Abstract
In the 2005-01 trial, we have demonstrated that bortezomib-dexamethasone as induction therapy before autologous stem cell transplantation was superior to vincristine-adriamycin-dexamethasone. We conducted a post-hoc analysis to assess the prognostic impact of initial characteristics as well as response to therapy in patients enrolled in this study. Multivariate analysis showed that ISS stages 2 and 3 and achievement of response less than very good partial response (VGPR) both after induction therapy and after autologous stem cell transplantation were adverse prognostic factors for progression-free survival, the most important one being achievement of response less than VGPR after induction. Progression-free survival was significantly improved with bortezomib-dexamethasone induction therapy in patients with poor-risk cytogenetics and ISS stages 2 and 3 compared with vincristine-adriamycin-dexamethasone. In these 2 groups of patients, achievement of at least VGPR after induction was of major importance. This study is registered with EudraCT (https://eudract.ema.europa.eu; EUDRACT 2005-000537-38) and http://clinicaltrials.gov (NCT00200681).
Introduction
High-dose therapy with autologous stem cell transplantation (HDT-ASCT) is the standard of care for previously untreated multiple myeloma (MM) patients younger than 65 years of age.1 Extensive evidence from studies in the transplant setting supports the relationship between achievement of complete response (CR) or very good partial response (VGPR) after transplant with substantially prolonged progression-free survival (PFS) and overall survival in previously untreated MM patients.2-10 However, with conventional induction regimens the prognostic impact of achieving CR or at least VGPR before ASCT remained controversial, mainly because this situation did not occur frequently enough.11-14 Nevertheless, the type of response achieved with novel agents as induction before ASCT might have an important prognostic impact.15
Combinations that use novel agents, including bortezomib-based therapy, are currently evaluated as induction treatment before ASCT, with the objective of increasing the CR or CR plus VGPR rate both before and after ASCT.16-18 In this setting, the recent phase 3 study by the Intergroupe Francophone du Myélome (IFM2005-01; NCT00200681) demonstrated the superiority of bortezomib-dexamethasone induction before HDT-ASCT compared with vincristine-adriamycin-dexamethasone (VAD), the previous standard of care. In this trial, bortezomib-dexamethasone resulted in greater postinduction and posttransplant rates of VGPR or greater and a trend toward improved PFS.19 The purposes of this post-hoc analysis were to assess the prognostic impact of initial characteristics as well as response to therapy in patients enrolled in the IFM 2005-01 study.
Methods
Patients and study design
IFM 2005-01 study details have been reported previously.19 In brief, 482 patients aged 65 years or younger with untreated symptomatic MM were randomized to VAD induction (n = 242), either without (arm A1) or with dexamethasone, cyclophosphamide, etoposide, and cis-platinum consolidation (A2), or bortezomib-dexamethasone induction (n = 240), without (B1) or with dexamethasone, cyclophosphamide, etoposide, and cis-platinum consolidation (B2). Patients were stratified by β2-microglobulin level and chromosome 13 abnormality by fluorescence in situ hybridization analysis. The study was approved by the University of Nantes Institutional Review Board and by the National Health authorities.
Patients achieving less than VGPR after the first transplant could receive a second transplant. A second transplant was not conducted in patients who achieved a VGPR or greater. Patients achieving a partial response or greater after transplant could be included in protocol 2005-02 consisting of 2 months' lenalidomide consolidation followed by lenalidomide maintenance or placebo. There was no difference between arm A (VAD induction) and arm B (bortezomib-dexamethasone induction) regarding post-ASCT type of treatment.
The primary end point was postinduction CR/nCR rate. Response was evaluated by the use of modified European Group for Blood and Marrow Transplantation criteria, including additional categories of near-CR and VGPR.20-22
We conducted post-hoc univariate and multivariate analyses in the intent-to-treat population to evaluate the impact on PFS (time from treatment start to progression/relapse or death) of the following prognostic factors: β2-microglobulin (≤ 3 mg/L vs > 3 mg/L), presence of t(4;14) and/or del(17p) versus absence, arm A1 + A2 versus B1+B2, hemoglobin (< 10 g/dL vs ≥ 10 g/dL), response to induction therapy (< VGPR vs ≥ VGPR), and best response to therapy including ASCT, International Staging System (ISS) disease stage. PFS comparisons between subgroups were performed by the use of the log-rank test; distributions were estimated by the use of Kaplan-Meier methodology. Factors associated with PFS were determined with Cox multivariate regression analyses.
Results and discussion
Patients and overall outcomes
The primary results from this study have been reported elsewhere.19 The conclusion was that bortezomib-dexamethasone significantly improved postinduction and posttransplantation response rates compared with VAD and resulted in longer PFS. Overall rates of VGPR or greater in the bortezomib-dexamethasone and VAD groups were 38% versus 15% after induction therapy, respectively (P < .0001) and 54% vs 37% after the first ASCT, respectively (P < .001); after a median follow-up of 32 months, median PFS was 36 months versus 29.7 months (P = .064).
Prognostic factors for PFS
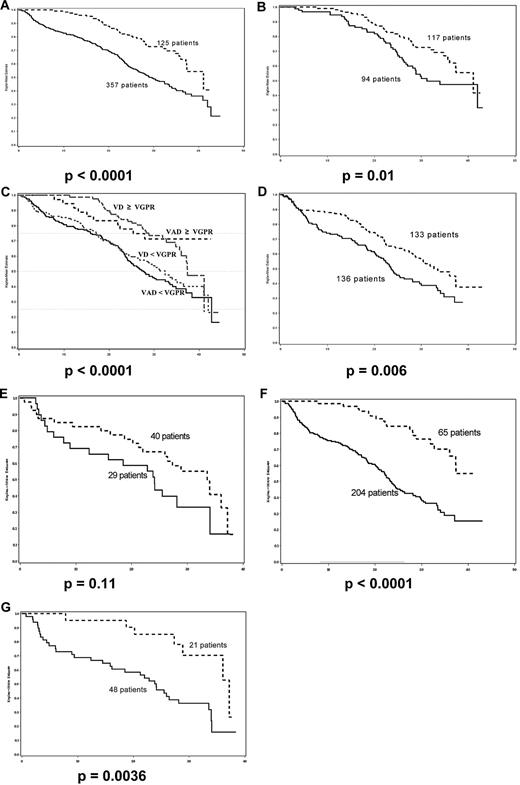
Univariate analysis performed on the whole group of 482 patients identified several prognostic factors that were associated with a significant negative impact on PFS (Table 1), including β2-microglobulin level > 3 mg/L (P < .0001), the presence of t(4;14) and/or del17p (P = .03), ISS stages 2 and 3 (P < .0001), achievement of less than a VGPR after induction (Figure 1A, P < .0001), and best response to therapy including ASCT < VGPR (P = .009).
Analysis of factors associated with PFS in the intent-to-treat population (N = 482)
| Variable . | Total patients evaluable . | Median PFS, mo (range) . | Relative risk (95% CI) . | P . |
|---|---|---|---|---|
| Univariate analysis | ||||
| Hemoglobin, < 10 g/dL | 480 | 30.9 (25.5-not reached) | 1.2 (0.9-1.5) | .2088 |
| t(4;14) and/or del(17p) | 482 | 26.4 (20.2-36.0) | 1.5 (1.0-2.2) | .0313 |
| Best response to therapy less than VGPR | 482 | 27.9 (23.6-33.2) | 1.7 (1.1-2.6) | .009 |
| β 2-microglobulin > 3 mg/L | 482 | 29.7 (24.4-33.5) | 1.7 (1.3-2.2) | .0001 |
| ISS stage 1 vs 2 and 3 | 468 | 28.8 (24.4-33.2) | 1.8 (1.4-2.4) | <.0001 |
| Response induction less than VGPR | 482 | 29.0 (26.4-33.5) | 2.2 (1.5-3.0) | <.0001 |
| Multivariate analysis | ||||
| t(4;14) and/or del(17p) | 1.5 (1.0-2.1) | .0621 | ||
| ISS stage 1 vs 2 and 3 | 1.8 (1.4-2.4) | <.0001 | ||
| Best response to therapy less than VGPR | 2.0 (1.5-2.7) | <.0001 | ||
| Response to induction less than VGPR | 2.3 (1.6-3.2) | <.0001 |
| Variable . | Total patients evaluable . | Median PFS, mo (range) . | Relative risk (95% CI) . | P . |
|---|---|---|---|---|
| Univariate analysis | ||||
| Hemoglobin, < 10 g/dL | 480 | 30.9 (25.5-not reached) | 1.2 (0.9-1.5) | .2088 |
| t(4;14) and/or del(17p) | 482 | 26.4 (20.2-36.0) | 1.5 (1.0-2.2) | .0313 |
| Best response to therapy less than VGPR | 482 | 27.9 (23.6-33.2) | 1.7 (1.1-2.6) | .009 |
| β 2-microglobulin > 3 mg/L | 482 | 29.7 (24.4-33.5) | 1.7 (1.3-2.2) | .0001 |
| ISS stage 1 vs 2 and 3 | 468 | 28.8 (24.4-33.2) | 1.8 (1.4-2.4) | <.0001 |
| Response induction less than VGPR | 482 | 29.0 (26.4-33.5) | 2.2 (1.5-3.0) | <.0001 |
| Multivariate analysis | ||||
| t(4;14) and/or del(17p) | 1.5 (1.0-2.1) | .0621 | ||
| ISS stage 1 vs 2 and 3 | 1.8 (1.4-2.4) | <.0001 | ||
| Best response to therapy less than VGPR | 2.0 (1.5-2.7) | <.0001 | ||
| Response to induction less than VGPR | 2.3 (1.6-3.2) | <.0001 |
ISS indicates International Staging System; PFS, progression-free survival; and VGPR, very good partial response.
Progression-free survival. (A) Achievement of VGPR after induction therapy versus no therapy; (B) achievement of VGPR after induction versus after high-dose therapy; (C) achievement of VGPR after induction in VAD and bortezomib-dexamethasone arms versus no induction; (D) ISS stages 2 and 3, bortezomib-dexamethasone induction versus VAD; (E) poor-risk cytogenetics, bortezomib-dexamethasone induction versus VAD; (F) achievement of VGPR after induction in ISS stages 2 and 3 versus no induction; (G) achievement of VGPR after induction in poor-risk cytogenetics versus no induction. (A) P < .0001; (B) P = .01; (C) P < .0001; (D) P = .006; (E) P = .11; (F) P < .0001; (G) P = .0036.
Progression-free survival. (A) Achievement of VGPR after induction therapy versus no therapy; (B) achievement of VGPR after induction versus after high-dose therapy; (C) achievement of VGPR after induction in VAD and bortezomib-dexamethasone arms versus no induction; (D) ISS stages 2 and 3, bortezomib-dexamethasone induction versus VAD; (E) poor-risk cytogenetics, bortezomib-dexamethasone induction versus VAD; (F) achievement of VGPR after induction in ISS stages 2 and 3 versus no induction; (G) achievement of VGPR after induction in poor-risk cytogenetics versus no induction. (A) P < .0001; (B) P = .01; (C) P < .0001; (D) P = .006; (E) P = .11; (F) P < .0001; (G) P = .0036.
Multivariate analysis identified 2 factors that were associated with increased risk of disease progression, ISS stages 2 and 3 (P < .0001), and achievement of response less than VGPR, with only a trend for adverse cytogenetics defined by t(4;14) and/or del17p (P = .06; Table 1). Achievement of less than VGPR was the major prognostic factor both after induction, and after ASCT (P < .0001), but the greater relative-risk was observed after induction (Table 1). When patients achieved VGPR after induction therapy, the median PFS was 41.2 months versus 31.1 months in patients who achieved VGPR only after high-dose therapy and ASCT (Figure 1B, P = .01). A 4-arm Kaplan-Meier estimate that included VAD versus bortezomib-dexamethasone broken down by less than VGPR or not shows remarkably the impact of this level of response on PFS (Figure 1C, P < .001). This clearly indicates that achievement of VGPR or better after induction is prognostic for better PFS.
We have previously shown that bortezomib-dexamethasone was superior to VAD across all prognostic groups.19 In particular, the CR + VGPR rate was dramatically improved with bortezomib-dexamethasone in patients with poor-risk initial characteristics, which translated into significantly improved PFS. For patients with ISS 2 and 3, the CR + VGPR rate was 37.6% in the bortezomib-dexamethasone arm versus 11% in the VAD arm, and the median PFS was 32.7 months versus 23.6 months (P = .006, Figure 1D), and for patients with poor-risk cytogenetics, the CR + VGPR rate was 40% with bortezomib-dexamethasone versus 17% with VAD, and the median PFS was 33.5 months vs 24.1 months, P = .11, Figure 1E). In these 2 groups of patients, achievement of at least VGPR after induction was of major importance: median PFS was 23 months in 204 patients with ISS 2 and 3 who did not achieve VGPR after induction therapy versus not reached in 65 patients who did (P < .0001, Figure 1F); similarly, median PFS was 24 months in 48 patients with poor-risk cytogenetics who did not achieve VGPR after induction therapy versus 37 months in 21 patients who did (P = .0036, Figure 1G). Although the median follow-up of our study is relatively short, it was possible to analyze the impact of induction on PFS because there was no difference between the 2 groups with regards to the type of post-ASCT treatment. These findings are in line with our previous experience with the long-term analysis of the IFM 99-02 and IFM99-04 trials with double ASCT.12 In this analysis we showed that achievement of at least a VGPR had a significant prognostic impact only in patients with ISS 2 and 3 and in patients with poor-risk cytogenetics.
With the introduction of novel agents, it is possible to achieve at least VGPR in significantly more patients and it is now possible to clearly show that achievement of VGPR or greater after induction is a major prognostic factor for improved PFS in previously untreated transplant-eligible MM patients. This might mean that for patients who are already in CR or VGPR after induction, HDT-ASCT represents a consolidation that further upgrades the level of remission. In this regard, bortezomib-dexamethasone is superior to VAD mostly because it increases CR + VGPR rates in patients with initial poor-risk characteristics, particularly those with ISS stages 2 and 3 and those with t(4;14) and/or del(17p). This level of response after induction, which is a major prognostic factor, is a key objective, and the choice of the best induction therapy is of great importance. In the era of novel agent, bortezomib-dexamethasone can be considered as the backbone of initial therapy. Recent results indicate that the use of triple drug combination, including the addition of an immunomodulatory drug such as thalidomide (VTD, ie, Velcade [Millenium Pharmaceuticals], thalidomide, and dexamethasone)16,17 or lenalidomide (RVD, ie, Revlimid [Celgene, Summit, NJ])23 or an alkylator such as cyclophosphamide (VCD, ie, bortezomib, cyclophosphamide, and dexamethasone)24 might further increase the response rate, translating into improved outcome. The authors of 3 randomized studies16,17,25 have already shown that VTD is superior to thalidomide-dexamethasone or bortezomib-dexamethasone in terms of CR or CR + VGPR, time to progression, and PFS and might therefore be considered as a new standard induction therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Maëlle Ningre (project manager) and Tanguy Roman (data manager), from the Center Hospitalier Universitaire de Nantes, and the IFM investigators (see supplemental Appendix).
This work was supported by Millennium Pharmaceuticals Inc.
Authorship
Contribution: P.M., M.A., H.A.-L., J.-L.H. designed the trial; all authors performed the research; P.M., LP, H.A.L., J.-L.H. analyzed data; P.M. and J.-L.H. wrote the paper; and all authors approved the manuscript.
Conflict-of-interest disclosure: P.M., M.A., C.H., T.F., H.A.-L., and J,-L.H. received honoraria from Janssen and Celgene; D.E. is employed by Millennium Pharmaceuticals, Cambridge, NJ; all other authors declare no competing financial interests.
For a complete list of IFM 2005-01 study investigators, please see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Professor Jean-Luc Harousseau, MD, Centre René Gauducheau, Bd Jacques Monod, 44805 Nantes/St Herblain, France; E-mail: jl-harousseau@nantes.fnclcc.fr.