a priming or promorphogenic molecule. FGF-2 has strong EC priming activity but does facilitate the action of promorphogenic hematopoietic cytokines. VEGF has strong EC priming activity and also facilitates promorphogenic hematopoietic cytokines during EC sprouting events. (B) Images of VEGF/FGF-primed EC cultures stimulated with VEGF-A versus the hematopoietic factors as morphogenic cues (top). Bar equals 20 μm. VEGF/FGF primed vitelline vessel explants stimulated with VEGF-A versus the hematopoietic factors as morphogenic cues (bottom). Bar equals 100 μm.
A 2-step model of vascular tube morphogenesis and sprouting: VEGF and FGF prime ECs to respond to hematopoietic stem cell cytokines to form tubes and sprout in 3D matrices. (A) A schematic is shown depicting the 2-step process of EC priming followed by tube morphogenesis and associated downstream effects connected to each step, including up-regulation of the hematopoietic cytokine receptors after VEGF-A/FGF-2 priming events, hematopoietic cytokine-induced morphogenesis, and vessel stabilization through the recruitment of pericytes and basement membrane matrix deposition. A Venn diagram compartmentalizes each growth factor on the basis of its primary function as either a priming or promorphogenic molecule. FGF-2 has strong EC priming activity but does facilitate the action of promorphogenic hematopoietic cytokines. VEGF has strong EC priming activity and also facilitates promorphogenic hematopoietic cytokines during EC sprouting events. (B) Images of VEGF/FGF-primed EC cultures stimulated with VEGF-A versus the hematopoietic factors as morphogenic cues (top). Bar equals 20 μm. VEGF/FGF primed vitelline vessel explants stimulated with VEGF-A versus the hematopoietic factors as morphogenic cues (bottom). Bar equals 100 μm.
Importantly, we demonstrate that each of these receptors is expressed on embryonic quail vessels in that we could detect them by using immunofluorescence microscopy and by Western blot (Figure 6), and furthermore, these vessels responded by sprouting and forming EC-lined tubes in 3D collagen matrices in response to hematopoietic cytokines (Figures 1,3,7). In support of our findings are previous studies in which authors31,33,41-44,46 detected these receptors on ECs during the earliest stages of vascular development in mice, chick, and zebrafish, as well as their ligands within or directly surrounding the vasculature. Thus, there is considerable evidence that hematopoietic stem cell cytokines are present during early development and that embryonic ECs express hematopoietic cytokine receptors that could allow them to respond and control developmental vascularization events like we demonstrate in this study. Of great interest is that there appears to be functional redundancy in that both Flt3L and M-CSF are able to substitute for the action of SCF in our in vitro system to support vascular morphogenic responses. VEGF and FGF priming also was shown to up-regulate Flt3 and CSFR1, which represent the Flt3L and M-CSF receptors, respectively, and induce morphogenic responses in 3D matrices.
VEGF is known to control both vascular development and hematopoiesis,14 and the authors of many recent studies11,13,14,16-18 reveal that ECs are precursors for hematopoietic stem cells. Thus, the studies reported here demonstrating that VEGF induces EC hematopoietic cytokine receptor expression are consistent with these previous findings. In addition, we found that EC Runx1, a critical transcriptional regulator for definitive hematopoiesis and vascular development in mice and zebrafish,12,17,18 controls expression of both priming (eg, VEGFR2) and promorphogenic (c-Kit, IL-3Rα, CXCR4) receptors. Previous authors22,23,46,47 have described a role for SDF-1α, in particular, in angiogenic responses both in developmental and adult contexts. This factor also has a diverse ability to affect a variety of stem cells (including primordial germ cells) to migrate within embryos through its activation of the G protein, Gαi. In addition, SDF-1α can recruit and retain circulating mononuclear cells in a perivascular location that affects angiogenic responses by production of proangiogenic cytokines.22,23
Here, our findings reveal that additional hematopoietic cytokines (eg, SCF, IL-3, M-CSF, Flt3L) are required to work in conjunction with SDF-1α under defined conditions to control vascular tube morphogenesis and sprouting. Another important finding is that these hematopoietic stem cell cytokines induce vascular tube morphogenesis but also allow for the recruitment of pericytes to these developing vessels and induce vessel maturation through vascular basement membrane matrix assembly. Furthermore, VEGF and FGF priming also enhances the ability of hematopoietic cytokines and pericytes to affect EC-pericyte tube coassembly and basement membrane assembly. Interestingly, we observed that laminin deposition around these tubes was strongly enhanced by VEGF and FGF priming.
Finally, our work has broad implications for the application of vascular therapeutics in a variety of clinical contexts and tissue engineering of microvascular tube networks because we demonstrate that major regulators of vascular tube morphogenesis and sprouting are hematopoietic stem cell cytokines. Currently, much of the focus of vascular therapeutics is directed toward modulation of VEGF activity. Here, we demonstrate that a major function of VEGF is to prime EC responsiveness to other factors that subsequently control vascular morphogenesis. In addition, our new findings suggest that agents that are currently effectively used in cancer therapeutics, such as imatinib48,49 or sunitinib,49,50 which block c-Kit, and c-Kit/Flt-3/CSFR1, respectively, might be efficacious in part because of their ability to affect vascular morphogenesis through inhibition of hematopoietic cytokine action on ECs. Combined blockade of c-Kit and CXCR4 or SCF and IL-3 leads to major defects in developmental vascularization, suggesting that such an approach might represent a new therapeutic strategy to block or modulate pathologic neovascularization. Clearly, it is important to reevaluate vascular therapeutic approaches and specific therapeutic agents with known action on hematopoietic cytokine receptor signaling in light of our new findings.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Kristine Malotte for excellent technical assistance.
This work was supported by National Institutes of Health grants HL59373 and HL79460 to G.E.D.
National Institutes of Health
Authorship
Contribution: A.N.S. performed and designed experiments, analyzed data, and wrote the paper; M.J.D. performed experiments and wrote the paper; and G.E.D. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: George E. Davis, MD, PhD, Mulligan Professor of Medical Research, Department of Medical Pharmacology and Physiology, School of Medicine, MA415 Medical Sciences Building, University of Missouri-Columbia, Columbia, MO 65212; e-mail: davisgeo@health.missouri.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal