Abstract
Radioimmunotherapy of indolent non-Hodgkin lymphoma (NHL) has achieved objective response rates in clinical trials comparable with standard rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy, but is relatively underused in routine practice. In this article, we report our clinical experience in 142 consecutive patients who received iodine-131 rituximab radioimmunotherapy for low-grade, predominantly follicular, relapsed NHL. Objective response rates of 67%, with complete response (CR) in 50% and median overall survival of 32 months, matched the response rates in a phase 2 clinical trial of 131I-rituximab radioimmunotherapy and compares favorably with those reported for 131I-tositumomab or 90Y-ibritumomab tiuxetan. Progression-free survival was 18 months overall and 32 months in CR or CR-unconfirmed patients. Our patients comprised 107 (75%) follicular lymphoma, 21 (15%) small lymphocytic lymphoma, 6 (4%) mucosa-associated lymphoid tissue/marginal zone lymphoma, and 8 (6%) mantle-cell lymphoma, with median follow-up of 32 months and 8-year overall survival of 48%. Toxicity was limited to hematologic grade 4 neutropenia, occurring in 10% and thrombocytopenia in 6%. There were no episodes of bleeding or infection requiring hospital admission. Radioimmunotherapy with 131I-rituximab in routine clinical outpatient practice provides cost-effective, safe treatment of relapsed/refractory indolent NHL, with half of patients achieving durable, complete remission with potential for repeat radioimmunotherapy on relapse.
Introduction
Low-grade lymphoma, before the availability of therapeutic monoclonal antibodies (mAbs), had a relentless progressive relapsing course,1 with a median survival for advanced non-Hodgkin lymphoma (NHL) of 8-10 years and median survival after relapse of 4-5 years.2 The advent of rituximab-based immunotherapy combined with chemotherapy, and subsequent maintenance with rituximab, has dramatically improved response rates and survival.3,4
Radioimmunotherapy (RIT) has become an important innovative treatment for relapsed and/or refractory indolent NHL, with many phase 2 studies reporting survival and quality-of-life benefits derived from the use of either the murine-based proprietary products, 90Y-ibritumomab tiuxetan (Zevalin; Spectrum Pharmaceuticals),5 131I-tositumomab (Bexxar; GlaxoSmithKline),6 or radiolabeled chimeric anti-CD20 antibody 131I–rituximab prepared in-house in university hospitals.7
Recent radiolabeled anti-CD20 mAb studies have demonstrated the value of RIT as an initial treatment for follicular lymphoma,8,9 and as consolidation after standard chemotherapy-induction protocols,10 in addition to the safe, effective therapy of relapsed/refractory NHL.7
There is, however, little evidence that RIT has been adopted, outside the context of clinical trials, for routine use in the management of relapsed/refractory indolent or aggressive NHL. Concern has been expressed that RIT may not be available in the future due to poor sales and a lack of promotion by pharmaceutical companies,11 which compounds logistic and regulatory constraints, leading to restricted use in the United States and other countries.11-14
In Australia, although 90Y-ibritumomab tiuxetan (Zevalin) has been approved by the Therapeutic Goods Administration, it is not funded by the Pharmaceutical Benefits Scheme and it is seldom used. However, the rapid, convenient, semiautomated routine preparation of 131I-rituximab in a university hospital nuclear medicine department makes cost-effective RIT readily available to patients in Australia as an outpatient treatment.15 There has been sporadic use of 131I-rituximab in Europe and South Korea for RIT of NHL, but national regulatory issues appear to have prevented routine clinical application.16,17 Nearly all the reported use of RIT is in the context of clinical trials. To have maximum benefit to a disease population, therapy needs to be accessible “off study,” but used in the context of consensus clinical guidelines.18
In a single-center study comparing experience with 90Y-ibritumomab tiuxetan and 131I-tositumomab in routine clinical practice outside the context of clinical trials, RIT was well tolerated, but the response rates were at the low end of those reported in the clinical trial literature with objective response rates (ORRs) of 47% and complete response (CR) of 13%.19 Toxicity was relatively high, with grade 3/4 neutropenia (55%) and thrombocytopenia (56%).19
We evaluated our 10-year, single-center clinical experience with 131I-rituximab for the therapy of relapsed or refractory indolent, mainly follicular NHL, in 142 patients treated on a compassionate patient-usage protocol, including 66 patients reported in a multicenter phase 2 clinical study.7 The prescribed radionuclide therapy activity was based upon prospective individualized dosimetry to the whole body as a surrogate for red marrow,20 which allowed a nonmyeloablative effective treatment without causing significant myelosuppression or other toxicity.
Methods
Patients
All 142 patients had relapsed lymphoma and comprised follicular (107 patients), mantle (8 patients), mucosa-associated lymphoid tissue (MALT; 6 patients), and small lymphocytic lymphoma (21 patients).
A pilot study of 10 patients had demonstrated efficacy and safety of 131I-rituximab RIT, and 132 consecutive patients were subsequently treated. The first 66 patients were enrolled at Fremantle Hospital as part of a multicenter phase 2 study and were included in this report to provide information regarding long-term follow-up of 131I-rituximab RIT.7 After completion of this study in 2005, a further 76 consecutive referred patients with relapsed indolent NHL were treated on the same protocol on a routine clinical basis, predominantly as outpatients,15 and followed up by their referring hematologist/oncologist.
Institutional human research ethics committee approval in accordance with Australian National Health and Medical Research Council guidelines and the Declaration of Helsinki, and written informed consent from each patient, was obtained. All patients were referred by a hematologist/oncologist and reviewed by the nuclear medicine physician to determine suitability for treatment. Treated patients were required to have had histologically confirmed disease within 1 year before treatment, have been aged more than 18 years, have had a World Health Organization performance status of less than 3, and a life expectancy of more than 3 months. Patients admitted to the phase 2 study were required to have relapsed after prior chemotherapy. Patients who had received previous rituximab (MabThera; Roche Products Pty Ltd) were eligible if more than 6 months had elapsed from prior treatment. Exclusion criteria included neutrophils less than 1.5 × 109/L or platelets less than 100 × 109/L, significant impairment of cardiac, renal, or hepatic function, or the administration of chemotherapy or radiotherapy within 6 weeks. The percentage of bone marrow involvement was not, in itself, an exclusion criterion. Patients with follicular lymphoma were evaluated further according to the Follicular Lymphoma International Prognostic Index (FLIPI) and were stratified into low-, intermediate-, and high-risk groups. After completion of the phase 2 study, patients were treated routinely in clinical practice on the same protocol in accordance with compassionate patient-usage provisions of the Special Access Scheme Therapeutic Goods Administration.
Such patients were required to have relapsed after prior treatments with chemotherapy with or without rituximab or external beam radiotherapy.
Repeat 131I-rituximab therapy was offered in the event of relapse and was, in fact, given to 16 patients.
Dosimetry and radioimmunotherapy
Rituximab was radiolabeled at Fremantle Hospital with 131I-sodium iodide. (Australian Radioisotopes and Industrials) using a chloramine T method as described previously.20
Radiolabeling yield was more than 98%, and the immunoreactive fraction of 131I-labeled anti-CD20 antibody was more than 80%.20 A tracer activity of 200MBq 131I-rituximab was given intravenously after administration of a standard dose of 375 mg/m2 rituximab unlabeled antibody. Within 1 hour, whole-body gamma imaging and background scans were performed and were repeated at 4 and 7 days under the same quantitative imaging conditions. The residence time of 131I-rituximab was calculated from whole-body gamma-camera counts at these time points.20 The radioimmunotherapeutic activity was administered 7 days later after a prior 375 mg/m2 loading dose of unlabeled rituximab. The administered activity was calculated to deliver a whole-body radiation-absorbed dose of 0.75 Gy. This prescribed radiation dose was based on the 131I-tositumomab dose-escalation studies of Kaminski et al6 and validated in pharmacokinetic and dosimetric studies of 131I-rituximab, which demonstrated red marrow dose < 2 Gy.21 To minimize the risk of hypothyroidism from free radioiodine, administration of Lugol iodine was commenced 24 hours before the tracer dosimetry study and continued daily, until 7 days after 131I-radioimmunotherapy in the first 35 patients and for the following 14 days in all subsequent patients, based on evidence of thyroid uptake on posttreatment imaging in the initial cohort. Patients received the standard 4-dose once-weekly regimen of 375 mg/m2 rituximab, with the tracer dose of 131I-rituximab given on week 1 and the therapy dose on week 2. Two further doses of nonradiolabeled “cold” rituximab were administered on weeks 3 and 4.
Patients retreated with 131I-rituximab RIT after relapse received only 2 doses of “cold” rituximab 375 mg/m2 along with the tracer and therapy doses of 131I-rituximab on weeks 1 and 2.
Administration and radiation safety
Clinical trial patients were treated in hospital, but subsequent compassionate-usage patients were treated as outpatients.15
Response and toxicity evaluation
Disease response after RIT was evaluated by (18F) fluorodeoxyglucose positron emission tomography (PET) scans or serial computed tomography (CT) scans of the chest, abdomen, and pelvis at 3 months after treatment.
Bone marrow biopsies, if bone marrow was involved at baseline, were also repeated at 3 months. Additional CT scans were performed at 6 and 12 months only for those on clinical trial and as clinically indicated for routine patient treatments. Response evaluation was in accord with the International Workshop of Standardized Response Criteria for NHL.22
Hematologic assessment with full blood counts was carried out weekly from treatment until count recovery, then at routine follow-up for 2 years.
The National Cancer Institute Common Toxicity Criteria Version 2 was used.23 Hepatic and renal function was assessed weekly for 6 weeks, then every 3 months. Thyroid function was monitored at 3-month intervals for 1 year, then every 6 months. Human antichimeric antibodies were not assayed, but tracer whole-body dosimetric imaging before radioimmunotherapy in each patient was routinely used to monitor biodistribution of 131I-rituximab in every patient.
Statistical analysis
The primary measurement of efficacy was the response rate 3 months after treatment. The Kaplan-Meier product-limit method was used to estimate OS and PFS. χ2 analysis was performed for comparison of response rates among subgroups. The log-rank analysis was used for comparison of survival between groups for OS and PFS. Multivariate analysis was performed according to the Cox proportional hazard regression model.
Results
All 142 patients (Table 1) received the prescribed whole-body radiation-absorbed dose of 0.75 Gy. No patient offered 131I-rituximab radioimmunotherapy declined treatment, and none was lost to follow-up. Administered therapeutic activities ranged from 1.36 to 5.34 GBq 131I-rituximab (median, 2.4 GBq). All patients were evaluated. Sixteen patients who relapsed received a repeat treatment of 131I-rituximab at standard dose.
Baseline characteristics of 142 consecutive patients receiving 131I-rituximab radioimmunotherapy
| Characteristic . | n . | % . |
|---|---|---|
| Age, y* | ||
| ≥ 60 | 82 | |
| < 60 | 60 | |
| Sex | ||
| Male | 78 | 54.93 |
| Female | 64 | 45.07 |
| Serum LDH | ||
| Normal | 82 | 57.75 |
| High | 48 | 33.80 |
| Unknown | 12 | 8.45 |
| Histology | ||
| Follicular grade | ||
| 1 | 39 | 27.46 |
| 2 | 39 | 27.46 |
| 3 | 16 | 11.27 |
| Mantle | 8 | 5.63 |
| MALT | 6 | 4.23 |
| SLL | 21 | 14.79 |
| Follicular unspecified/low grade | 13 | 9.15 |
| FLIPI | ||
| 0-1 | 37 | 26.06 |
| 2 | 39 | 27.46 |
| ≥ 3 | 29 | 20.42 |
| NA | 36 | 25.35 |
| Unknown | 1 | 0.70 |
| Bone marrow involvement | ||
| Yes | 46 | 32.39 |
| No | 94 | 66.20 |
| Unknown | 2 | 1.41 |
| Prior chemotherapy | ||
| 1 | 87 | 61.27 |
| 2 | 31 | 21.83 |
| ≥ 3 | 24 | 16.90 |
| Last chemo to RIT, mo | ||
| ≤ 6 | 41 | 31.3 |
| > 6 | 90 | 68.7 |
| Prior rituximab | ||
| Yes | 80 | 56.34 |
| No | 60 | 42.25 |
| Unknown | 2 | 1.41 |
| Prior DXRT | ||
| Yes | 42 | 29.58 |
| No | 99 | 69.71 |
| Unknown | 1 | 0.7 |
| Stage at study entry | ||
| I/II | 40 | 28.17 |
| III/IV | 102 | 71.83 |
| Bulky disease (≥ 5 cm) | ||
| Yes | 28 | 19.72 |
| No | 105 | 73.94 |
| Unknown | 9 | 6.34 |
| B2 microglobulin | ||
| Normal | 83 | 58.44 |
| Elevated | 21 | 14.78 |
| Unknown | 38 | 26.76 |
| Characteristic . | n . | % . |
|---|---|---|
| Age, y* | ||
| ≥ 60 | 82 | |
| < 60 | 60 | |
| Sex | ||
| Male | 78 | 54.93 |
| Female | 64 | 45.07 |
| Serum LDH | ||
| Normal | 82 | 57.75 |
| High | 48 | 33.80 |
| Unknown | 12 | 8.45 |
| Histology | ||
| Follicular grade | ||
| 1 | 39 | 27.46 |
| 2 | 39 | 27.46 |
| 3 | 16 | 11.27 |
| Mantle | 8 | 5.63 |
| MALT | 6 | 4.23 |
| SLL | 21 | 14.79 |
| Follicular unspecified/low grade | 13 | 9.15 |
| FLIPI | ||
| 0-1 | 37 | 26.06 |
| 2 | 39 | 27.46 |
| ≥ 3 | 29 | 20.42 |
| NA | 36 | 25.35 |
| Unknown | 1 | 0.70 |
| Bone marrow involvement | ||
| Yes | 46 | 32.39 |
| No | 94 | 66.20 |
| Unknown | 2 | 1.41 |
| Prior chemotherapy | ||
| 1 | 87 | 61.27 |
| 2 | 31 | 21.83 |
| ≥ 3 | 24 | 16.90 |
| Last chemo to RIT, mo | ||
| ≤ 6 | 41 | 31.3 |
| > 6 | 90 | 68.7 |
| Prior rituximab | ||
| Yes | 80 | 56.34 |
| No | 60 | 42.25 |
| Unknown | 2 | 1.41 |
| Prior DXRT | ||
| Yes | 42 | 29.58 |
| No | 99 | 69.71 |
| Unknown | 1 | 0.7 |
| Stage at study entry | ||
| I/II | 40 | 28.17 |
| III/IV | 102 | 71.83 |
| Bulky disease (≥ 5 cm) | ||
| Yes | 28 | 19.72 |
| No | 105 | 73.94 |
| Unknown | 9 | 6.34 |
| B2 microglobulin | ||
| Normal | 83 | 58.44 |
| Elevated | 21 | 14.78 |
| Unknown | 38 | 26.76 |
LDH indicates lactate dehydrogenase; MALT, mucosa-associated lymphoid tissue; DXRT, external beam radiotherapy; and FLIPI, Follicular Lymphoma International Prognostic Index.
Median age, 61 years (range, 30-85 years).
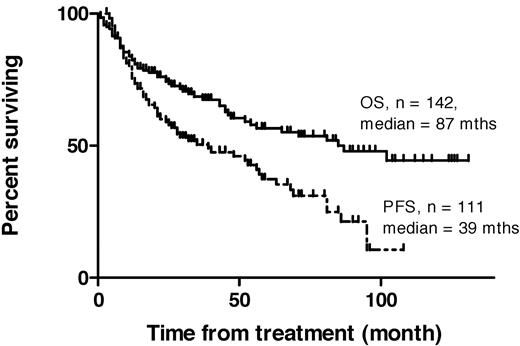
An objective response was observed in 97 patients (ORR, 68%). Seventy-one patients (50%) achieved CR or CRu at the 12-week assessment. The median OS was 87 months (range, 1-131), the median PFS for all responding patients and those with stable disease was 39 months (range, 3-108 months; Figure 1), and for patients in CR/CRu, median PFS was 63 months (range, 3-108 months). For partial responders (PR), median PFS was 13 months (range, 4-95; see Figure 2).
Kaplan-Meier plots of overall survival (OS) and progression-free survival (PFS) in 142 consecutive patients receiving 131I-rituximab radioimmunotherapy for relapsed indolent lymphoma.
Kaplan-Meier plots of overall survival (OS) and progression-free survival (PFS) in 142 consecutive patients receiving 131I-rituximab radioimmunotherapy for relapsed indolent lymphoma.
Kaplan-Meier plots of PFS by response in 142 consecutive patients receiving 131I-rituximab radioimmunotherapy for relapsed indolent lymphoma. CR/CRu indicates complete response; PR, partial response; and NR, no response.
Kaplan-Meier plots of PFS by response in 142 consecutive patients receiving 131I-rituximab radioimmunotherapy for relapsed indolent lymphoma. CR/CRu indicates complete response; PR, partial response; and NR, no response.
The 3-month response was assessed in 80 patients by CT and in 44 patients by PET/CT, along with further scans at 6 and 12 months for those on clinical trial and thereafter as clinically indicated. In those patients treated on the compassionate-usage program, follow-up scans after the 3-month response assessment were only performed as clinically indicated.
In 18 of the patients treated off-study on the compassionate-usage program, no 3-month assessment scan was available, with the response being assessed on clinical examination by the referring hematologist or oncologist. If these 18 patients in whom the 3-month CT or CT/PET scans were not performed are censored, the ORR was 63% and CR/CRu was 43% in the 124 patients evaluated.
Those 66 patients who were originally treated as part of the multicenter phase 2 study7 had a median follow-up time of 66 months (range, 1-118 months), showed a median OS of 71 months (95% CI not reached) and median PFS of 32 months (95% CI, 15-60 months).
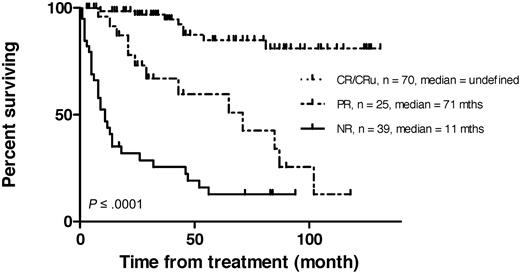
All patients were followed up, median 32 months (range, 1-131 months). Survival of complete responders was significantly longer than that of nonresponders. (P < .001; Figure 3) In a univariate analysis (Table 2), a significantly higher ORR was associated with age < 60 years (P = .004) and low-grade follicular histology (P = .012). Age < 60 years and low FLIPI score also predicted for a high CR/CRu rate. All 6 patients with MALT/marginal zone NHL responded, but only 2 of 8 with mantle-cell disease. Prior rituximab therapy made no statistical difference to ORR, but was associated with a significantly reduced CR/CRu (P = .001) rate.
Kaplan-Meier plots of survival by remission status in 142 consecutive patients receiving 131I-rituximab radioimmunotherapy for relapsed indolent lymphoma. CR/CRu indicates complete response; PR, partial response; and NR, no response.
Kaplan-Meier plots of survival by remission status in 142 consecutive patients receiving 131I-rituximab radioimmunotherapy for relapsed indolent lymphoma. CR/CRu indicates complete response; PR, partial response; and NR, no response.
Analysis of response by characteristic in 142 consecutive patients receiving 131I-rituximab radioimmunotherapy
| Characteristic . | No. of patients . | ORR (68%, n = 97) . | CR + CRu (50%, n = 71) . | ||||
|---|---|---|---|---|---|---|---|
| No. . | % . | P . | No. . | % . | P . | ||
| Age, y | |||||||
| < 60 | 60 | 49 | 81.7 | .004 | 38 | 63.3 | .011 |
| ≥ 60 | 82 | 48 | 58.5 | 33 | 40.2 | ||
| Stage at entry | |||||||
| I or II | 40 | 30 | 75.0 | .322 | 24 | 60.0 | .191 |
| III or IV | 102 | 67 | 65.7 | 47 | 46.1 | ||
| Serum LDH | |||||||
| Normal | 82 | 61 | 74.4 | .164 | 44 | 53.7 | .349 |
| High | 48 | 28 | 58.3 | 20 | 41.7 | ||
| Unknown | 12 | 8 | 66.7 | 7 | 58.3 | ||
| FLIPI | |||||||
| 0-1 | 37 | 29 | 78.4 | .227 | 26 | 70.3 | .021 |
| 2 | 39 | 28 | 71.8 | 19 | 48.7 | ||
| ≥ 3 | 29 | 19 | 65.5 | 13 | 44.8 | ||
| NA | 36 | 20 | 55.6 | 12 | 33.3 | ||
| Unknown | 1 | 1 | 100.0 | 1 | 100.0 | ||
| Histology | |||||||
| Follicular grade 1 | 39 | 31 | 79.5 | .012 | 23 | 59.0 | .105 |
| Follicular grade 2 | 39 | 29 | 74.4 | 24 | 61.5 | ||
| Follicular grade 3 | 16 | 8 | 50.0 | 7 | 43.8 | ||
| Mantle | 8 | 2 | 25.0 | 1 | 12.5 | ||
| MALT | 6 | 6 | 100.0 | 3 | 50.0 | ||
| SLL | 21 | 12 | 57.1 | 7 | 33.3 | ||
| Follicular unspecified/low grade | 13 | 9 | 69.2 | 6 | 46.2 | ||
| Bone marrow involvement | |||||||
| Yes | 46 | 33 | 71.7 | .699 | 24 | 52.2 | .857 |
| No | 94 | 63 | 67.0 | 46 | 48.9 | ||
| Unknown | 2 | 1 | 50.0 | 1 | 50.0 | ||
| Prior chemotherapy | |||||||
| 1 | 87 | 26 | 29.9 | .0161 | 48 | 55.2 | .1451 |
| 2 | 31 | 18 | 58.1 | 14 | 45.2 | ||
| ≥ 3 | 24 | 11 | 45.8 | 8 | 33.3 | ||
| Last chemo to I-131 RIT, mo | |||||||
| ≤ 6 | 41 | 20 | 48.8 | .0095 | 17 | 41.5 | .4510 |
| > 6 | 90 | 66 | 73.3 | 45 | 50.0 | ||
| Prior rituximab | |||||||
| Yes | 80 | 50 | 62.5 | .098 | 30 | 37.5 | .001 |
| No | 60 | 46 | 76.7 | 40 | 66.7 | ||
| Unknown | 2 | 1 | 50.0 | 1 | 50.0 | ||
| Prior DXRT | |||||||
| Yes | 42 | 32 | 76.2 | .240 | 27 | 64.3 | .042 |
| No | 99 | 65 | 65.7 | 44 | 44.4 | ||
| Unknown | 1 | 0 | 0.0 | 0 | 0.0 | ||
| Tumor diameter, cm | |||||||
| > 5 | 28 | 16 | 57.1 | .913 | 10 | 35.7 | .109 |
| ≤ 5 | 105 | 77 | 73.3 | 58 | 55.2 | ||
| Unknown | 9 | 4 | 44.4 | 3 | 33.3 | ||
| B2 microglobulin | |||||||
| Normal | 83 | 57 | 68.7 | .028 | 46 | 55.4 | .002 |
| Elevated | 21 | 9 | 42.9 | 3 | 14.3 | ||
| Unknown | 38 | 29 | 76.3 | 21 | 55.3 | ||
| Characteristic . | No. of patients . | ORR (68%, n = 97) . | CR + CRu (50%, n = 71) . | ||||
|---|---|---|---|---|---|---|---|
| No. . | % . | P . | No. . | % . | P . | ||
| Age, y | |||||||
| < 60 | 60 | 49 | 81.7 | .004 | 38 | 63.3 | .011 |
| ≥ 60 | 82 | 48 | 58.5 | 33 | 40.2 | ||
| Stage at entry | |||||||
| I or II | 40 | 30 | 75.0 | .322 | 24 | 60.0 | .191 |
| III or IV | 102 | 67 | 65.7 | 47 | 46.1 | ||
| Serum LDH | |||||||
| Normal | 82 | 61 | 74.4 | .164 | 44 | 53.7 | .349 |
| High | 48 | 28 | 58.3 | 20 | 41.7 | ||
| Unknown | 12 | 8 | 66.7 | 7 | 58.3 | ||
| FLIPI | |||||||
| 0-1 | 37 | 29 | 78.4 | .227 | 26 | 70.3 | .021 |
| 2 | 39 | 28 | 71.8 | 19 | 48.7 | ||
| ≥ 3 | 29 | 19 | 65.5 | 13 | 44.8 | ||
| NA | 36 | 20 | 55.6 | 12 | 33.3 | ||
| Unknown | 1 | 1 | 100.0 | 1 | 100.0 | ||
| Histology | |||||||
| Follicular grade 1 | 39 | 31 | 79.5 | .012 | 23 | 59.0 | .105 |
| Follicular grade 2 | 39 | 29 | 74.4 | 24 | 61.5 | ||
| Follicular grade 3 | 16 | 8 | 50.0 | 7 | 43.8 | ||
| Mantle | 8 | 2 | 25.0 | 1 | 12.5 | ||
| MALT | 6 | 6 | 100.0 | 3 | 50.0 | ||
| SLL | 21 | 12 | 57.1 | 7 | 33.3 | ||
| Follicular unspecified/low grade | 13 | 9 | 69.2 | 6 | 46.2 | ||
| Bone marrow involvement | |||||||
| Yes | 46 | 33 | 71.7 | .699 | 24 | 52.2 | .857 |
| No | 94 | 63 | 67.0 | 46 | 48.9 | ||
| Unknown | 2 | 1 | 50.0 | 1 | 50.0 | ||
| Prior chemotherapy | |||||||
| 1 | 87 | 26 | 29.9 | .0161 | 48 | 55.2 | .1451 |
| 2 | 31 | 18 | 58.1 | 14 | 45.2 | ||
| ≥ 3 | 24 | 11 | 45.8 | 8 | 33.3 | ||
| Last chemo to I-131 RIT, mo | |||||||
| ≤ 6 | 41 | 20 | 48.8 | .0095 | 17 | 41.5 | .4510 |
| > 6 | 90 | 66 | 73.3 | 45 | 50.0 | ||
| Prior rituximab | |||||||
| Yes | 80 | 50 | 62.5 | .098 | 30 | 37.5 | .001 |
| No | 60 | 46 | 76.7 | 40 | 66.7 | ||
| Unknown | 2 | 1 | 50.0 | 1 | 50.0 | ||
| Prior DXRT | |||||||
| Yes | 42 | 32 | 76.2 | .240 | 27 | 64.3 | .042 |
| No | 99 | 65 | 65.7 | 44 | 44.4 | ||
| Unknown | 1 | 0 | 0.0 | 0 | 0.0 | ||
| Tumor diameter, cm | |||||||
| > 5 | 28 | 16 | 57.1 | .913 | 10 | 35.7 | .109 |
| ≤ 5 | 105 | 77 | 73.3 | 58 | 55.2 | ||
| Unknown | 9 | 4 | 44.4 | 3 | 33.3 | ||
| B2 microglobulin | |||||||
| Normal | 83 | 57 | 68.7 | .028 | 46 | 55.4 | .002 |
| Elevated | 21 | 9 | 42.9 | 3 | 14.3 | ||
| Unknown | 38 | 29 | 76.3 | 21 | 55.3 | ||
FLIPI indicates Follicular Lymphoma International Prognostic Index; MALT, mucosa-associated lymphoid tissue; ORR, overall response rate; CR, complete response; CRu, unconfirmed complete response; DXRT, external beam radiotherapy; and LDH, lactate dehydrogenase.
χ2 analysis for comparison of response rates among subgroups.
Two or more prior chemotherapy courses were associated with significantly reduced ORR (P = .0161). The presence of marrow involvement or elevated serum levels of lactate dehydrogenase was not associated with a reduction in ORR or CR/CRu. Elevated beta 2 microglobulin levels were associated with reduced ORR (P = .028) and CR/CRu (P = .002). Six months or less of time from last chemotherapy was associated with a significantly reduced ORR, compared with those whose last chemotherapy was given more than 6 months before RIT (P = .0095).
The median OS for the intermediate- and high-risk FLIPI groups was 87 and 81 months, respectively, but was not reached for the low-risk group. However, neither OS nor PFS were significantly different between the high-, intermediate-, or low-risk groups.
Forty-six patients (32%) with a median follow-up of 33 months (range, 3-108 months) have maintained remission after 131I-rituximab RIT. Thirty-five of these are in CR/CRu, 7 in PR, and 4 with stable disease.
In a multivariate analysis assessing all the characteristics (Table 3) using the Cox proportional hazard regression model, only 2 factors predicted for relapse. Bone marrow involvement (P = .05; HR, 0.42; 95% CI, 0.18-0.99) predicted for a higher relapse rate, compared with no involvement, and those patients who received chemotherapy more than 6 months before RIT were less likely to relapse (P = .009; HR, 2.95; 95% CI, 1.31-6.64).
Risk of not relapsing: multivariate analysis
| Variable . | HR . | P . | 95% CI . |
|---|---|---|---|
| Age, y | |||
| < 60 | 1.0 | ||
| ≥ 60 | 0.82 | .55 | 0.43-1.57 |
| Female | 1.20 | .60 | 0.61-2.36 |
| Bone marrow involvement | |||
| No | 1.0 | ||
| Yes | 0.42 | .05 | 0.18-0.99 |
| Last chemo, mo | |||
| ≤ 6 | 1.0 | ||
| > 6 | 2.95 | .009 | 1.31-6.64 |
| Variable . | HR . | P . | 95% CI . |
|---|---|---|---|
| Age, y | |||
| < 60 | 1.0 | ||
| ≥ 60 | 0.82 | .55 | 0.43-1.57 |
| Female | 1.20 | .60 | 0.61-2.36 |
| Bone marrow involvement | |||
| No | 1.0 | ||
| Yes | 0.42 | .05 | 0.18-0.99 |
| Last chemo, mo | |||
| ≤ 6 | 1.0 | ||
| > 6 | 2.95 | .009 | 1.31-6.64 |
In a multivariate analysis using a Cox proportional hazard regression model, patients without bone marrow involvement or chemotherapy administered more than 6 months before 131I-rituximab radioimmunotherapy were less likely to relapse.
Toxicity
Toxicity was mainly self-limited myelosuppression (Table 4), with grade 4 neutropenia in 14 (10%) patients and thrombocytopenia in 9 (6%). The median time to platelet nadir was 5 weeks (53 × 109/L; range, 2-591 × 109/L), 7 weeks for neutrophils (0.91 × 109/L; range, 0.03-12.7 × 109/L), and 5 weeks for hemoglobin (117 g/L; range, 63-169).
Summary of hematologic toxicity in 142 consecutive patients treated with 131I-rituximab radioimmunotherapy
| Parameter . | Median nadir . | Range . | Time to nadir, wk . | Grade 4 toxicity, n (%) . |
|---|---|---|---|---|
| Neutrophils | 0.91 × 109/L | 0.03-12.7 × 109/L | 7 | 14 (10) |
| Thrombocytopenia | 53 × 109/L | 2-591 × 109/L | 5 | 9 (6) |
| Hemoglobin | 117 g/L | 63-169 g/L | 5 | 1 (1) |
| Parameter . | Median nadir . | Range . | Time to nadir, wk . | Grade 4 toxicity, n (%) . |
|---|---|---|---|---|
| Neutrophils | 0.91 × 109/L | 0.03-12.7 × 109/L | 7 | 14 (10) |
| Thrombocytopenia | 53 × 109/L | 2-591 × 109/L | 5 | 9 (6) |
| Hemoglobin | 117 g/L | 63-169 g/L | 5 | 1 (1) |
Above data are according to National Cancer Institute Common Toxicity Criteria Version 2.
Three patients required platelet transfusions, 1 patient developed grade 4 anemia and required packed red blood cells, and 1 patient received granulocyte colony-stimulating factor. Oral antibiotics were administered to 2 patients for febrile neutropenia, but no patient was hospitalized with infection or required intravenous antibiotics. No episode of bleeding occurred.
Six patients (4%), with a median age of 63 years (range, 58-82 years), developed a myelodysplastic syndrome (MDS) after a median follow-up of 43 months (range, 8-105 months) after RIT. One patient experienced disease progression to acute myeloid leukemia (AML). All of the MDS patients had received prior chemotherapy (median, 2 prior therapies; range, 1-4 prior therapies); 2 had also received external-beam radiotherapy, and 1 had received 2 prior treatments with 131I-rituximab.
At the time of study entry, an elevated thyroid-stimulating hormone, or pre-existing clinical hypothyroidism requiring thyroxine replacement, was present in 12 patients. At follow-up, an additional 20 patients had an elevated TSH, representing a 15% incidence of treatment-related subclinical or clinical hypothyroidism.
In the first 35 patients who received a 7-day course of Lugol iodine after RIT, 8 of 28 (29%) patients developed subclinical or clinical hypothyroidism. Subsequently, in the remaining 95 eligible patients who received 14 days of Lugol iodine after RIT, 12 of 87 (14%) developed subclinical or clinical hypothyroidism. There was no significant difference in the development of subclinical or clinical hypothyroidism between the 2 groups (P = .0885).
Retreatment
Sixteen patients who relapsed after RIT were given a second course of 131I-rituximab. The first treatments were included in the analysis of the whole 142 patient group, but the repeat treatments have been analyzed separately.
In the retreatment group, the ORR was 94% (15/16), CR/CRu was achieved in 69% (11/16), PR in 25% (4/16), and one patient had stable disease after the initial RIT. The overall median duration of response for the first treatment was 21 months (range, 8-81 months), with a median response duration of 21 months (range, 12-81 months) for CR/CRu and 11 months (range, 8-18 months) for PR. Ten of these 16 retreated patients subsequently achieved a second response (ORR 63%, 7 CR, and 3 PR). In 2 patients, the disease remained stable, in 2 the lymphoma progressed, and in 2 patients the follow-up response at 3 months had yet to be evaluated.
The median response duration of the second response was 22 months (range, 1-40 months), with a median duration for CR/CRu of 21 months (range, 2-26 months) and a median duration for PR of 37 months (range, 12-40 months).
There was no significant difference in the incidence of grade 3/4 hematologic toxicity between the 2 groups. After the first treatment, grade 3/4 neutropenia occurred in 6 patients (37%) and thrombocytopenia in 3 (19%), compared with 2 (12%; P = .22) and 5 (31%; P = .658) patients, respectively, after the repeat treatment.
Discussion
Rituximab anti-CD20 mAb has delivered an increased survival in advanced-stage, low-grade follicular NHL when given with standard chemotherapy regimens, such as rituximab with cyclophosphamide, vincristine, and prednisone3 and rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone,24 and is the current standard of care. However, despite the improvement in survival for follicular lymphoma over the last 20 years, NHL remains incurable, and the majority of patients will relapse and require further treatment with standard or salvage regimens, including stem cell transplantation.25
RIT with an anti-CD20 mAb conjugated to a beta-emitting radioisotope will deliver radiation not only to tumor cells that bind the antibody, but also, due to a cross-fire effect, to neighboring tumor cells inaccessible to the antibody or with insufficient target-antigen expression. Indolent NHL cells are inherently radiosensitive, and rituximab synergistically enhances radiation-induced apoptosis.26 The current status of nonmyeloablative RIT with the commercially approved murine anti-CD20 agents, 131I-tositumomab or 90Y-ibritumomab tiuxetan, is induction of remissions in 50%-80% of patients with either recurrent or refractory indolent lymphomas and 15%-50% can achieve complete remission.13 Unfortunately, these remissions last only 6-15 months.27
Proprietary murine anti-CD20 mAb RIT agents have availability constraints in the United States and other countries, principally due to cost reimbursement and logistic issues,28 and these drugs remain grossly underused by the clinical community.13,14
131I-rituximab has been used in relapsed and refractory NHL in small studies in Germany16 and South Korea17 in small numbers of heavily pretreated patients with a variety of NHL types, reporting ORRs of 30%-50%, which approached that of 131I-tositumomab (47%-68%) in similar patients.27 Five years after we commenced RIT of NHL with 131I-rituximab, Bienert et al16 described their experience in Germany with 131I-rituximab in 9 heavily pretreated patients with B-cell lymphoma. Four patients had received prior high-dose chemotherapy, followed by autologous stem cell transplantation, and 8 had received prior rituximab therapy. Treatment was well tolerated, with an ORR of 40%. Patients with bulky disease were least likely to respond.
In 2006, Kang et al17 used 131I-rituximab in 17 patients with relapsed or refractory B-cell NHL. Fifteen patients were evaluated for response and toxicities. The ORR was 33%. A response occurred in all 4 low-grade lymphomas and in only 1 of the 11 diffuse large B-cell lymphoma patients. Grade 3 and 4 neutropenia, thrombocytopenia, and anemia occurred in 20%, 43.3%, and 13.3%, respectively.
An Australian multicenter phase 2 study of 131I-rituximab RIT in relapsed or refractory indolent lymphoma in 91 patients not responding to chemoimmunotherapy, resulted in a high ORR (76%) and CR/CRu (53%).7 Median duration of response was 10 months for all responders and 20 months for CR/CRu. These results compared favorably with those reported in clinical trials of the Food and Drug Administration–approved murine anti-CD20 mAb preparations, 131I-tositumomab and 90Y-ibritumomab tiuxetan.29,30
There are few published reports of RIT of NHL in routine clinical practice. In a retrospective observational study from the Johns Hopkins Hospital, 38 patients with refractory or relapsed B-cell NHL were treated with 90Y-ibritumomab tiuxetan or 131I-tositumomab.19 The ORR was 47% and CR 13%, with similar 12-week response rates for 90Y-ibritumomab tiuxetan and 131I-tositumomab in this unselected group of patients. This ORR in a clinical setting is lower than that reported in formal phase 2 trials, which have more rigid entry criteria and which probably eliminate treatment of patients with poor performance status. Treatment of sicker patients may also have led to the increased hematologic toxicity reported in this group, with grade 3/4 neutropenia in 55% and thrombocytopenia in 56%.19
Our 142 unselected consecutive patients achieved ORR 67% and CR/CRu 50% with lower rates of myelosuppression in an NHL patient population, of which 66 were included in a phase 2 clinical trial and, after closure of the formal study, 76 who were managed on routine referral basis on a compassionate patient-usage program using the same protocol. In this observational assessment, the CR in those 18 patients treated on a compassionate-usage basis not assessed by PET/CT or CT at 3 months was likely to have been overestimated. However, the response rates were only slightly lower (ORR, 63%; CR/Cru, 43%) when these patients were not included in the 3-month assessments.
Patients on the compassionate-usage program had follow-up scans after the 3-month assessment scans and those on clinical trial after the 12-month scan only as clinically indicated. In the absence of regular scans in either group, the PFS may have been an overestimate.
In our patients, significantly higher ORR was associated with low FLIPI score, age < 60 years, and low-grade follicular histology. Age < 60 years and low FLIPI score also predicted for a high CR/CRu rate. All 6 patients with MALT/marginal zone NHL responded, but only 2 of 8 with mantle-cell disease.
Two or more prior chemotherapy courses were associated with significantly reduced ORR and CR/CRu. The presence of marrow involvement or elevated serum levels of lactate dehydrogenase was not associated with a reduction in ORR or CR/CRu. Elevated beta 2 microglobulin levels were associated with reduced ORR and CR/CRu. Six months or less time from last chemotherapy was associated with a reduced ORR, compared with those whose last chemotherapy was given more than 6 months before RIT. Bulky disease made no difference to the response rates.
Interestingly, there was no significant difference in survival in the low-, intermediate-, and high-risk FLIPI groups
Forty-six patients have maintained remission without relapse after RIT. In a multivariate analysis, the only characteristics that predicted for relapse were bone marrow involvement and chemotherapy administered less than 6 months before the RIT, generally considered poor prognostic features.
There are no distinguishing characteristics relating to nonrelapsing patients.27 There appear to be 15%-20% of patients who have durable remissions lasting many years.
In our patients with a median follow-up of 32 months (range, 1-131 months), 32% (46/142) have maintained remission, although, especially in those treated recently, this is likely to decline.
In our patients, prior rituximab therapy made no statistical difference to ORR, but was associated with a significantly reduced CR/CRu (P = .001) rate.
Predosing with rituximab is thought to increase tumor targeting of the radiolabeled mAb by blocking nonspecific binding sites, such as circulating and splenic B cells. Without predosing, a substantial proportion of the administered radioimmunoconjugate may be sequestered in the spleen.31 After infusion of 375 mg/m2 rituximab, a more favorable biodistribution with selective tumor targeting of 131I-rituximab at sites of nodal disease was seen.31 In vitro studies imply that the sequential use of anti-CD20 mAb may result in the inhibition of binding of the second anti-CD20 mAb,32 but prior rituximab did not adversely affect the response to 131I-rituximab in a phase 2 clinical study,7 compared with rituximab-naïve patients. Whether the pre-RIT rituximab dose affects the response to RIT in vivo has not been conclusively demonstrated, but recent studies suggest an enhanced efficacy.31
Induction therapy with high-dose rituximab administered before 131I-rituximab RIT increased the effective circulating half-life from 45 to more than 90 hours.31 Induction therapy did not appear to compromise the clinical effects of RIT with 131I-rituximab or increase myelotoxicity.31
The dose-limiting toxicity of RIT is transient myelosuppression. 90Y-ibritumomab tiuxetan RIT studies report reversible myelosuppression, with grade 4 neutropenia in 32%, thrombocytopenia in 5%, and anemia in 3% of patients.30 Grade 3 and 4 myelosuppression occurred in 30%-40% of patients with relapsed NHL receiving 131I-tositumomab, with nadirs typically at weeks 4-6. Grade 4 neutropenia occurred in 16%, grade 4 thrombocytopenia in 3%, and grade 4 anemia in 2% of 677 patients previously treated with chemotherapy.29
Prospective individual dosimetry was performed in each of our patients using the maximum tolerated dose paradigm of Kaminski to limit the whole-body radiation-absorbed dose to 0.75 Gy.6 This dose is a function of body surface area and whole-body clearance of 131I-tositumomab and was designed to limit the radiation-absorbed dose in red marrow to 1.5-2 Gy. Our pharmacokinetic studies of tracer activities of 131I-rituximab in patients with NHL confirm that, while the circulation time (mean, 86 hours) is longer than that of 131I-tositumomab (mean, 56 hours), the absorbed radiation dose is comparable.20 In particular, direct measurement of red marrow dose in a cohort of our patients demonstrated that a whole-body radiation-absorbed dose of 0.75 Gy correlated with a radiation-absorbed dose to hemopoietic marrow, which did not exceed the toxicity threshold of 2 Gy.21 Furthermore, the bone marrow clearance of 131I-rituximab appeared to be the same as the whole-body clearance and is likely to reflect that of 131I-tositumomab.21 Our toxicity profile data suggest that direct targeting of 131I-rituximab on marrow tumor cells has relatively less effect on absorbed dose; therefore, we did not withhold treatment with 131I-rituximab from NHL patients with high marrow involvement, nor did we reduce the prescribed 0.75 Gy whole-body radiation absorbed dose in these patients. The range of administered therapeutic activities of 131I-rituximab was wide (1.5-5.2 GBq), with each being predicated upon the prospectively measured prescribed whole-body dose of 0.75 Gy. This large patient variation emphasizes the need for personalized individual dosimetry, in the absence of which patients may be significantly under- or overdosed by up to 20%-30%.20
The reported myelotoxicity of 131I-tositumomab is similar to our experience of 131I-rituximab, in which self-limited grade 4 neutropenia occurred in 16% and grade 4 thrombocytopenia in 4% of patients undergoing RIT. There is little evidence to suggest significant long-term toxicities attributable to RIT.13 Studies with prolonged monitoring have shown that the overall incidence of treatment-related myelodysplasia and AML (tMDS/tAML) after RIT with 131I-tositumomab and 90Y-ibritumomab tiuxetan remains 2.5%-3.5%, with a median follow-up of 5 years,5,6 with an annualized incidence of 0.7%-1.6% in patients who had relapsed after chemotherapy. The incidence is similar to rates of tMDS/tAML expected after chemotherapy alone and is frequently associated with structural alterations of chromosomes 5 and 7, which are recognized as being associated with alkylating agent-induced tMDS or tAML and occurred only in heavily pretreated patients.33 Follow-up of 76 patients treated with 131I-tositumomab as initial treatment for follicular lymphoma without prior chemotherapy has not been associated with any myelodysplasia over a median follow-up of 5.1 years,8 again suggesting that prior chemotherapy, especially with alkylating agents causing DNA damage, may be responsible for the progression to myelodysplasia. However, follow-up is relatively short in the evolution of MDS, and RIT by itself cannot be entirely discounted as a cause of MDS in the absence of prior chemotherapy.
Retreatment in low-grade lymphoma, with either 90Y-ibritumomab tiuxetan or 131I-tositumomab, is not approved due to the potential risk associated with induction of human antimouse antibodies (HAMAs). However, small published studies of repeat treatment showed it to be well tolerated and effective, with ORRs of 77% and 56%, respectively, similar to initial RIT with these murine-based agents. The incidence of grade 3/4 toxicity was not increased by retreatment.34-36 Whole-body gamma imaging of tracer 200-MBq activities of 131I-rituximab performed in all our patients confirmed the expected biodistribution of those radiolabeled mAbs, including those patients in whom previous RIT may theoretically have given rise to human antichimeric antibody. Such evidence of human-mouse immunoreaction was not seen in any of our patients, which contrasts with a significant risk of HAMA formation after RIT with murine-based mAbs, which, however, does not preclude effective retreatment of those patients who did not have HAMA.35,36 Our use of 131I-rituximab chimeric mAb allowed retreatment of indolent lymphoma without potential problems of HAMA and achieved ORR 63%, with 7 of 10 responding patients achieving a CR/CRu having the same or longer duration of response. The median event-free survival, and the severity of myelosuppression, was not significantly different between the initial and repeat treatments. Similar findings are reported in the study of Bishton.34
The median survival of 87 months for relapsed or refractory advanced indolent NHL treated with 131I-rituximab RIT almost doubles the survival, compared with historical pre-RIT era data of 4-year median survival after first relapse.1,5
In those countries, including Australia, where Food and Drug Administration–approved proprietary murine anti-CD20 mAb preparations are unavailable, because of cost or regulatory issues, 131I rituximab prepared in a university hospital nuclear medicine department provides a practical, cost-effective RIT treatment option for the management of lymphoma. The additional expense of radioiodinating rituximab in-house, above the acquisition cost of the drug, is approximately US$10007 and has the additional advantage of avoiding radiolysis and consequent antibody degradation, since the rituximab is radioiodinated immediately before administration to the patient using a standard 20-minute kit procedure.20 In Western Australia, 131I-rituximab has been approved for administration to patients in an ambulatory setting, just as 131I-tositumomab may be given to outpatients in the United States, which avoids the expense and radiation isolation exigencies of inpatient treatment.14
An ongoing study at Fremantle Hospital using 131I-rituximab RIT as first-line treatment for advanced indolent lymphoma in 50 patients showed interim ORR of 98% and CR of 78%,9 which is comparable with the encouraging results of first-line RIT with 131I tositumomab in advanced follicular lymphoma.8
Despite the overwhelming body of evidence that has accumulated regarding the benefits of RIT in respect of disease response and quality of life, RIT remains underused in the United States and other countries.11-14
The reasons for this failure to apply RIT in routine clinical practice appear to be attributable, at least partially, to logistic issues relating to transfer of care from the hematologist/oncologist to the nuclear medicine physician, concerns about inadequate reimbursement for RIT by medical insurance agencies, and perceptions of potential delayed toxicity, such as marrow damage and secondary malignancies, which have not been encountered in practice.12,13,28
In the recommendations of a recent European Consensus Guidelines workshop (Windsor, United Kingdom), RIT was incorporated as an effective treatment of NHL.18 In particular, use of RIT early in the course of follicular lymphoma was recommended to optimize patient outcomes.18 In clinical practice, however, RIT frequently remains a treatment of last resort, when it is least likely to be effective.14
Radioimmunotherapy is a safe, effective treatment of low-grade lymphoma and increases OS while preserving quality of life. Radioiodinated rituximab offers practical, cost-effective radioimmunotherapy for routine clinical applications where regulatory or cost constraints limit the availability of proprietary radiolabeled murine anti-CD20 mAbs, and it also has the potential for safe, effective repeated radioimmunotherapy upon relapse.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Anna Chiam for collation of data, Suet Mei Yu for statistical analysis, and Professor Max Bulsara, Department of Biostatistics, University of Notre Dame, Fremantle, Australia, for performing the multivariate analysis.
The Western Australia Cancer and Palliative Care Network provided funding for data management.
Authorship
Contribution: J.H.T. treated the patients; M.F.L. correlated the data; and M.F.L. and J.H.T. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael Leahy, Department of Hematology, The University of Western Australia, Fremantle Hospital, Fremantle 6160, Australia; e-mail: Michael.Leahy@health.wa.gov.au.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal