Abstract
The β-hemoglobinopathies sickle cell disease and β-thalassemia are among the most common human genetic disorders worldwide. Hemoglobin A2 (HbA2, α2δ2) and fetal hemoglobin (HbF, α2γ2) both inhibit the polymerization of hemoglobin S, which results in erythrocyte sickling. Expression of erythroid Kruppel-like factor (EKLF) and GATA1 is critical for transitioning hemoglobin from HbF to hemoglobin A (HbA, α2β2) and HbA2. The lower levels of δ-globin expression compared with β-globin expression seen in adulthood are likely due to the absence of an EKLF-binding motif in the δ-globin proximal promoter. In an effort to up-regulate δ-globin to increase HbA2 expression, we created a series of EKLF-GATA1 fusion constructs composed of the transactivation domain of EKLF and the DNA-binding domain of GATA1, and then tested their effects on hemoglobin expression. EKLF-GATA1 fusion proteins activated δ-, γ-, and β-globin promoters in K562 cells, and significantly up-regulated δ- and γ-globin RNA transcript and protein expression in K562 and/or CD34+ cells. The binding of EKLF-GATA1 fusion proteins at the GATA1 consensus site in the δ-globin promoter was confirmed by chromatin immunoprecipitation assay. Our studies demonstrate that EKLF-GATA1 fusion proteins can enhance δ-globin expression through interaction with the δ-globin promoter, and may represent a new genetic therapeutic approach to β-hemoglobinopathies.
Introduction
Sickle cell disease (SCD) and β-thalassemia are among the most common genetic diseases worldwide, affecting global health and mortality.1 Therefore, these β-hemoglobinopathies represent a major public health challenge. In SCD, a point mutation in the β-globin chain leads to abnormal production of sickle hemoglobin (HbS, α2βS2), which polymerizes and precipitates in red blood cells when deoxygenated, decreasing cell flexibility and damaging the cell membrane. These stiff sickle cells lead to hemolytic anemia and vaso-occlusion, causing severe clinical complications.2,3 Genetic alterations in β-thalassemia cause defective production of the β-globin chain and result in an imbalanced accumulation of the α-globin chain.4 These 2 disorders both produce a variable degree of hemolytic anemia and transfusion-related complications.
Activation of γ-globin to increase fetal hemoglobin (HbF, α2γ2) is currently a strategy used in the management of β-hemoglobin disorders. Hydroxyurea has been successfully used in the treatment of SCD and β-thalassemia by augmenting the production of HbF, which interferes with HbS polymerization, preventing red blood cells from sickling in SCD5 and reducing the α-globin chain imbalance in β-thalassemia.6 Whereas both hemoglobin A2 (HbA2, α2δ2, 2%-2.5% of total Hb) and HbF (≤ 2% of total Hb) are minor components in adult blood, they have been proven to be equally effective in inhibiting intracellular deoxy-HbS polymerization.7 However, unlike HbF, which is restricted to a small population of erythrocytes (5%-10%),8 the distribution of HbA2 is pancellular.9 Therefore, increased expression of HbA2 may compensate for the impaired β-globin production inherent in β-hemoglobinopathies and ameliorate the clinical severity of these diseases. We and others have previously shown that the low expression of δ-globin in adult blood is due to a mutation in the erythroid Kruppel-like factor (EKLF)–binding site (CACCC box) within the δ-globin proximal promoter region (Figure 1A). Restoration of this binding site activates δ-globin promoter activity to levels equivalent to that of β-globin promoter activity in K562 cells and human adult erythroid cells.10-12
EKLF is an erythroid cell–specific DNA-binding protein with the DNA-binding domains at the C-terminus and the transactivation domain at the N-terminus. It binds to the β-globin CACCC box and is essential for β-globin expression. The transactivation domain of EKLF serves as a positive regulator,13 and when fused with a GAL-binding domain can significantly stimulate δ-globin expression in HS2-δ GAL4-β–transfected MEL cells.12 However, because this approach involves an alteration within the δ-globin promoter, it is not feasible for clinical use. GATA1 is a transcription factor that is essential for red blood cell formation, development, and hemoglobin production. It contains 3 transactivation domains and 2 DNA-binding domains. The carboxyl-terminal zinc-finger domain (GATA1 CF) constitutes the primary DNA-binding domain that is capable of independently binding the consensus motif A/TGATAA/G with high affinity.14,15 The amino-terminal zinc-finger domain (GATA1 NF) independently binds to a motif containing a GATC core sequence with lower affinity, stabilizing DNA binding of multiple GATA sites.16,17 A known functional GATA1-binding motif is close to the mutated CACCC box in the δ-globin proximal region18,19 (Figure 1A). In addition, GATA1 is highly hematopoietic cell specific and is only expressed in hematopoietic lineages (except for Sertorli cells of the pubescent testis).20,21 Finally, it has been reported that GATA1's zinc-finger domain has a strong binding affinity and that GATA1 has a unique function in erythropoiesis.22 Based on these characteristics, we have chosen to link EKLF to GATA1 to produce EKLF-GATA1 fusion proteins to be examined for better transactivation of the δ-globin gene.
In the present study, we constructed and characterized a series of EKLF-GATA1 chimeric proteins composed of different combinations of EKLF-transactivation domains and GATA1 DNA–binding domains. We then used these constructs to examine how these modules affect the promoter activity and expression of various globins in K562 erythroleukemia cells and CD34+ primary cells. We found that EKLF-GATA1 fusion proteins significantly increased hemoglobin production, with profound effects on δ-globin expression. These findings may provide a fundamental basis for future development of new genetic therapeutic approaches for the treatment of SCD and β-thalassemia.
Methods
Plasmid construction
The construction of luciferase reporter plasmids containing the δ-, β-, or γ-globin promoter has been described previously.10,23 The δ-globin reporter construct contains a 680-bp fragment of the human δ-globin promoter (from −612 to +68 relative to the cap site). The β-globin reporter construct contains a 690-bp fragment of the human β-globin promoter (from −640 to +50). The γ-globin reporter construct contains a 316-bp fragment of the human γ-globin promoter (from −268 to +48) with the known functional GATA1- and EKLF-binding sites indicated (Figure 1B).18,19,24-27 The mutation of the GATA1-binding motif in the δ-globin promoter reporter construct was generated by QuikChange site-directed mutagenesis (Stratagene) and verified by sequencing.
The coding regions of EKLF (isolated from a plasmid pSG5-EKLF provided by Dr Jim Bieker, Mt Sinai Medical School, New York, NY) or GATA1 were directly cloned into the pLenti V5 topo expression vector by polymerase chain reaction (PCR) using a high-fidelity PCR mixture (Invitrogen). Three fusion pLenti V5 EKLF-GATA1 expression vectors (long, medium, and short forms; Figure 1C) were constructed. First, a PCR fragment containing the full coding region of EKLF (1-362 amino acids) or the transactivation domain of EKLF (1-275 amino acids) was cloned into the pLenti V5 topo expression vector. A PCR fragment containing the coding region of GATA1 (145-413 amino acids) or the DNA-binding domain of GATA1 (190-320 amino acids or 190-272 amino acids) was then cloned into the PCR2.1 topo vector. Finally, the fragment of GATA1 was digested from PCR2.1 GATA1 and in-frame subcloned into pLenti V5 EKLF. All inserts were fused with the V5 epitope tag at the C-terminus. Plasmids were sequenced to verify the integrity of the insert.
Lentiviral production and gene transduction
Lentivirus encoding EKLF, GATA1, EKLF-GATA1, or vector only was packaged into 293FT cells according to the ViraPower Lentiviral Expression Systems instructions (Invitrogen). The viral product was concentrated 100 times by Lenti-X (Clontech) and titers were determined. For gene transduction in CD34+ stem cells, 4 × 106 viral particles were preloaded onto RetroNectin-coated plates (Takara Shuzo) and incubated at 37°C for 5 hours. Just before infection, the viral supernatant was discarded, 4 × 105 CD34+ cells in StemSpan serum-free expansion medium (StemCell Technologies) supplemented with 1× CC100 cytokine mix (StemCell Technologies) was added to the viral-preloaded plate, and the plate was incubated at 37°C in 5% CO2 for 2 days.
Cell culture
293FT cells (Invitrogen) were grown in Dulbecco modified Eagle medium containing 10% fetal bovine serum supplemented with 0.1mM minimum essential medium nonessential amino acids, 1mM sodium pyruvate, 2mM l-glutamine, and 500 μg/mL geneticin. K562 cells were maintained in RPMI with 10% fetal bovine serum. Bone marrow CD34+ cells were purchased from Poietic Technologies and grown in StemSpan serum-free expansion medium supplemented with 1× CC100 cytokine mix for 6 days (expansion stage). Lentiviral gene transduction was performed on day 4. On day 6, gene-transduced CD34+ cells were reseeded into Eagle minimum essential medium containing 30% fetal bovine serum, 4 U/mL erythropoietin (EPO), 1% deionized bovine serum albumin, 40mM glutamine, 100μM β-mercaptoethanol, 1μM dexamethasone, 0.3 mg/mL holotransferrin, and 3 μg/mL blasticidin (Invitrogen). CD34+ cells were harvested for chromatin immunoprecipitation (ChIP) assay and hemoglobin gene expression studies after 7 days of culturing with EPO (differentiation stage).
RNA isolation and real-time PCR
RNA was isolated with the RNeasy Mini Plus Kit (QIAQEN) and reverse transcription PCR was performed using SuperScript Reverse Transcriptase II and Taq DNA polymerase (Invitrogen) according to the manufacturer's instructions. Real-time PCR was performed with Platinum QPCR SuperMix-UDG (Invitrogen) using the primer pairs and probes for γ- and β-globin.28 The primers and probes for δ-globin and control β-actin were from Applied Biosystems.
Reporter construct transfection and reporter gene assays
The δ-, γ-, or β-globin reporter construct was cotransfected with fusion EKLF, GATA1, EKLF-GATA1, or vector-only constructs into K562 cells by electroporation (Amaxa Biosystems). K562 cells transfected with wild-type or mutant globin gene reporter construct alone were defined as mock-transfected cells. pRL-TK (Promega) was cotransfected as an internal control. The cells were harvested for luciferase assays 48 hours after transfection. The level of promoter activity was evaluated by measurement of the firefly luciferase activity relative to the internal control renilla luciferase activity using the dual luciferase assay system as described by the manufacturer (Promega). A minimum of 3 independent transfections were performed in triplicate. Results are reported as the means ± SD.
Statistical analysis
The statistical analysis of the results were obtained using the Student t test for significance of the difference of individual pairs and the Pearson correlation coefficient for the closeness of linear relationship.
Western blot
For Western blots, cell lysates containing 5-50 μg of protein were loaded onto tris(hydroxymethyl)aminomethane (Bis-Tris) gels (Invitrogen), subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes (Invitrogen). Membranes were probed with antibodies to δ-globin (provided by Dr Ning Fu, Southern Medical University, Guangzhou, China)29 ; γ-globin, β-globin, EKLF, or GATA1 (Santa Cruz Biotechnology); or V5 (Invitrogen) overnight at 4°C. Membranes were washed and incubated with horseradish peroxidase–conjugated secondary antibody. The protein bands that reacted with the antibodies were detected on radiographic film (GE Healthcare) 1-60 seconds after exposure. The bands on radiographic films were scanned and analyzed densitometrically using a ChemiImager (Alpha Innotech). Blots were stripped and reprobed with a β-actin antibody (Santa Cruz Biotechnology) as a loading control.
ChIP
ChIP was performed using the Magna ChIP kit (Millipore) according to the manufacturer's instructions with minor modifications. Briefly, 0.5-1.0 × 107 CD34+ cells (transduced with EKLF, GATA1, EKLF-GATA1, or vector only) were fixed using a 1% final concentration of formaldehyde for 10 minutes. The cross-linking was then stopped by adding glycine to a final concentration of 125mM. We typically obtained fragments in the range of 200-1000 bp after sonication. Anti-V5 (Invitrogen) or GATA1 (Santa Cruz Biotechnology) antibody was used to immunoprecipitate chromatin and protein complexes, which were subsequently isolated with magnetic protein G beads according to the manufacturer's instructions (Millipore). The ChIP samples were analyzed by PCR. Primers were designed to amplify the fragment from −152 to +2 of the δ-globin gene-promoter region containing the GATA1-binding motif. The forward primer sequence was 5′-ATCTCAGGGCAAGTTAAGGG-3′ and the reverse primer sequence was 5′-GTAAGCAACAGTCGACTCTG-3′. A pair of primers designed to amplify the fragment of the δ-globin gene-promoter region from −619 to −473 that does not contain the GATA1-binding motif were used in the ChIP assay as a negative control; the forward primer sequence was 5′-AATTCATGTTTCCAGAACCTATTTC-3′ and the reverse primer sequence was 5′- CTTGCTTGCTTCCTCCTTC-3′.
Results
Effect of the EKLF-GATA1 fusion protein on globin gene promoter activity
To test whether the EKLF-GATA1 fusion constructs can express proper proteins, expressional vectors containing EKLF, GATA1, fusion EKLF-GATA1 (long, medium, or short form), or vector only were transfected into 293FT cells. Protein expression was examined by Western blotting using anti-V5, anti-EKLF, or anti-GATA1 antibodies (Figure 1D). All construct proteins were recognized by the anti-V5 antibody. Construct proteins containing EKLF or fusion EKLF-GATA1 were recognized by the anti-EKLF antibody. Construct proteins containing the fusion EKLF-GATA1 long form or GATA1 alone were recognized by an antibody directed at the C-terminus of GATA1. At present, a suitable anti-GATA1 antibody capable of detecting medium- and short-form fusion proteins is unavailable.
Schematic diagram of the structure of human hemoglobin reporter constructs and EKLF, GATA1, and EKLF-GATA1 fusion constructs. (A) Comparison of the δ- and β-globin promoter proximal regions; GATA1- and EKLF-binding sites are indicated. (B) The β-globin locus on human chromosome 11. Schematic diagram of δ-, β-, and γ-globin promoter regions in a luciferase reporter construct with the known functional EKLF- or GATA1-binding sites in each promoter indicated.18,19,24-27 (C) Schematic diagram of the structure of EKLF, GATA1, and 3 different EKLF-GATA1 fusion pLenti V5 topo expression constructs. All inserts were fused to a V5 epitope at the C-terminus with the transactivation and DNA-binding domains indicated. F1, F2, and F3 represent 3 finger domains of EKLF; CF and NF represent C- and N-fingers of GATA1; R1, R2, and R3 represent 3 regions of the transactivation domain of GATA1. (D) Long (L), medium (M), or short (S) form of EKLF, GATA1, EKLF-GATA1, or vector only (V) were transiently transfected into 293FT cells and then harvested 48 hours after transfection and subjected to Western blotting analysis using anti-V5, EKLF, or GATA1 antibodies. Mo indicates mock-transfected 293FT cells.
Schematic diagram of the structure of human hemoglobin reporter constructs and EKLF, GATA1, and EKLF-GATA1 fusion constructs. (A) Comparison of the δ- and β-globin promoter proximal regions; GATA1- and EKLF-binding sites are indicated. (B) The β-globin locus on human chromosome 11. Schematic diagram of δ-, β-, and γ-globin promoter regions in a luciferase reporter construct with the known functional EKLF- or GATA1-binding sites in each promoter indicated.18,19,24-27 (C) Schematic diagram of the structure of EKLF, GATA1, and 3 different EKLF-GATA1 fusion pLenti V5 topo expression constructs. All inserts were fused to a V5 epitope at the C-terminus with the transactivation and DNA-binding domains indicated. F1, F2, and F3 represent 3 finger domains of EKLF; CF and NF represent C- and N-fingers of GATA1; R1, R2, and R3 represent 3 regions of the transactivation domain of GATA1. (D) Long (L), medium (M), or short (S) form of EKLF, GATA1, EKLF-GATA1, or vector only (V) were transiently transfected into 293FT cells and then harvested 48 hours after transfection and subjected to Western blotting analysis using anti-V5, EKLF, or GATA1 antibodies. Mo indicates mock-transfected 293FT cells.
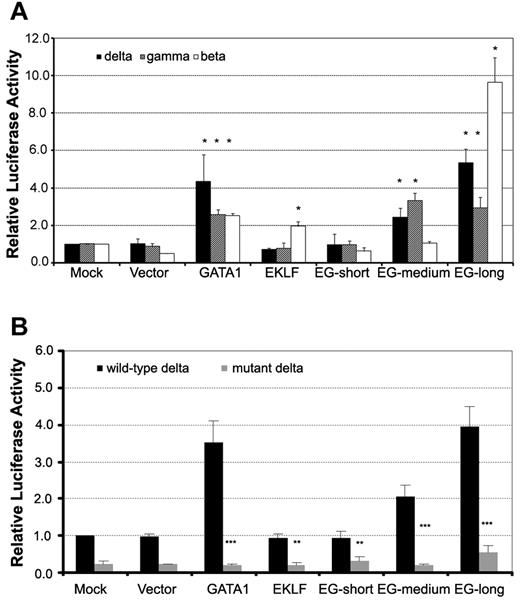
We next examined the functions of the EKLF-GATA1 fusion protein. Vector expressing EKLF-GATA1, EKLF, or GATA1 or empty vector was cotransfected with δ-, γ-, or β-globin reporter constructs or empty vector into K562 cells. The pRL-TK vector was cotransfected as an internal control. Reporter activity was assessed at 48 hours after transfection (Figure 2A). We found that the expression of long-form EKLF-GATA1 increased δ-, γ-, and β-globin promoter activity 5.4-, 2.9-, and 9.4-fold, respectively, after transfection compared with mock transfection. The effect of medium-form EKLF-GATA1 expression on globin promoter activity was less profound than that of long-form EKLF-GATA1, with a 2.5- and 3.2-fold increase for δ- and γ-globin promoter activity, respectively. Both the short-form EKLF-GATA1 and vector only had no appreciable effect on globin promoter activity. GATA1 expression increased δ-globin promoter activity ∼ 4.3-fold; EKLF induced β-globin promoter activity ∼ 2-fold. These results indicate that the long- and medium-form EKLF-GATA1 fusion proteins, which contain the N-finger and C-finger of the GATA1-binding domain, may well bind to and activate the δ-globin promoter. In contrast, the short-form EKLF-GATA1 fusion protein, which lacked the intact C-finger, was not able to bind to the δ-globin promoter and thus had no impact on globin expression.
EKLF-GATA1 fusion proteins activated δ-, γ-, and β-globin promoter activity in K562 cells. ELKF, GATA1, fusion EKLF-GATA1 (EG) vector, or vector only was cotransfected with δ-, γ-, or β-globin promoter reporter constructs (A) or wild-type or mutant δ-globin promoter reporter constructs (B) into K562 cells. The level of promoter activity was evaluated 48 hours after transfection by measurement of firefly luciferase activity relative to the internal control renilla luciferase activity using the dual luciferase assay system essentially as described by the manufacturer. (A) Fold increase was calculated compared with expression in mock-transfected K562 cells. *P < .05 versus mock-transfected cells. (B) Fold increase was calculated relative to expression in mock-transfected cells. **P < .01 or ***P < .001 versus corresponding wild-type transfected cells. Error bars indicate SD of the mean of 3 independent experiments.
EKLF-GATA1 fusion proteins activated δ-, γ-, and β-globin promoter activity in K562 cells. ELKF, GATA1, fusion EKLF-GATA1 (EG) vector, or vector only was cotransfected with δ-, γ-, or β-globin promoter reporter constructs (A) or wild-type or mutant δ-globin promoter reporter constructs (B) into K562 cells. The level of promoter activity was evaluated 48 hours after transfection by measurement of firefly luciferase activity relative to the internal control renilla luciferase activity using the dual luciferase assay system essentially as described by the manufacturer. (A) Fold increase was calculated compared with expression in mock-transfected K562 cells. *P < .05 versus mock-transfected cells. (B) Fold increase was calculated relative to expression in mock-transfected cells. **P < .01 or ***P < .001 versus corresponding wild-type transfected cells. Error bars indicate SD of the mean of 3 independent experiments.
To determine whether the transactivation of the δ-globin promoter by the EKLF-GATA1 fusion protein depends on the GATA1-binding motif found in its proximal region, we mutated this GATA1-binding site (TTATCT was mutated to CTCGAG) and examined the effect of fusion protein on mutated δ-globin promoter activity. Vector expressing EKLF, GATA1, EKLF-GATA1, or empty vector was cotransfected with δ-globin reporter constructs (wild-type or mutant) or empty vector into K562 cells. The pRL-TK vector was cotransfected as an internal control. Reporter activity was assessed at 48 hours after transfection. The mutation in the δ-globin promoter GATA1-binding motif not only reduced the basal promoter activity, but also had an impact on the effect of GATA1 and EKLF-GATA1 fusion proteins on δ-globin promoter activity (Figure 2B). Mutation of the GATA1 motif in the δ-globin promoter resulted in a 75% and 72% reduction in the basal promoter activity in mock-transfected (from 1.0 to 0.25) and vector-transfected cells (from 0.96 to 0.24), respectively. In GATA1-transfected cells, the magnitude was reduced from 3.5-fold to 0.2-fold, whereas in EKLF-transfected cells, it was reduced from 0.94 to 0.30. Mutation of the GATA1 motif also abolished the induction resulting from EKLF-GATA1 fusion protein transfection. The promoter activity in EKLF-GATA1–transfected cells dropped from 0.95, 2.10, and 4.00 to 0.31, 0.21, and 0.53 for the short-, medium- and long-form fusion proteins, respectively. These results indicated that the transactivation of the δ-globin promoter by the EKLF-GATA1 fusion protein is largely dependent on the GATA1-binding motif in the promoter.
EKLF-GATA1 protein up-regulates δ-globin gene expression in K562 cells
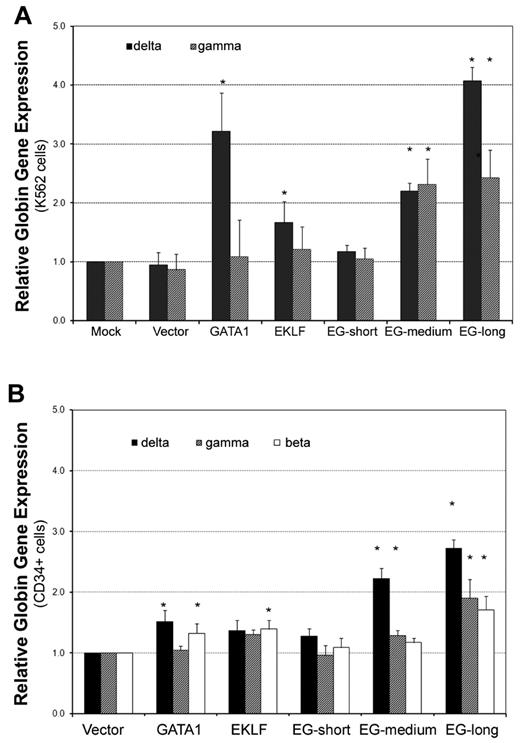
After we determined that the EKLF-GATA1 fusion protein could activate globin promoter activity, we further investigated its effect on endogenous globin transcriptional expression. K562 cells were stably transfected with expression vectors containing EKLF, GATA1, fusion EKLF-GATA1, or empty vector. Real-time PCR analysis revealed that both the long and medium forms of EKLF-GATA1 up-regulated δ- and γ-globin gene expression (Figure 3A). The long-form EKLF-GATA1 induced δ- and γ-globin expression 4.1- and 2.4-fold, respectively, whereas the medium-form EKLF-GATA1 induced δ- and γ-globin expression 2.2- and 2.3-fold, respectively, compared with the mock-transfection control. EKLF and GATA1 alone increased δ-globin gene expression 1.7- and 3.2-fold, respectively, but did not significantly affect γ-globin gene expression. In contrast, the short form and vector only had no impact on δ- or γ-globin expression. Because K562 cells lack β-globin expression,30 we were unable to assess the effects of the fusion EKLF-GATA1 proteins on β-globin expression in these cells.
The EKLF-GATA1 fusion proteins enhanced δ-globin gene expression in K562 and CD34+ cells. (A) K562 cells were transfected with EKLF, GATA1, fusion EKLF-GATA1 (EG), or vector only and cultured in medium with 6 μg/mL of blasticidin for 2 weeks. RNA was isolated and real-time PCR analysis performed. Data represent real-time PCR analysis of δ- and γ-globin gene expression in EKLF-, GATA1-, or fusion EKLF-GATA1–transfected cells normalized to β-actin gene expression. Fold increase was calculated compared with expression in mock-transfected cells. *P < .05 versus mock-transfected cells. Error bars indicate SD of the mean of 3 independent experiments. (B) CD34+ bone marrow cells were infected by lentivirus encoding EKLF, GATA1, fusion EKLF-GATA1 (EG) constructs, or vector only at day 4 of the expansion stage, On day 6, gene-transduced CD34+ cells were reseeded and grown in differentiation medium with 3 μg/mL of blasticidin for 7 days. RNA was isolated and real-time PCR analysis performed. Data represent real-time PCR analysis of δ-, γ-, and β-globin gene expression in EKLF-, GATA1-, or fusion EKLF-GATA1–transduced cells normalized to β-actin gene expression. Fold increase was calculated compared with vector only–transduced cells. *P < .05 versus vector only–transduced cells. Error bars indicate SD of the mean of 3 independent experiments.
The EKLF-GATA1 fusion proteins enhanced δ-globin gene expression in K562 and CD34+ cells. (A) K562 cells were transfected with EKLF, GATA1, fusion EKLF-GATA1 (EG), or vector only and cultured in medium with 6 μg/mL of blasticidin for 2 weeks. RNA was isolated and real-time PCR analysis performed. Data represent real-time PCR analysis of δ- and γ-globin gene expression in EKLF-, GATA1-, or fusion EKLF-GATA1–transfected cells normalized to β-actin gene expression. Fold increase was calculated compared with expression in mock-transfected cells. *P < .05 versus mock-transfected cells. Error bars indicate SD of the mean of 3 independent experiments. (B) CD34+ bone marrow cells were infected by lentivirus encoding EKLF, GATA1, fusion EKLF-GATA1 (EG) constructs, or vector only at day 4 of the expansion stage, On day 6, gene-transduced CD34+ cells were reseeded and grown in differentiation medium with 3 μg/mL of blasticidin for 7 days. RNA was isolated and real-time PCR analysis performed. Data represent real-time PCR analysis of δ-, γ-, and β-globin gene expression in EKLF-, GATA1-, or fusion EKLF-GATA1–transduced cells normalized to β-actin gene expression. Fold increase was calculated compared with vector only–transduced cells. *P < .05 versus vector only–transduced cells. Error bars indicate SD of the mean of 3 independent experiments.
EKLF-GATA1 protein up-regulates δ-globin gene expression in CD34+ cells
Given that K562 cells have several limitations, including the fact that they are leukemic cells with impaired development and/or maturation, their proliferation is EPO independent, and they have no detectable β-globin expression,30 we extended our studies to examine the effect of EKLF-GATA1 fusion proteins on δ-, γ-, and β-globin expression in the clinically relevant CD34+ bone marrow stem cells. CD34+ cells were infected by lentivirus encoding EKLF, GATA1, or EKLF-GATA1 proteins at day 4 of the expansion stage. To enrich the numbers of transduced cells, the cells were grown in medium with blasticidin for 7 days at the differentiation stage. Transcription of δ-, γ-, and β-globin genes was then examined by real-time PCR (Figure 3B). We found that both the long and medium form of the fusion EKLF-GATA1 protein enhanced globin gene expression compared with vector control. The long-form EKLF-GATA1 up-regulated β-globin gene expression 1.7-fold, δ-globin gene expression 2.7-fold, and γ-globin gene expression 1.9-fold. The medium-form EKLF-GATA1 up-regulated δ-globin gene expression 2.2-fold and γ-globin 1.3-fold, but had no significant effect on β-globin gene expression. We also observed that EKLF only–transduced CD34+ cells expressed 1.4-fold higher levels of β-globin, and that GATA1 only–transduced cells expressed 1.5-fold higher levels of δ-globin and 1.3-fold higher levels of β-globin. In contrast, the short form of EKLF-GATA1 had no significant effect on globin expression. Specific to the δ-globin gene, the long-form EKLF-GATA1 induced expression 1.8-fold (P < .05) more than GATA1 alone, whereas the medium-form EKLF-GATA1 induced expression 1.5-fold (P < .05) more than GATA1 alone.
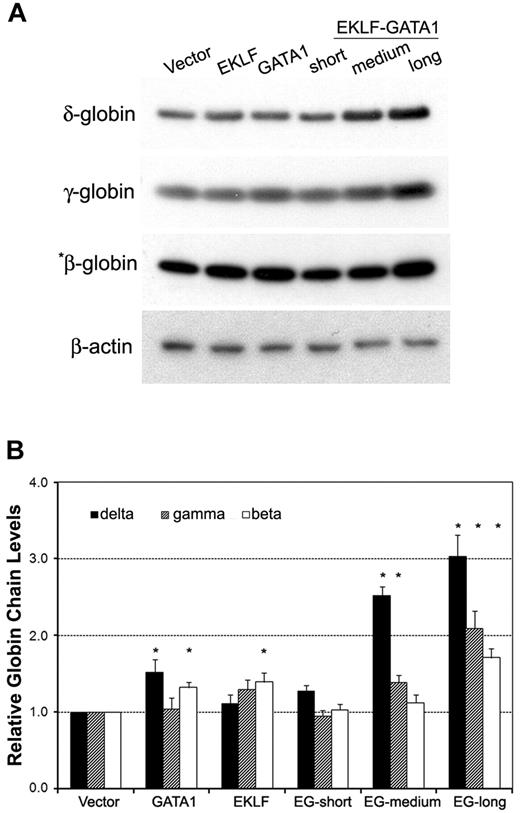
The effects of EKLF-GATA1 fusion protein on δ-, γ-, and β-globin protein expression were measured by Western blotting analysis of transduced CD34+ cells (Figure 4). Transduction of the long-form EKLF-GATA1 increased δ-globin protein expression 3-fold over that observed with vector only–transduced cells. The effect of this EKLF-GATA1 fusion protein on δ-globin was greater than its effects on γ- and β-globin protein expression (2.1- and 1.7-fold, respectively). The medium-form EKLF-GATA1 increased δ- and γ-globin protein expression 2.5- and 1.4-fold over vector only–transduced cells, and showed no significant effect on β-globin expression. Likewise, the short-form EKLF-GATA1 had no impact on the protein expression of all 3 isoforms of hemoglobin. These results are in agreement with the assumption that the EKLF-GATA1 fusion proteins affect endogenous globin transcription in erythroid cells.
The EKLF-GATA1 fusion proteins regulated hemoglobin protein expression in CD34+ cells. (A) CD34+ bone marrow cells were transduced with vector expressing EKLF, GATA1, EKLF-GATA1 (EG), or vector only. Cells were harvested 7 days after transduction and cell lysates subjected to Western blotting analysis. Samples containing 50 μg (for γ-globin, δ-globin, and β-actin) or 5 μg (for *β-globin) of protein were electrophoresed, transferred to nitrocellulose, and probed with antibodies directed against γ-, δ-, or β-globin or β-actin. β-Actin served as an internal control. (B) Graphical representation of data in panel A (normalized to the β-actin) measured by densitometry to indicate the fold increase in globin protein expression over vector only–transduced cells. *P < .05 versus vector only–transduced cells. Error bars indicate SD of the mean of 3 independent experiments.
The EKLF-GATA1 fusion proteins regulated hemoglobin protein expression in CD34+ cells. (A) CD34+ bone marrow cells were transduced with vector expressing EKLF, GATA1, EKLF-GATA1 (EG), or vector only. Cells were harvested 7 days after transduction and cell lysates subjected to Western blotting analysis. Samples containing 50 μg (for γ-globin, δ-globin, and β-actin) or 5 μg (for *β-globin) of protein were electrophoresed, transferred to nitrocellulose, and probed with antibodies directed against γ-, δ-, or β-globin or β-actin. β-Actin served as an internal control. (B) Graphical representation of data in panel A (normalized to the β-actin) measured by densitometry to indicate the fold increase in globin protein expression over vector only–transduced cells. *P < .05 versus vector only–transduced cells. Error bars indicate SD of the mean of 3 independent experiments.
EKLF-GATA1 fusion proteins can occupy the GATA1-binding motif of the δ-globin promoter
The GATA1-binding motif in the δ-globin proximal promoter is a functional regulator for δ-globin expression.18,19 To investigate whether EKLF-GATA1 fusion proteins were recruited to this region, we performed a ChIP assay in EKLF-, GATA1-, EKLF-GATA1-, or vector only–transduced CD34+ cells using antibody against V5 to immunoprecipitate protein-chromatin complexes, and then subjected the precipitated DNA to PCR for the δ-globin gene promoter region containing the GATA1-binding motif (Figure 5A top 3 panels, B). A pair of primers designed to amplify the fragment of the δ-globin gene promoter region that does not contain the GATA1-binding motif were used in the ChIP assay as a negative control (Figure 5A bottom 2 panels). The results showed that both the long- and medium- forms of EKLF-GATA1, as well as wild-type GATA1, can strongly bind to the δ-globin proximal promoter region. The mean binding of long- and medium-form EKLF-GATA1 to the δ-globin promoter was ∼ 80% and 49% of the GATA1's occupancy at this region, respectively. However, the short form of EKLF-GATA1 and wild-type EKLF were unable to bind to the same region. A ChIP assay using mouse immunoglobulin G (IgG) to immunoprecipitate protein-chromatin complexes was performed in parallel as a ChIP assay control. These results indicate that EKLF-GATA1 fusion proteins containing the transactivation domain of EKLF and the DNA-binding domain of GATA1 (C-finger and N-finger) can interact with the δ-globin promoter and activate δ-globin expression in erythroid cells. The fact that the long-form EKLF-GATA1 increased δ-globin gene expression more than the medium-form did may be attributable to the presence of the EKLF DNA F1-3–binding domain and/or the GATA1 R3 domain in the long form (Figure 1C).
The EKLF-GATA1 fusion protein occupied the GATA1-binding motif of the δ-globin promoter proximal region and competed with GATA1 for binding to the promoter. (A) CD34+ bone marrow cells were transduced with EKLF, GATA1, EKLF-GATA1 (EG), or vector only at day 4 of the expansion stage. On day 6, transduced CD34+ cells were reseeded into differentiation medium with 3 μg/mL of blasticidin for 7 days. Cells were then harvested and subjected to ChIP assay using antibody against V5 (top first panel) to immunoprecipitate chromatin-protein complexes. A parallel ChIP assay was performed using mouse IgG for the immunoprecipitation (IP) step as a ChIP assay control (top second panel). DNA was amplified and quantitated by PCR with specific primers flanking the δ-globin gene promoter from −152 to +2 (which contains the GATA1-binding motif) and a pair of control primers flanking the δ-globin gene promoter from −619 to −473 that does not contain the GATA1-binding motif (bottom second-to-last panel). PCR using input DNA as template served as an internal control (top third panel and bottom last panel). (B) Graphical representation of data in panel A. Results are expressed as relative proportions of immunoprecipitated DNA (ratios of immunoprecipitated versus input DNA) normalized to the ratio obtained for the δ-globin promoter in GATA1-transduced CD34+ cells (arbitrarily set at 100%). *P < .05 versus vector only–transduced cells. (C) CD34+ bone marrow cells were transduced with various MOIs of medium-form EKLF-GATA1 (EG) or vector only at day 4 of the expansion stage. On day 6, transduced CD34+ cells were reseeded into differentiation medium with 3 μg/mL of blasticidin for 5 days. Cells were then harvested and subjected to ChIP assay using antibody against V5 (top panel) or GATA1 (directed at the C-terminus of GATA1; middle panel) to immunoprecipitate chromatin-protein complexes. DNA was amplified and quantitated by PCR. A parallel ChIP assay was performed using mouse IgG for the immunoprecipitation (IP) step as a negative control. (D) Graphical representation of data in panel C. Relative level of immunoprecipitated DNA (ratios of immunoprecipitated vs input DNA) normalized to the ratio obtained for the δ-globin promoter in mock CD34+ cells (arbitrarily set at 1). Error bars indicate SD of the mean of 3 independent experiments.
The EKLF-GATA1 fusion protein occupied the GATA1-binding motif of the δ-globin promoter proximal region and competed with GATA1 for binding to the promoter. (A) CD34+ bone marrow cells were transduced with EKLF, GATA1, EKLF-GATA1 (EG), or vector only at day 4 of the expansion stage. On day 6, transduced CD34+ cells were reseeded into differentiation medium with 3 μg/mL of blasticidin for 7 days. Cells were then harvested and subjected to ChIP assay using antibody against V5 (top first panel) to immunoprecipitate chromatin-protein complexes. A parallel ChIP assay was performed using mouse IgG for the immunoprecipitation (IP) step as a ChIP assay control (top second panel). DNA was amplified and quantitated by PCR with specific primers flanking the δ-globin gene promoter from −152 to +2 (which contains the GATA1-binding motif) and a pair of control primers flanking the δ-globin gene promoter from −619 to −473 that does not contain the GATA1-binding motif (bottom second-to-last panel). PCR using input DNA as template served as an internal control (top third panel and bottom last panel). (B) Graphical representation of data in panel A. Results are expressed as relative proportions of immunoprecipitated DNA (ratios of immunoprecipitated versus input DNA) normalized to the ratio obtained for the δ-globin promoter in GATA1-transduced CD34+ cells (arbitrarily set at 100%). *P < .05 versus vector only–transduced cells. (C) CD34+ bone marrow cells were transduced with various MOIs of medium-form EKLF-GATA1 (EG) or vector only at day 4 of the expansion stage. On day 6, transduced CD34+ cells were reseeded into differentiation medium with 3 μg/mL of blasticidin for 5 days. Cells were then harvested and subjected to ChIP assay using antibody against V5 (top panel) or GATA1 (directed at the C-terminus of GATA1; middle panel) to immunoprecipitate chromatin-protein complexes. DNA was amplified and quantitated by PCR. A parallel ChIP assay was performed using mouse IgG for the immunoprecipitation (IP) step as a negative control. (D) Graphical representation of data in panel C. Relative level of immunoprecipitated DNA (ratios of immunoprecipitated vs input DNA) normalized to the ratio obtained for the δ-globin promoter in mock CD34+ cells (arbitrarily set at 1). Error bars indicate SD of the mean of 3 independent experiments.
EKLF-GATA1 protein competes with endogenous GATA1 for binding to the δ-globin promoter
To further investigate EKLF-GATA1 fusion protein binding to the δ-globin promoter, we examined whether EKLF-GATA1 protein could compete with endogenous GATA1 to bind to the δ-globin promoter. CD34+ cells were infected with the lentivirus encoding medium-form EKLF-GATA1 protein at various multiplicities of infection (MOI; 0, 1, 5, 10, and 20). ChIP assays were performed in EKLF-GATA1- or vector only–transduced CD34+ cells using antibody against V5 or GATA1 (directed at the C-terminus of GATA1 recognizing endogenous GATA1 only) to immunoprecipitate protein-chromatin complexes. A ChIP assay using mouse IgG to immunoprecipitate protein-chromatin complexes was performed in parallel as a control. The results showed that when CD34+ cells were infected with EKLF-GATA1 lentiviral particles at an MOI from 1-20, the occupancy of EKLF-GATA1 on the δ-globin promoter increased in an MOI-dependent manner, reaching a maximum of ∼ 61% of endogenous GATA1's occupancy in mock-transduced CD34+ cells at an MOI of 20. As expected, the binding of endogenous GATA1 protein to the δ-globin promoter at this region was reduced to 40% compared with mock-transduced cells (Figure 5C-D). This finding indicates that overexpressed EKLF-GATA1 fusion protein could compete with endogenous GATA1 binding to the δ-globin promoter at certain levels, but was not able to totally displace it under these experimental conditions.
Discussion
In the present study, we found that novel EKLF-GATA1 fusion proteins composed of the DNA-binding domain of GATA1 and the transactivation domain of EKLF can interact with the δ-globin promoter and increase δ-globin expression both in K562 cells and in primary CD34+ cells. These fusion proteins exhibit potential clinical value as a form of genetic therapy for β-globin gene disorders.
The programmed expression of hemoglobin is controlled by the complex interaction between cis-acting sequences and trans-acting factors.31 The transcription factors EKLF and GATA1 are among the trans-acting factors that can bind to the β-globin locus control region (β-LCR) or erythroid cell-specific genes to regulate erythroid differentiation and hemoglobin expression. EKLF binds to the CACCC box in the β-globin proximal promoter and activates β-globin transcription. EKLF can also activate δ-globin promoter activity when the CACCC box is inserted in the corresponding position of the β-globin promoter.18,19 In addition, the EKLF transactivation domain serves as a potent activator when fused to a nonrelated DNA-binding module.13 We sought to determine whether a modified EKLF protein could bind to the endogenous δ-globin promoter and activate δ-globin transcription. Considering that a functional GATA1-binding site is close to the mutated CACCC box in the δ-globin promoter,18,19 we hypothesized that an EKLF protein containing GATA1-binding domains could bind to the GATA1 consensus sequence in the δ-globin promoter and act as a positive regulator of δ-globin expression. We generated various forms of EKLF-GATA1 fusion constructs (long, medium, and short) and characterized how these constructs affected the promoter activity and expression of δ-, γ-, and β-globin. We report for the first time that EKLF-GATA1 fusion proteins can bind to and activate the δ-globin promoter and subsequently induce δ-globin transcription and protein expression in erythroid cells.
With this fusion-protein approach, we can increase HbF levels by 2-fold and HbA2 levels by 3-fold, which would initially be expected to result in an increase from 2% to 4% for HbF levels and from 2%-2.5% to 6%-8% for HAb2 levels in adult blood. This moderate degree of elevation in HbA2 and HbF could have further significance, because under these conditions the cells would survive longer in the peripheral circulation and therefore their number would be amplified.32 Data from the Cooperative Study of Sickle Cell Disease provide convincing evidence that increases in levels of HbF result in partial amelioration of clinical severity and mortality, and that elevations to a level as low as 4% HbF should have clinically significant effects in this disease.33,34 The double effects of EKLF-GATA1 fusion proteins on HbF and HbA2 are speculated to have greater potency in ameliorating the clinical severity.
In addition to their effects on δ-globin expression, EKLF-GATA1 fusion proteins also had an effect on γ- and β-globin expression, although to a lesser extent than that observed for δ-globin. In particular, the long-form EKLF-GATA1 fusion protein exhibited a strong activation of β-globin promoter activity. These findings may be explained by the fact that the long-form EKLF-GATA1 fusion protein, which contains the full-length EKLF and the 145-413 amino acid fragment of GATA1 (Figure 1C) should retain the full function of EKLF and partial features of GATA1. Thus, when EKLF-GATA1 bound to the β-globin promoter in our studies, it may have activated exogenous promoter in a synergistic manner, which is consistent with previous reports.35,36 The expression of hemoglobin is controlled by multiple factors, such as β-LCR, promoters, and transcriptional factors.31 This complex control mechanism may be the reason that we did not observe the same effects with the long-form EKLF-GATA1 fusion protein on endogenous β-globin expression in CD34+ cells that we observed on exogenous β-globin promoter activity in K562 cells. The 2-fold increase in the β-globin promoter activity in K562 cells activated by our EKLF is similar to the observation made by Ristaldi et al,19 but lower than that by Donze et al.30 The difference between our results and those of Donze et al is most likely related to the different constructs used, in particular to the fact that the HS2 enhancer was used in their research, whereas we used SV40 in our constructs.
Using artificially designed transcription factors to regulate the expression of endogenous genes is an emerging field. For example, engineered zinc-finger transcription factors in which the zinc-finger protein is coupled with an activation domain provide a new approach for altering disturbed gene expression to treat numerous diseases.37-39 Graslund et al fused zinc-finger protein to the activation domain of VP64, and found that this fusion protein could bind to the γ-globin promoter and enhance its expression in K562 cells38 ; the EKLF-GATA1 fusion protein containing GATA1 zinc-finger (DNA-binding) domains and an EKLF transactivation domain increased both δ- and γ-globin expression in K562 cells, as well as in the more clinically relevant CD34+ cells. Other researchers have also designed different modified EKLF proteins that can repress β-globin or activate δ-globin expression in erythroid cells and/or transgenic mice.12,40 Repression of impaired β-globin expression alone as a treatment for β-hemoglobinopathies is not a practical approach, because it can result in insufficient hemoglobin levels. Donze et al reported that their fusion protein containing a GAL4 DNA-binding domain and EKLF (EKLF-Gal4 [1-147]) increased δ-globin mRNA expression 3-fold, but a caveat is that this effect was based on an artificial expression system in which a GAL4-binding site was created in front of the δ-promoter.12 In our system, the increase of δ-globin is accomplished through the exogenous expression of the EKLF-GATA1 fusion proteins and does not rely on any alteration of the targeted human genome structures. Therefore, our strategy of using artificial EKLF-GATA1 fusion proteins to bind to the endogenous defective δ-globin promoter and stimulate its expression may be more viable in future clinical applications for the treatment of β-globin disorders.
It has been reported that a mutation in the GATA1-binding motif in the δ-globin proximal promoter results in δ-thalassemia,18 and that disruption of this GATA1 consensus sequence significantly reduces δ-globin promoter activity.19 Our results showed that EKLF-GATA1 fusion proteins interacted with this GATA1-binding motif to activate δ-globin expression, further supporting the importance of GATA1 in δ-globin expression and remaining consistent with the notion that the transactivation domain of EKLF is a strong positive regulator when binding to the target gene.30 Several GATA1-binding sites exist in the β-LCR and upstream of the δ-globin gene, suggesting that the EKLF-GATA1 fusion proteins could interact with these regions and the δ-globin promoter and strongly activate δ-globin expression. It is plausible to speculate that transduction of stem cells with the EKLF-GATA1 fusion proteins to activate endogenous δ-globin may have a better efficacy in the management of β-globin disorders than the current gene-therapeutic approach, which involves hematopoietic stem cell target-gene transfer by transplantation with autologous progenitors that have been genetically modified with an antisickling globin gene to replace defective β-globin.41,42 Moreover, our results suggest that EKLF-GATA1 augments δ- and γ-globin gene expression, which could then lead to increased HbA2 and HbF protein levels. These increases in HbA2 and HbF would lead theoretically to the intracellular increase in 2 β-like globin chains with equivalent efficacy in inhibiting HbS polymerization in SCD, or improving the β-(like) to non-β chain ratio in the β-thalassemias.
In summary, we present 2 functional EKLF-GATA1 fusion proteins containing the GATA1 primary binding domain that could bind to and activate δ-globin promoter and significantly increase δ-globin expression in K562 cells and CD34+ bone marrow cells. Although the long-form EKLF-GATA1 fusion protein also increased β-globin expression in CD34+ cells, its major effects were on δ- and γ-globin induction; the medium-form EKLF-GATA1 elevated δ- and γ-globin expression without an effect on β-globin expression. Thus, the medium form may have more applicability as a treatment approach for SCD, for which the beneficial effects of increasing HbA2 and HbF might be offset by a corresponding increase in HbS that may accompany the use of the long form. In contrast, the medium and long forms may be suitable for application to β-thalassemia, particularly β-thalassemia intermedia, and a simultaneous induction of γ-, δ- and β-globin would be expected to greatly improve the non–α-globin/α-globin chain ration. Induction of both δ- and γ-globin expression may be beneficial for an antisickling effect and may compensate for impaired β-globin production. These EKLF-GATA1 fusion proteins could prove useful as a genetic therapeutic tool for SCD and β-thalassemia, and warrant further preclinical evaluation. As a next step in considering the feasibility of this genetic therapeutic approach, we are designing a series of in vitro and in vivo experiments to evaluate the possible off-target effects of the EKLF-GATA1 fusion protein constructs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr James Bieker for kindly providing the pSG5-EKLF construct and Dr Ning Fu for kindly providing the antibody against δ-globin.
This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: J.Z. designed and performed research, analyzed data, and wrote the manuscript; K.C. designed and performed research and analyzed data; W.A. performed research; C.T. contributed to the GATA1 construct; P.G. contributed to construct design; and G.P.R. conceived and participated in the design of the study and contributed to the evaluation of the results and to the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Griffin P. Rodgers, MD, Bldg 10, Rm 9N119, Molecular and Clinical Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, 10 Center Dr, Bethesda, MD 20892; e-mail: gr5n@nih.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal