Case presentation
A 47-year-old man presented to his primary care physician with increasing fatigue and spontaneous bruising. Laboratory evaluation revealed a white blood cell count (WBC) of 80 000/mm3, hematocrit of 25.6%, platelet count of 9000/mm3, and lactic dehydrogenase of 1075 U/L (reference range 110-210 U/L). He was immediately transferred to a local hospital. The peripheral blood smear showed predominantly myeloblasts with high nuclear:cytoplasm ratio, fine chromatin, prominent nucleoli, and numerous cells with an Auer rod. The platelets were significantly decreased in number and no nucleated red blood cells were identified. The bone marrow aspirate and biopsy revealed sheets of large blasts with fine chromatin and numerous Auer rods without normal hematopoiesis (Figure 1A). Immunophenotypic analysis of the blast population showed expression of CD13, CD19, CD33, CD34, CD117, and HLA-DR with partial expression of CD11b and CD33. The cytogenetic analysis demonstrated t(8;21)(q22;q22) in 20 of 20 metaphases examined. Molecular studies did not identify a FLT3 or NPM1 mutation. These findings were consistent with a diagnosis of core binding factor acute myeloid leukemia (CBF AML).
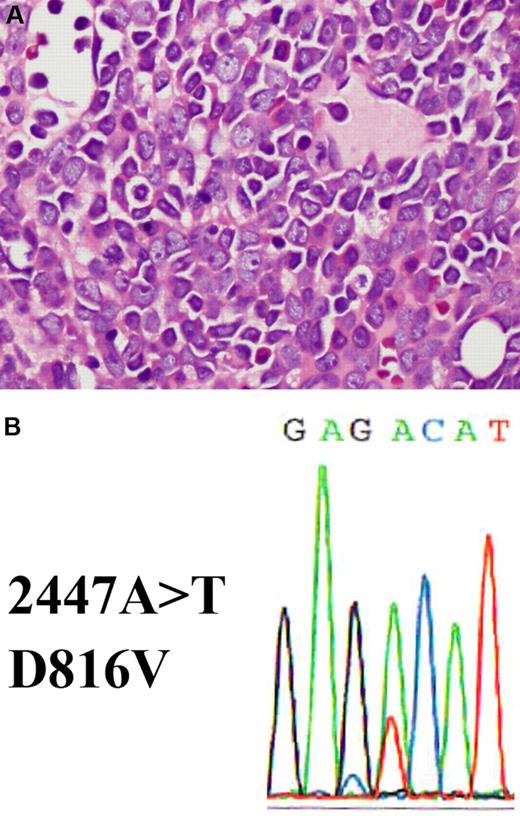
Bone marrow section showing acute myeloid leukemia with a c-KIT D816V mutation. (A) The hematoxylin and eosin–stained bone marrow biopsy shows replacement by blasts with prominent nucleoli and eosinophilic cytoplasm (shown at ×400 magnification). (B) c-KIT D816V mutation detected in bone marrow. DNA was extracted from bone marrow and exon 17 of c-KIT was amplified by polymerase chain reaction (PCR). The PCR product was purified and analyzed by standard direct DNA sequencing procedures using an Applied Biosystems 3730 DNA Genetic Analyzer. A missense A>T mutation is detected at position 2447, which results in a D816V amino acid change.
Bone marrow section showing acute myeloid leukemia with a c-KIT D816V mutation. (A) The hematoxylin and eosin–stained bone marrow biopsy shows replacement by blasts with prominent nucleoli and eosinophilic cytoplasm (shown at ×400 magnification). (B) c-KIT D816V mutation detected in bone marrow. DNA was extracted from bone marrow and exon 17 of c-KIT was amplified by polymerase chain reaction (PCR). The PCR product was purified and analyzed by standard direct DNA sequencing procedures using an Applied Biosystems 3730 DNA Genetic Analyzer. A missense A>T mutation is detected at position 2447, which results in a D816V amino acid change.
The patient received induction chemotherapy consisting of daunorubicin 90 mg/m2 for 3 days and cytarabine at 200 mg/m2 by continuous infusion for 7 days. A bone marrow aspirate and biopsy at day 14 after the beginning of induction revealed 10% cellularity with 7% residual blasts. On day 16 he subsequently received reinduction chemotherapy consisting of daunorubicin 45 mg/m2 for 3 days and cytarabine 100 mg/m2 by continuous infusion for 7 days. A repeat bone marrow aspirate and biopsy at day 14 after the reinduction chemotherapy showed a hypocellular bone marrow with no blast cells. He received granulocyte colony-stimulating factor (G-CSF) while in the hospital with reconstitution of the WBC, but the platelet count remained decreased. At the time of discharge, his laboratory evaluation showed a WBC of 4000/mm3, hematocrit of 26%, and platelet count of 14 000/mm3.
Following discharge from the hospital, he continued to require weekly red blood cell and platelet transfusions and remained pancytopenic. A bone marrow aspirate and biopsy were performed at 2 months following the reinduction chemotherapy, which revealed a mildly hypercellular bone marrow (60% cellularity) with trilineage hematopoiesis and no evidence of leukemia. Megakaryocytes were decreased in number and normal in morphology and no dysplastic erythroid cells were identified. A repeat bone marrow aspirate and biopsy 1 month later revealed the same finding with no evidence of residual leukemia. Fluorescence in situ hybridization (FISH) analysis did not reveal the RUNX1/RUNX1T1 (formerly known as AML1/ETO) rearrangement.
He now presents to our institution for a second opinion regarding postremission treatment options. His medical history is notable only for hypertension and a previous surgical procedure to repair an ankle fracture. His medications include valsartan and metoprolol. He has no known drug allergies. He denies any smoking history or known toxin exposure. He is married and lives with his wife and 3 children, all of whom are in good health. There is no family history of malignancy.
The patient appeared well. The physical examination was completely normal. Laboratory test results revealed a WBC of 3000/mm3 with an absolute neutrophil count of 900/mm3, hematocrit of 34.6%, and platelet count of 37 000/mm3. Specimens from all the previous bone marrow aspirates and biopsies were reviewed and a management decision was made after additional test results were obtained.
Discussion
This patient with AML and the t(8;21), one of the CBF AMLs, was in apparent first complete remission (CR) (although the persistent cytopenias were unexplained) and sought advice regarding the best postremission strategy. The patient was given induction elsewhere initially with a cytarabine dose of 200 mg/m2. A randomized trial has demonstrated that doses of cytarabine of 100 mg/m2 and 200 mg/m2 when combined with daunorubicin lead to equivalent CR rates. Any potential benefit for the higher dose remains to be proven in a clinical trial. The dose of daunorubicin of 90 mg/m2/d for 3 days was appropriate considering that a large randomized trial in younger patients showed an improved outcome compared with the standard approved dose of 45 mg/m2.1 He required 2 cycles of induction to achieve CR, which is the case in approximately 30% of patients with AML and the t(8;21)(q22;q22), and necessitating 2 cycles does not confer a less favorable prognosis compared with patients who achieve CR with a single cycle.2 It appears that no change in postremission therapy is required.
Historically, patients with CBF AML have been reported to have a relatively favorable outcome when treated with conventional induction and intensive consolidation chemotherapy.3 The standard postremission approach for a patient with a CBF AML is to administer 3-4 cycles of high-dose cytarabine or other intensive chemotherapy. Many, but not all, studies have suggested that leukemia cells from patients with CBF AML are particularly sensitive to high-dose cytarabine and that multiple cycles lead to a higher cure rate than 1. However, no definitive randomized trial testing this hypothesis has been conducted. Furthermore, other studies have demonstrated that the same relatively favorable outcome can be achieved with multiple cycles of intensive chemotherapy other than high-dose cytarabine.
During the past decade, studies have indicated that not all patients with the t(8;21)(q22;22) have the same relatively favorable prognosis. For example, a less favorable outcome has been reported among patients who present with a high WBC, expression of the CD56 antigen,4 extramedullary disease,5 and concomitant trisomy 4.6 Most recently, c-KIT mutations have been found in a variable but relatively high frequency (up to 50%) in patients with CBF AML including both t(8;21) and the other major type of CBF AML inv(16)(p13q22) or t(16;16)(p13;q22) and appear to confer a quite unfavorable prognosis,7,8 particularly those that occur in exon 17. Such patients may warrant more aggressive or alternative therapy.
A search for the c-KIT mutation had not been carried out in this patient. To determine whether this patient had a c-KIT mutation, we retrieved material from his initial bone marrow for molecular studies. Indeed, we discovered his leukemia cells harbored the D816V c-KIT mutation (Figure 1B). It is possible that this mutation accounted for the lack of CR with a single cycle of induction chemotherapy. Whether or not this was the case, the c-KIT mutation was very likely to confer a significantly less favorable prognosis. This patient's persistently low peripheral blood counts remained puzzling. The possibility that this represented occult relapse was foremost in our minds despite a recent normal bone marrow aspirate and biopsy.
Although matched sibling donor allogeneic hematopoietic cell transplantation (HCT) has not been shown to improve the outcome in general among patients with the t(8;21)(q22;q22),9 such a strategy provides the most potent antileukemia effect compared with either consolidation chemotherapy or autologous HCT. Among young patients (who still have a risk of nonrelapse mortality of at least 20%) with the c-KIT mutation, one can speculate that allogeneic HCT may be the most potentially curative approach. Studies evaluating the benefits of HCT in specific populations of patients with the t(8;21)(q22;q222) or with various prognostic factors are needed.
The presence of the c-KIT mutation is also important because it provides a target for novel tyrosine kinase inhibitor (TKI) therapy.10 Although it is not clear how c-KIT and its mutations precisely contribute to the development of AML, the remarkable example of tyrosine kinase inhibition of BCR-ABL in CML suggests that c-KIT is a rational target to explore therapeutically. Prospective studies testing this hypothesis are under way.
If this patient had the t(8;21)(q22;q22) without the c-KIT mutation, we would have recommended 3-4 cycles of intensive postremission chemotherapy and our preference would have been high-dose cytarabine at 3 g/m2 twice daily on days 1, 3, and 5. An alternative approach would be to give 2 cycles of high-dose cytarabine followed by autologous HCT. However, because the c-KIT mutation was present at diagnosis, we recommended that the patient proceed to HLA-identical sibling allogeneic HCT if feasible because such a suitable donor was identified. If time is needed to make appropriate arrangements, we would recommend that the patient receive 1 cycle of intensive consolidation chemotherapy followed by allogeneic HCT. If such a donor had not been available, we would have proceeded with an HCT from a matched unrelated donor because the outcome in recent studies of HCT using such a graft source appears to approach or be equivalent to that associated with a matched sibling donor. Such a decision would require considerable further discussion. If the presence of the c-KIT mutation had been known at diagnosis, we would have recommended participation in a clinical trial exploring the benefits of TKI therapy such as dasatinib or nilotinib, or a multikinase inhibitor such as sorafenib during induction in combination with chemotherapy to exploit potential synergism if one had been available. This case demonstrates that a search for known specific molecular mutation(s) should be carried out at diagnosis once the chromosome results are known, which will allow early and fully informed planning of the overall treatment strategy.
Authorship
Contribution: M.S.T. and J.H.P. wrote the manuscript; and C.V.H. edited the manuscript and provided the figure.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin S. Tallman, MD, Memorial Sloan Kettering Cancer Center, 1275 York Ave, Box 380, New York, NY 10065; e-mail: Tallmanm@mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal