Abstract
Macrophages contribute to tumor growth through the secretion of the proangiogenic molecule vascular endothelial growth factor (VEGF). We previously observed that monocytes treated with the cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) produce a soluble form of the VEGF receptor-1 (sVEGFR-1), which neutralizes VEGF biologic activity. The VEGF and VEGFR-1 promoters both contain a hypoxia regulatory element, which binds the hypoxia-inducible factor (HIF) transcription factors under hypoxic conditions. Based on this observation, we examined VEGF and sVEGFR-1 production from monocytes cultured at various O2 concentrations. The amount of sVEGFR-1 production observed from GM-CSF-treated monocytes increased with decreasing levels of O2. This sVEGFR-1 was biologically active and sequestered VEGF. To evaluate the role of the HIFs in sVEGFR-1 production, we used macrophages with a genetic deletion of HIF-1α. HIF-1α−/− macrophages cultured with GM-CSF at hypoxia secreted diminished amounts of VEGF compared with HIF-1α+/+ macrophages, whereas sVEGFR-1 secretion was unaffected. In contrast, siRNA-mediated knockdown of HIF-2α inhibited the production of sVEGFR-1 in response to GM-CSF and low O2, whereas VEGF production was unaffected. These studies suggest that hypoxia, generally thought to promote angiogenesis, can induce antiangiogenic behavior from macrophages within a GM-CSF–rich environment. Furthermore, these results suggest specific and independent roles for HIF-1α and HIF-2α in hypoxic macrophages.
Introduction
Reduced oxygen diffusion within growing tumors activates angiogenesis. Hypoxia triggers this angiogenic program in a tumor microenvironment to nurture new blood vessels, which transport oxygen and rid waste.1 This mechanism is transcriptionally mediated by the hypoxia-inducible factors (HIFs), HIF-1α and HIF-2α.2,3 As the master regulator of vascular endothelial growth factor (VEGF) during hypoxia, HIF-1α is well studied.4 Recently, the more obscure HIF-2α has been named an important transcriptional effecter in macrophages during hypoxia.5
Soluble VEGF receptor-1 (sVEGFR-1) is an alternatively spliced variant of the membrane-bound VEGFR-1 (VEGFR-1) expressed on endothelial cells and mononuclear phagocytes.6 On endothelial cells, both sVEGFR-1 and VEGFR-1 act as negative regulators for VEGF activity by sequestering VEGF from VEGFR-2, the main signaling receptor responsible for the cellular effects of VEGF, which include proliferation, migration, and survival. However, VEGFR-1 serves as the signaling receptor for VEGF on mononuclear phagocytes, which lack VEGFR-2, whereas sVEGFR-1 acts as the negative regulator of VEGF. The sVEGFR-1/VEGF ratio in tumor extracts correlates with prognosis, as a low ratio favors tumor angiogenesis and progression.7,8
Previously, we demonstrated, in a murine model of breast cancer, that local administration of granulocyte-macrophage colony-stimulating factor (GM-CSF) induces mononuclear phagocytes to overexpress sVEGFR-1 and suppress angiogenesis.9 In the current study, we exposed mononuclear phagocytes to hypoxia and found that lower oxygen significantly enhanced GM-CSF–induced sVEGFR-1 production from these cells. Further, we coupled studies in bone marrow–derived murine macrophages deficient in HIF-1α with siRNA targeting of HIF-2α and found that augmented sVEGFR-1 levels were dependent on HIF-2α, whereas HIF-1α appeared to regulate VEGF production. This study highlights the possibility that hypoxia, canonically thought to promote angiogenesis, can selectively stimulate antiangiogenic molecules from mononuclear phagocytes in a GM-CSF–rich environment. Furthermore, these results suggest that HIF-2α could be an effective and novel target to regulate angiogenesis in oxygen-deprived environments, such as myocardial disease, wound healing, and cancer.
Methods
Purification of peripheral blood monocytes
Human monocytes were purified from total peripheral blood mononuclear cells by layering over fetal bovine serum (FBS), as previously described.10 Monocytes were cultured in endotoxin-free RPMI 1640 supplemented with 1% FBS and 10 μg/mL of the endotoxin inhibitor polymyxin B. Monocytes were left untreated or were stimulated with 100 ng/mL GM-CSF at 21% (ambient) O2, 5% O2, or 0.5% O2. In experiments investigating JAK2 inhibition, monocytes were pretreated with 100μM of the JAK2/JAK3 inhibitor AG490 (Calbiochem) or an equivalent volume of dimethyl sulfoxide (vehicle control) for 1 hour at ambient O2 before exposure to GM-CSF and hypoxia. To chemically stabilize HIFs, monocytes were treated with GM-CSF at ambient O2 in media containing 100μM CoCl2 (Sigma-Aldrich) or an equivalent volume of diH2O (vehicle control). In some experiments, freshly isolated monocytes were differentiated into macrophages by 3-day culture in media containing 10% FBS, 1% PSA (penicillin G sodium, streptomycin sulfate, and amphotericin B), and 20 ng/mL macrophage colony-stimulating factor (M-CSF). Macrophages were serum-starved for 2 hours before stimulation with 100 ng/mL GM-CSF at ambient O2 or 0.5% O2.
Purification of neutrophils
Leukocyte source packs were subjected to density gradient centrifugation. The plasma and lymphocyte layers were removed, and the cell pellet was mixed with 20 mL of a 3% dextran sulfate solution and allowed to separate for 1 hour at room temperature, after which the top (neutrophil-containing) layer was harvested. Purity of the neutrophil preparations was routinely more than 90%, as determined by flow cytometry for the cell surface marker CD66b.
Endothelial tube formation assay
Human umbilical vein endothelial cells (HUVECs) were maintained in M200 media supplemented with the low serum growth supplement kit (Cascade Biologics). To quantify endothelial tube formation, cell-free culture supernatants from stimulated monocytes stimulated with GM-CSF at normoxia or 0.5% O2 were harvested and VEGF/sVEGFR-1 production was confirmed by enzyme-linked immunosorbent assay (ELISA). HUVECs were then cultured in these supernatants with growth factor-depleted Matrigel, as previously described.9 Entire wells were photographed, and angiogenic activity was quantified by counting the total number of tubes formed between discrete endothelial cells.
Real-time PCR
At various time points, cells were harvested in Trizol reagent (Invitrogen) and RNA was extracted in chloroform and then purified using the RNeasy Minikit (QIAGEN). cDNA was generated from 1 μg of RNA using the Superscript First Strand Synthesis System (Invitrogen) and used for real-time polymerase chain reaction (PCR) using SYBR Green PCR Master Mix (Applied Biosciences). Primer sets were designed using Primer Express, Version 3.0 software (ABI Prism; PerkinElmer Life and Analytical Sciences) and synthesized by Invitrogen. Data were analyzed according to the comparative threshold method and normalized against the β-actin internal control transcript. Data are semiquantitative and represent the fold difference in transcript levels in a particular sample compared with levels in untreated cells from the same donor.
Intracellular flow cytometry for total and phospho-STAT5
Intracellular flow cytometry was used to evaluate STAT5 signaling. The staining was performed using the Caltag Fixation and Permeabilization kit (Invitrogen) according to the manufacturer's instructions. Briefly, cells were fixed with 100 μL of Reagent A from the kit and incubated for 10 minutes in 3 mL of cold 100% methanol. Cells were washed in flow buffer (phosphate-buffered saline containing 5% FBS) and resuspended in 100 μL of Reagent B. For phospho (p)-STAT5 analysis, cells were labeled with 10 μL of an AlexaFluor 488–conjugated mouse anti–human p-STAT5a (Y694; BD Biosciences PharMingen) or an equivalent amount of an isotype-matched control antibody for 60 minutes at room temperature. For total STAT5, cells were first incubated with mouse anti–human STAT5 (BD Biosciences PharMingen) or an isotype control antibody for 30 minutes at room temperature. Cells were then washed once in flow buffer, blocked for 10 minutes with polyclonal goat IgG (Sigma-Aldrich), and stained for 30 minutes at room temperature with a 1:200 dilution of goat anti–mouse AlexaFlour 488. After the antibody labeling, cells were washed with flow buffer, fixed in 1% formalin, and stored at 4°C until flow cytometric analysis.
Flow cytometry for VEGFR-1 and VEGFR-2 surface expression
Expression of the membrane-bound VEGFR-1 and VEGFR-2 on monocytes or HUVECs was assessed. Cells were resuspended in 100 μL of flow buffer and blocked for 10 minutes on ice with murine IgG1 (Sigma-Aldrich) before staining for 30 minutes on ice with 10 μL of allophycocyanin-conjugated murine anti–human VEGFR-1, phycoerythrin-conjugated murine anti–human VEGFR-2, or the appropriate isotype control antibodies. Cells were then washed in flow buffer, fixed in 1% formalin, and stored at 4°C until analysis.
Culture of bone marrow–derived macrophages
The HIF-1αflox/flox/LysMcre knockout mice were a gift from Dr Phillip Popovich (Ohio State University). All animal protocols were approved by the Ohio State University Animal Care and Use Committee, and mice were treated in accordance with institutional guidelines for animal care. The mice were generated by crossing a C57BL/6 mouse containing loxP sequences on either side of the HIF-1α gene with a C57BL/6 mouse expressing Cre recombinase from the lysozyme M (LysM) promoter, which is expressed only in myeloid lineage cells.11 The result is a homozygous mouse that is deficient in HIF-1α in the monocytes and macrophages. Femoral bone marrow was isolated and progenitor cells were plated in RPMI 1640 supplemented with 10% FBS, 1% PSA (penicillin G sodium, streptomycin sulfate, and amphotericin B), 10 μg/mL polymyxin B, and 20 ng/mL recombinant murine M-CSF. Cells were cultured for 5 days with the addition of fresh M-CSF every other day. Differentiated bone marrow-derived macrophages were serum-starved for 2 hours and then treated with 100 ng/mL murine GM-CSF in RPMI 1640 containing 1% FBS and 10 μg/mL polymixin B. Culture supernatants were collected after 72 hours and assayed for VEGF and sVEGFR-1 content by ELISA (R&D Systems). Mice containing the floxed HIF-1α allele, which do not express the cre recombinase gene (HIF-1αflox/flox), served as the control mice in these studies. Deletion of HIF-1α in HIF-1αflox/flox/LysMcre macrophages, but not HIF-1αflox/flox control macrophages, was confirmed at the transcript level by real-time PCR.
Transfection of macrophages with siRNA
Bone marrow–derived macrophages from wild-type C57BL/6 mice were cultured for 12 hours in 1.1 mL of media containing 6 μL of HiPerFect Transfection Reagent (QIAGEN) and 50nM control siRNA or a cocktail of siRNAs specific for HIF-2α (QIAGEN). Murine GM-CSF was then added and cells were incubated for an additional 48 hours at ambient O2 or 0.5% O2. Cell-free supernatants were collected for VEGF and sVEGFR-1 ELISAs and the cells resuspended in Trizol for RNA isolation. The efficiency of transfection was assessed by real-time PCR for HIF-2α, and the specificity of transfection was confirmed by real-time PCR for HIF-1α.
Results
Production of the soluble VEGF receptor by GM-CSF–stimulated monocytes is enhanced under hypoxic conditions
We previously reported that monocytes stimulated with GM-CSF secrete sVEGFR-1, which sequesters VEGF from biologic activity.9 Because both the VEGF and the VEGFR-1 promoters contain a hypoxia regulatory element promoter element, we examined the effect of hypoxia on GM-CSF-induced sVEGFR-1 production. Human monocytes were left untreated or were stimulated with 100 ng/mL GM-CSF at 21% O2 (ambient O2), 5% O2 (mild hypoxia), or 0.5% O2 (severe hypoxia). Culture supernatants were harvested at 48 hours and analyzed for sVEGFR-1 content by ELISA. Consistent with our previous observations, monocytes stimulated with GM-CSF at ambient O2 secreted significant amounts of sVEGFR-1 (P < .001). In addition, sVEGFR-1 production increased when monocytes were cultured under hypoxia (P < .001 for untreated monocytes at 0.5% O2, compared with untreated monocytes at normoxia). Monocytes stimulated with GM-CSF at 5% O2 secreted increased amounts of sVEGFR-1 compared with monocytes stimulated with GM-CSF at ambient O2, and the greatest sVEGFR-1 production was observed from GM-CSF–stimulated monocytes cultured at 0.5% O2 (P = .001; Figure 1A). Monocyte production of sVEGFR-1 began within 5 hours of GM-CSF exposure and peaked at approximately 20 hours (Figure 1B).
Production of sVEGFR-1 by mononuclear phagocytes increases with decreasing O2 concentration. (A) Human peripheral blood monocytes were cultured at 21% (ambient) O2, 5% O2 (mild hypoxia), or 0.5% O2 (severe hypoxia) in media alone or media containing 100 ng/mL GM-CSF. After 48 hours, culture supernatants were harvested and analyzed for sVEGFR-1 content by ELISA. Data are mean ± SEM of 7 normal donors. (B) Monocytes were stimulated with GM-CSF at ambient O2 or at 0.5% O2. Culture supernatants were harvested at the indicated time points and analyzed for sVEGFR-1 content by ELISA. Data are the mean ± SEM of 6 normal donors. (C) Monocytes were stimulated as in panel B and analyzed for sVEGFR-1 transcript by real-time PCR at the indicated time points. Data are the mean ± SEM of 6 normal donors. (D) Supernatants from the same cells as in panel A were measured using an ELISA that detects only bioavailable VEGF (ie, VEGF that is not bound to sVEGFR-1). (E) VEGF levels under the same conditions as in panel B. (F) VEGF transcript levels under the same conditions as in panel B. (G) Monocytes were cultured at ambient O2 or 0.5% O2 with increasing concentrations of GM-CSF (0.1 ng/mL to 100 ng/mL). sVEGFR-1 secretion after 48 hours was measured by ELISA. (H) VEGF levels in the same supernatants as in panel G. (I) HUVECs were cultured in Matrigel in unsupplemented media (negative control), media supplemented with 10 ng/mL VEGF (positive control), or supernatants derived from monocytes cultured at 0.5% O2 in the presence or absence of GM-CSF. A representative image from each condition is shown (left). The total number of tubes formed between discrete endothelial cells were counted in each well. Graphed results (right) represent the average ± SEM number of tubes/field using supernatants from 4 different monocyte donors. (J) Peripheral blood monocytes were differentiated into macrophages by 3-day culture with M-CSF before stimulation with 100 ng/mL GM-CSF at either ambient O2 or 0.5% O2. Culture supernatants were harvested after 48 hours, and sVEGFR-1 concentration was measured by ELISA. Data are mean ± SEM of 3 donors. (K) VEGF levels under the same conditions as in panel J. *P < .001 versus all other conditions at the same time point. **P < .05 versus all other conditions at the same time point. Utx indicates untreated.
Production of sVEGFR-1 by mononuclear phagocytes increases with decreasing O2 concentration. (A) Human peripheral blood monocytes were cultured at 21% (ambient) O2, 5% O2 (mild hypoxia), or 0.5% O2 (severe hypoxia) in media alone or media containing 100 ng/mL GM-CSF. After 48 hours, culture supernatants were harvested and analyzed for sVEGFR-1 content by ELISA. Data are mean ± SEM of 7 normal donors. (B) Monocytes were stimulated with GM-CSF at ambient O2 or at 0.5% O2. Culture supernatants were harvested at the indicated time points and analyzed for sVEGFR-1 content by ELISA. Data are the mean ± SEM of 6 normal donors. (C) Monocytes were stimulated as in panel B and analyzed for sVEGFR-1 transcript by real-time PCR at the indicated time points. Data are the mean ± SEM of 6 normal donors. (D) Supernatants from the same cells as in panel A were measured using an ELISA that detects only bioavailable VEGF (ie, VEGF that is not bound to sVEGFR-1). (E) VEGF levels under the same conditions as in panel B. (F) VEGF transcript levels under the same conditions as in panel B. (G) Monocytes were cultured at ambient O2 or 0.5% O2 with increasing concentrations of GM-CSF (0.1 ng/mL to 100 ng/mL). sVEGFR-1 secretion after 48 hours was measured by ELISA. (H) VEGF levels in the same supernatants as in panel G. (I) HUVECs were cultured in Matrigel in unsupplemented media (negative control), media supplemented with 10 ng/mL VEGF (positive control), or supernatants derived from monocytes cultured at 0.5% O2 in the presence or absence of GM-CSF. A representative image from each condition is shown (left). The total number of tubes formed between discrete endothelial cells were counted in each well. Graphed results (right) represent the average ± SEM number of tubes/field using supernatants from 4 different monocyte donors. (J) Peripheral blood monocytes were differentiated into macrophages by 3-day culture with M-CSF before stimulation with 100 ng/mL GM-CSF at either ambient O2 or 0.5% O2. Culture supernatants were harvested after 48 hours, and sVEGFR-1 concentration was measured by ELISA. Data are mean ± SEM of 3 donors. (K) VEGF levels under the same conditions as in panel J. *P < .001 versus all other conditions at the same time point. **P < .05 versus all other conditions at the same time point. Utx indicates untreated.
To confirm the transcriptional up-regulation of sVEGFR-1, we conducted real-time PCR using primers specific for the unique 93-base pair sequence that is present within the soluble VEGFR-1 transcript but absent from the transcript of membrane-bound VEGFR-112 (Figure 1C). Increased sVEGFR-1 mRNA production in response to GM-CSF was evident by 5 hours after stimulation, at which time cells treated with GM-CSF at normoxia exhibited a 48- ± 13-fold increase in sVEGFR-1 mRNA compared with unstimulated cells at normoxia (P = .002), whereas cells stimulated with GM-CSF at 0.5% O2 exhibited a 163- ± 82-fold increase (P < .001). By 20 hours after stimulation, sVEGFR-1 mRNA in cells stimulated with GM-CSF at normoxia was elevated 364- ± 164-fold over untreated cells at normoxia (P = .038), whereas sVEGFR-1 mRNA in cells stimulated with GM-CSF at 0.5% O2 was increased 1848- ± 961-fold (P < .001). GM-CSF and hypoxia each resulted in a significant increase in sVEGFR-1 transcription across all time points examined (5-48 hours; P < .001 for GM-CSF alone and P < .01 for hypoxia alone). Furthermore, monocytes stimulated with GM-CSF at 0.5% O2 contained significantly higher levels of sVEGFR-1 transcript compared with monocytes stimulated with GM-CSF at normoxia (P < .001).
To determine whether the sVEGFR-1 was capable of neutralizing endogenously produced VEGF, we analyzed the same supernatants for VEGF using an ELISA that detects free (bioavailable) VEGF, but not VEGF bound to sVEGFR-1. As shown in Figure 1D, untreated monocytes secreted a basal amount of VEGF, which increased with decreasing O2 concentration (P = .008). VEGF detection from GM-CSF–stimulated monocytes was significantly inhibited at all O2 levels (P < .001). VEGF was detected within the supernatants of unstimulated monocytes beginning at 9 hours (Figure 1E). However, sVEGFR-1 levels within the supernatants of monocytes stimulated with GM-CSF at 0.5% O2 were insufficient to fully inhibit VEGF detection until approximately 12 hours after stimulation. To confirm that the lack of VEGF detection in supernatants from GM-CSF–stimulated monocytes was the result of neutralization of VEGF and not the result of a decrease in VEGF production, we also assessed VEGF transcription by real-time PCR. GM-CSF did not inhibit VEGF transcription but actually enhanced it, as monocytes cultured at 0.5% O2 in the presence of GM-CSF produced more VEGF transcripts than monocytes cultured at 0.5% O2 in media alone (P = .017; Figure 1F). These results demonstrate that lack of detectable VEGF in the supernatants of GM-CSF–treated monocytes was the result of the ability of the secreted sVEGFR-1 to neutralize VEGF.
We next conducted a dose-response experiment to determine the optimal concentration of GM-CSF to costimulate monocyte sVEGFR-1 production under hypoxia. Of note, sVEGFR-1 secretion in response to GM-CSF was significantly greater at 0.5% O2 than at normoxia for all doses of GM-CSF tested (Figure 1G; P = .002). Furthermore, sVEGFR-1 production increased with increasing dose of GM-CSF, at both normoxia and hypoxia (P < .001). This resulted in a corresponding decrease in VEGF detection with increasing dose of GM-CSF (Figure 1H; P < .001).
We next examined the ability of supernatants from monocytes to induce angiogenesis in an endothelial cell tube formation assay. Human monocytes were cultured at 0.5% O2 in the presence or absence of 100 ng/mL GM-CSF, and supernatants were harvested after 48 hours. HUVECs were plated on Matrigel and were cultured overnight with the monocyte-derived supernatants. The formation of endothelial tubules was then quantified. As shown in Figure 1I, we observed a significant reduction in tube formation from HUVECs cultured with supernatants from monocytes cultured with GM-CSF at 0.5% O2 compared with monocytes cultured at 0.5% O2 in the absence of GM-CSF (P = .002). These results confirm that hypoxia can have an antiangiogenic effect on monocytes in the presence of GM-CSF.
Hypoxia enhances sVEGFR-1 production by GM-CSF–stimulated macrophages
To determine whether the effect of hypoxia on sVEGFR-1 production was limited to monocytes, freshly isolated peripheral blood monocytes were differentiated into macrophages by 3-day culture in low-dose M-CSF before starvation and stimulation with GM-CSF at low O2. As with monocytes, increased sVEGFR-1 production was observed from human peripheral blood monocyte-derived macrophages stimulated with GM-CSF at 0.5% O2 (P = .001; Figure 1J), correlating with a significant decrease in VEGF detection (P < .001; Figure 1K).
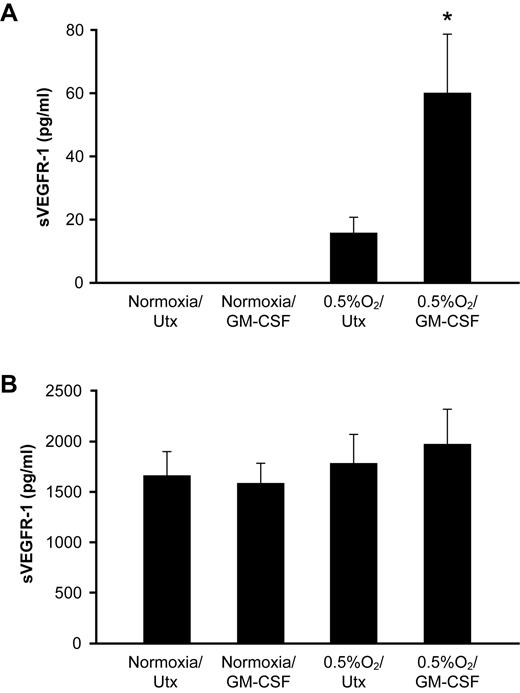
To determine whether the effect of GM-CSF and hypoxia was specific to mononuclear phagocytes, we also assessed sVEGFR-1 secretion from human neutrophils, which also express the GM-CSF receptor machinery, and HUVECs, which are known to secrete sVEGFR-1.13 Although neutrophils cultured with GM-CSF at 0.5% O2 secreted more sVEGFR-1 than neutrophils cultured under either condition alone (P < .001), the overall amount of sVEGFR-1 production from neutrophils was much less than from an equivalent number of monocytes (60 ± 15 pg/mL sVEGFR-1 in supernatants of neutrophils stimulated with GM-CSF at 0.5% O2, vs 12 000 ± 2800 pg/mL from monocytes at the same conditions; Figure 2A), suggesting that, despite expression of GM-CSF receptors, neutrophils are not an important source of sVEGFR-1 in response to GM-CSF. Furthermore, the low amount of sVEGFR-1 produced from GM-CSF–stimulated neutrophils was insufficient to neutralize endogenously secreted VEGF, as VEGF levels were equivalent across all conditions tested (P = .998; data not shown). The lack of VEGF production in response to hypoxia is consistent with published data concerning the failure of hypoxia to induce VEGF release from neutrophils.14 HUVECs secreted a basal amount of sVEGFR-1, which did not increase in response to GM-CSF, low O2, or the combination (P = .556; Figure 2B). Expression of the soluble form of VEGFR-2, which is expressed by endothelial cells but not mononuclear phagocytes, was below the limit of detection of the ELISA in all conditions tested (< 10 pg/mL; data not shown).
Hypoxia does not induce sVEGFR-1 production from neutrophils or HUVECs. (A) Human peripheral blood neutrophils were stimulated with 100 ng/mL GM-CSF at ambient O2 or 0.5% O2. Cell-free culture supernatants were collected at 48 hours and analyzed for sVEGFR-1 content by ELISA. (B) HUVECs were treated with 100 ng/mL GM-CSF at ambient O2 or 0.5% O2. sVEGFR-1 secretion after 48 hours was assessed by ELISA. *P < .001 versus all conditions shown.
Hypoxia does not induce sVEGFR-1 production from neutrophils or HUVECs. (A) Human peripheral blood neutrophils were stimulated with 100 ng/mL GM-CSF at ambient O2 or 0.5% O2. Cell-free culture supernatants were collected at 48 hours and analyzed for sVEGFR-1 content by ELISA. (B) HUVECs were treated with 100 ng/mL GM-CSF at ambient O2 or 0.5% O2. sVEGFR-1 secretion after 48 hours was assessed by ELISA. *P < .001 versus all conditions shown.
Hypoxia increases expression of VEGFR-1 on the surface of monocytes but not endothelial cells
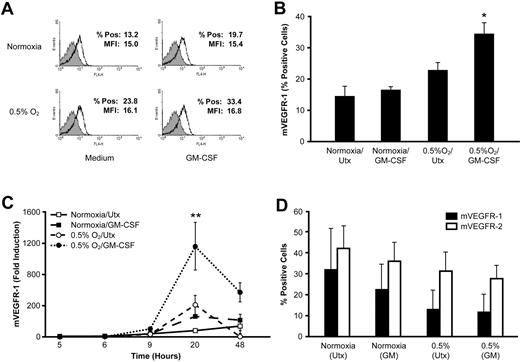
As the soluble and membrane-bound form of the VEGF receptor (mVEGFR-1) are derived from the same prespliced RNA transcript,15 we examined the effect of GM-CSF and hypoxia on monocyte expression of mVEGFR-1. Freshly isolated monocytes were stimulated with GM-CSF at ambient O2 or 0.5% O2 and stained for surface VEGFR-1 expression by flow cytometry. Hypoxia alone or GM-CSF alone did not increase mVEGFR-1 expression (P = .278 and P = .136, respectively), but the combination resulted in an approximately 20% increase in the percentage of cells expressing mVEGFR-1 (P < .001; Figure 3A-B). However, the mean fluorescence index for the positively stained cell population was unchanged (P = .71), indicating that hypoxia and GM-CSF did not increase the amount of mVEGFR-1 expressed by individual cells (data not shown). Analysis by real-time PCR using primers specific for the membrane-bound VEGFR-1 revealed a significant induction in mVEGFR-1 transcript in cells cultured with GM-CSF at low O2 compared with cells cultured under either stimulus alone (P < .001; Figure 3C).
Hypoxia up-regulates expression of the membrane-bound VEGF-1 receptor from monocytes but not HUVECs. (A) Human peripheral blood monocytes were cultured at ambient O2 or 0.5% O2 in the presence of 100 ng/mL GM-CSF. After 48 hours, cells were analyzed for expression of mVEGFR-1 by flow cytometry. Results from a representative donor are shown. The shaded peak represents the staining of the isotype control. (B) mVEGFR-1 expression was analyzed on human peripheral blood monocytes treated as in panel A. Data are the mean ± SEM of 6 normal donors. (C) Expression of mVEGFR-1 mRNA in monocytes by real-time PCR. Data are mean ± SEM of 6 normal donors. (D) HUVECs were cultured at ambient O2 or 0.5% O2 in the presence of 100 ng/mL GM-CSF, and the surface expression of VEGFR-1 and VEGFR-2 was assessed by flow cytometry. Data are mean ± SEM of 4 independent experiments. *P < .001 versus untreated cells at normoxia. **P < .001 versus all other conditions at the same time point.
Hypoxia up-regulates expression of the membrane-bound VEGF-1 receptor from monocytes but not HUVECs. (A) Human peripheral blood monocytes were cultured at ambient O2 or 0.5% O2 in the presence of 100 ng/mL GM-CSF. After 48 hours, cells were analyzed for expression of mVEGFR-1 by flow cytometry. Results from a representative donor are shown. The shaded peak represents the staining of the isotype control. (B) mVEGFR-1 expression was analyzed on human peripheral blood monocytes treated as in panel A. Data are the mean ± SEM of 6 normal donors. (C) Expression of mVEGFR-1 mRNA in monocytes by real-time PCR. Data are mean ± SEM of 6 normal donors. (D) HUVECs were cultured at ambient O2 or 0.5% O2 in the presence of 100 ng/mL GM-CSF, and the surface expression of VEGFR-1 and VEGFR-2 was assessed by flow cytometry. Data are mean ± SEM of 4 independent experiments. *P < .001 versus untreated cells at normoxia. **P < .001 versus all other conditions at the same time point.
We also examined the expression of membrane-bound VEGFR-1 and VEGFR-2 on endothelial cells. HUVECs were cultured for 48 hours at normoxia or 0.5% O2 and stained for VEGFR-1 and VEGFR-2 levels by flow cytometry. GM-CSF treatment had no effect on HUVEC expression of VEGFR-1 or VEGFR-2 regardless of O2 concentration, consistent with the lack of GM-CSF receptor expression on these cells.16 Exposure to 0.5% O2 did not increase the percentage of HUVECs expressing either VEGFR-1 (P = .22) or VEGFR-2 (P = .19; Figure 3D). Hypoxia also did not alter the level of VEGFR-1 or VEGFR-2 expressed on individual cells (P = .70 and P = .62 for the mean fluorescence index of VEGFR-1 and VEGFR-2, respectively; data not shown).
Monocyte production of sVEGFR-1 in response to GM-CSF is dependent on STAT5 activation
The signal transducer and activation of transcription 5 (STAT5) mediates sVEGFR-1 production from GM-CSF–stimulated monocytes.9 We therefore examined the ability of hypoxia to enhance GM-CSF–mediated sVEGFR-1 production in the absence of STAT5 activation. Monocytes were pretreated with dimethyl sulfoxide (vehicle control) or 100μM of the JAK2/JAK3 inhibitor AG490 before stimulation with GM-CSF. Cell viability was confirmed by Trypan blue staining at the end of the culture period and was routinely more than 90%. Vehicle-treated monocytes cultured at 0.5% O2 in the presence of GM-CSF secreted greater amounts of sVEGFR-1 than GM-CSF–stimulated monocytes at normoxia, as previously observed. At both normoxia and 0.5% O2, monocytes treated with GM-CSF and AG490 secreted significantly less sVEGFR-1 than the vehicle-treated cells (Figure 4A). The inhibition of sVEGFR-1 production in the presence of AG490 restored VEGF detection in the supernatants of AG490/GM-CSF–treated monocytes (Figure 4B). Because hypoxia has been shown to activate STAT5 in some cell types,17 we considered STAT5 as a possible point of interaction between the GM-CSF and the hypoxia-induced signaling pathways. Peripheral blood monocytes were stimulated with GM-CSF at normoxia or at 0.5% O2 for various time points (5 minutes to 48 hours), and p-STAT5 levels were assessed by flow cytometry. Stimulation of monocytes with GM-CSF at normoxia induced strong STAT5 phosphorylation. However, low O2 alone did not induce STAT5 activation in monocytes, and there was no difference in STAT5 activation between the cells stimulated with GM-CSF at normoxia and those stimulated at 0.5% O2 at any time point up to 24 hours after stimulation (Figure 4C-D). This was the case both in terms of the percentage of p-STAT5-positive cells and in terms of the mean fluorescence index of the positively stained cell population (Figure 4D; and data not shown). By 48 hours after stimulation, there was an approximately 10% difference in the percentage of p-STAT5-positive cells at normoxia and at hypoxia, indicating that hypoxia did slightly extend the duration of STAT5 signaling within GM-CSF–stimulated monocytes (Figure 4D). However, this difference was only apparent at 48 hours after stimulation, indicating that it is unrelated to the mechanism of synergy. Levels of total STAT5 were also unchanged in response to GM-CSF, hypoxia, or the combination (data not shown).
Monocyte production of sVEGFR-1 is dependent on JAK2/STAT5 signaling. (A) Human peripheral blood monocytes were treated with the JAK2/JAK3 inhibitor, AG490, before stimulation with GM-CSF at ambient O2 or 0.5% O2. Cell-free culture supernatants were collected after 48 hours and analyzed for sVEGFR-1 content by ELISA. (B) VEGF levels under the same conditions as in panel A. (C) Human peripheral blood monocytes were stimulated with GM-CSF for 5 minutes at ambient or 0.5% O2, and levels of p-STAT5 (Y694) were measured by flow cytometry. The shaded peak represents the staining of the isotype control. Results from a representative donor are shown. (D) Monocytes were stimulated with GM-CSF at ambient O2 or 0.5% O2 for various lengths of time (5 minutes to 48 hours), and p-STAT5 levels were measured by flow cytometry. Data are expressed as the percentage of positively stained cells at the indicated time points. All results represent the mean ± SEM of 4 normal donors.
Monocyte production of sVEGFR-1 is dependent on JAK2/STAT5 signaling. (A) Human peripheral blood monocytes were treated with the JAK2/JAK3 inhibitor, AG490, before stimulation with GM-CSF at ambient O2 or 0.5% O2. Cell-free culture supernatants were collected after 48 hours and analyzed for sVEGFR-1 content by ELISA. (B) VEGF levels under the same conditions as in panel A. (C) Human peripheral blood monocytes were stimulated with GM-CSF for 5 minutes at ambient or 0.5% O2, and levels of p-STAT5 (Y694) were measured by flow cytometry. The shaded peak represents the staining of the isotype control. Results from a representative donor are shown. (D) Monocytes were stimulated with GM-CSF at ambient O2 or 0.5% O2 for various lengths of time (5 minutes to 48 hours), and p-STAT5 levels were measured by flow cytometry. Data are expressed as the percentage of positively stained cells at the indicated time points. All results represent the mean ± SEM of 4 normal donors.
CoCl2 enhances sVEGFR-1 production from GM-CSF–stimulated monocytes
To determine whether the HIF transcription factors are involved in the enhanced sVEGFR-1 production observed at low O2 concentrations, we treated monocytes with GM-CSF in the presence of CoCl2, which inhibits the O2-mediated degradation of HIF-1α and HIF-2α and stabilizes the HIFs at normoxia.18 CoCl2 enhanced monocyte secretion of sVEGFR-1 in response to GM-CSF (P < .001; Figure 5A) and inhibited VEGF detection (P < .001; Figure 5B). Treatment of monocytes with the prolyl hydroxylase inhibitor DMOG produced similar results (data not shown), suggesting that low O2 enhanced sVEGFR-1 production by activating the HIF pathway.
Cobalt chloride enhances sVEGFR-1 production from monocytes stimulated with GM-CSF. (A) Human peripheral blood monocytes were stimulated with 100 ng/mL GM-CSF in the presence or absence of 100μM CoCl2. Cell-free culture supernatants were harvested at 48 hours, and sVEGFR-1 content was determined by ELISA. Data are mean ± SEM of 7 normal donors. (B) VEGF levels under the same conditions as in panel A.
Cobalt chloride enhances sVEGFR-1 production from monocytes stimulated with GM-CSF. (A) Human peripheral blood monocytes were stimulated with 100 ng/mL GM-CSF in the presence or absence of 100μM CoCl2. Cell-free culture supernatants were harvested at 48 hours, and sVEGFR-1 content was determined by ELISA. Data are mean ± SEM of 7 normal donors. (B) VEGF levels under the same conditions as in panel A.
HIF-1α deficiency does not affect sVEGFR-1 production from GM-CSF–stimulated macrophages
To determine which of the HIFs were responsible for sVEGFR-1 production in response to GM-CSF and low O2, we used HIF-1αflox/flox/LysMcre mice, in which the HIF-1α allele is floxed and the cre recombinase is driven from the LysM promoter, resulting in a deletion of HIF-1α in the monocyte/macrophage compartment.11 We first derived macrophages from bone marrow of HIF-1αflox/flox/LysMcre mice or HIF-1αflox/flox control mice and assessed their HIF-1α and HIF-2α expression via real-time PCR. As expected, HIF-1α mRNA was detected at a significantly lower level within bone marrow–derived macrophages derived from HIF-1αflox/flox/LysMcre mice (P < .001), whereas HIF-2α expression in the HIF-1αflox/flox/LysMcre mice was comparable with that of HIF-1αflox/flox control mice (P = .278; Figure 6A). Bone marrow–derived macrophages from HIF-1αflox/flox/LysMcre mice (HIF-1α−/− macrophages) or control mice (HIF-1α+/+ macrophages) were cultured for 48 hours at ambient O2 or 0.5% O2, in media alone or media containing 100 ng/mL GM-CSF. HIF-1α+/+and HIF-1α−/− macrophages secreted equivalent amounts of sVEGFR-1 in response to hypoxia, GM-CSF, or the combination (P = .779; Figure 6B). However, HIF-1α−/− macrophages secreted significantly less VEGF than control macrophages under all conditions examined (P = .002; Figure 6C). Because the sVEGFR-1 content of the supernatants from control and HIF-1α–deficient macrophages was equivalent, the difference in VEGF detection must be the result of a difference in total VEGF levels and not the result of sVEGFR-1 masking the VEGF from detection. To confirm this finding, we assessed levels of sVEGFR-1 and VEGF transcripts by real-time PCR. Consistent with our ELISA results, HIF-1α−/− macrophages cultured with GM-CSF at low O2 had comparable levels of sVEGFR-1 mRNA as HIF-1α+/+ macrophages (P = .937; Figure 6D) but substantially reduced levels of VEGF mRNA (P < .001; Figure 6E). These results suggest that VEGF, but not sVEGFR-1, production in response to GM-CSF and hypoxia is dependent on HIF-1α.
Production of VEGF, but not sVEGFR-1, is inhibited in HIF-α−/− macrophages. (A) HIF-1α and HIF-2α expression was assessed by real-time PCR in bone marrow–derived macrophages from HIF-1αflox/flox/LysMcre mice or HIF-1αflox/flox control mice. (B) Bone marrow–derived macrophages from HIF-1αflox/flox/LysMcre mice (HIF-1α−/− macrophages) or from HIF-1αflox/flox control mice (HIF-1α+/+ macrophages) were left untreated or were stimulated with 100 ng/mL GM-CSF, at ambient O2 or at 0.5% O2. Cell-free culture supernatants were assessed for sVEGFR-1 (B) after 48 hours. (C) VEGF levels from the same supernatants as in panel B. Cells were also assessed for levels of sVEGFR-1 (D) and VEGF (E) transcript by real-time PCR. Data are mean ± SEM of 8 mice per group.
Production of VEGF, but not sVEGFR-1, is inhibited in HIF-α−/− macrophages. (A) HIF-1α and HIF-2α expression was assessed by real-time PCR in bone marrow–derived macrophages from HIF-1αflox/flox/LysMcre mice or HIF-1αflox/flox control mice. (B) Bone marrow–derived macrophages from HIF-1αflox/flox/LysMcre mice (HIF-1α−/− macrophages) or from HIF-1αflox/flox control mice (HIF-1α+/+ macrophages) were left untreated or were stimulated with 100 ng/mL GM-CSF, at ambient O2 or at 0.5% O2. Cell-free culture supernatants were assessed for sVEGFR-1 (B) after 48 hours. (C) VEGF levels from the same supernatants as in panel B. Cells were also assessed for levels of sVEGFR-1 (D) and VEGF (E) transcript by real-time PCR. Data are mean ± SEM of 8 mice per group.
sVEGFR-1 production from GM-CSF–stimulated macrophages is dependent on HIF-2α
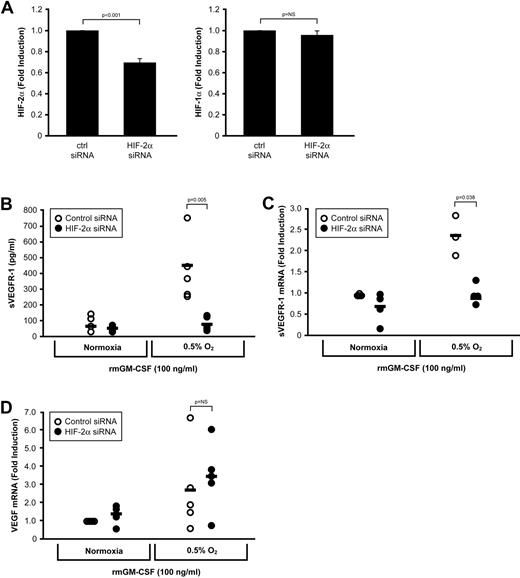
Because sVEGFR-1 was produced independently of HIF-1α, we next examined the effect of HIF-2α deletion on sVEGFR-1 production from GM-CSF–stimulated macrophages. Bone marrow-derived macrophages from wild-type C57Bl/6 mice were incubated for 12 hours with an HIF-2α–specific siRNA cocktail (or a scrambled siRNA sequence). Transfected macrophages were then left untreated or treated with 100 ng/mL GM-CSF at ambient O2 or 0.5% O2. After 48 hours, culture supernatants were harvested and RNA from these cells collected to assess the efficiency of siRNA knockdown of HIF-2α. Transfection with the siRNA targeting HIF-2α resulted in an approximate 35% reduction in HIF-2α mRNA levels as measured by real-time PCR (P < .001), with no effect on HIF-1α expression (P = .41; Figure 7A). Knockdown of HIF-2α resulted in a significant decrease in sVEGFR-1 production in response to the combination of GM-CSF and low O2 at the protein level (P = .005; Figure 7B) and at the transcript level (P = .038; Figure 7C). However, HIF-2α knockdown had no effect on VEGF production under the same conditions (P = .272; Figure 7C). Because our data indicated that a relatively modest (30%) knockdown in HIF-2α mRNA effectively abrogated sVEGFR-1 secretion in response to GM-CSF and 0.5% O2, we examined sVEGFR-1 production from HIF-2α+/flox/LysMcre mice. These mice are heterozygous for the floxed HIF-2α allele, resulting in an approximately 50% reduction in HIF-2α siRNA compared with control macrophages, as determined by real-time PCR (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). HIF-2α+/− macrophages stimulated with GM-CSF at 0.5% O2 secreted comparable amounts of VEGF to control macrophages but significantly less sVEGFR-1 (P = .004; supplemental Figure 1). Taken together, these results suggest that HIF-2α drives macrophage production of sVEGFR-1, whereas HIF-1α drives macrophage production of VEGF.
Neutralization of HIF-2α inhibits sVEGFR-1, but not VEGF, production. (A) Bone marrow-derived macrophages from wild-type mice were transfected with a control siRNA or a HIF-2α–specific siRNA cocktail and stimulated with GM-CSF for 48 hours at ambient O2 or at 0.5% O2. Transfection resulted in an approximate 35% decrease in HIF-2α transcript levels by real-time PCR, whereas HIF-1α was unaffected. (B) Cell-free culture supernatants were assessed for sVEGFR-1 after 48 hours. Levels of (C) sVEGFR-1 and (D) VEGF were also assessed via real-time PCR. Data are mean ± SEM of 5 mice per group.
Neutralization of HIF-2α inhibits sVEGFR-1, but not VEGF, production. (A) Bone marrow-derived macrophages from wild-type mice were transfected with a control siRNA or a HIF-2α–specific siRNA cocktail and stimulated with GM-CSF for 48 hours at ambient O2 or at 0.5% O2. Transfection resulted in an approximate 35% decrease in HIF-2α transcript levels by real-time PCR, whereas HIF-1α was unaffected. (B) Cell-free culture supernatants were assessed for sVEGFR-1 after 48 hours. Levels of (C) sVEGFR-1 and (D) VEGF were also assessed via real-time PCR. Data are mean ± SEM of 5 mice per group.
Discussion
Previously, we reported that GM-CSF induces sVEGFR-1 production from human monocytes.9 Because the intratumoral sVEGFR-1/VEGF ratio is a potent prognostic indicator in patients with breast cancer,8 we hypothesized that administration of GM-CSF into murine breast tumors would inhibit angiogenesis by inducing sVEGFR-1, which would in turn sequester free VEGF.19 During in situ hybridization analysis of the GM-CSF–treated mammary tumors from that study, we were surprised to note that the major contributor of sVEGFR-1 was macrophages residing within the perinecrotic, hypoxic regions, more so than those macrophages residing in tissue with higher oxygen tension. Tumor-associated macrophages (TAMs) are known to migrate to areas of hypoxia, lose migratory ability, and display a protumor phenotype because of a distinct hypoxia-induced gene expression profile characterized by abundant production of VEGF.15 For this reason, increased numbers of TAMs generally correlate with increased microvascular density, tumor progression, and metastases.20 Surprisingly, we observed an increase in TAM infiltration in GM-CSF–treated tumors, which grew at a slower rate than those not receiving GM-CSF.19 We therefore speculated that hypoxia synergized with GM-CSF to up-regulate sVEGFR-1 from TAMs.
To address this hypothesis, we assayed human monocytes to determine whether hypoxia augmented GM-CSF–induced sVEGFR-1 production. Indeed, we observed a significant increase in sVEGFR-1 production in response to hypoxia alone. In addition, GM-CSF–stimulated monocytes exposed to hypoxia secreted approximately 4-fold more sVEGFR-1 than cells stimulated with GM-CSF at normoxia. To understand whether macrophages similarly produce sVEGFR-1 in response to GM-CSF and hypoxia, we differentiated macrophages from peripheral blood monocytes and found the same effect on sVEGFR-1 production, only to a lesser degree. Because neutrophils express the receptor machinery for GM-CSF and because endothelial cells express both membrane-bound VEGF receptors-1 and -2, we asked whether these cells would behave the same as the mononuclear phagocytes in their production of sVEGFR-1. Although hypoxia did enhance sVEGFR-1 production from GM-CSF–stimulated neutrophils, the amount of sVEGFR-1 produced by neutrophils was approximately 2.7% of that produced by the same number of monocytes over the same time. Furthermore, even though the endothelial cells we assayed exhibited a low basal level of sVEGFR-1 production, these levels were not augmented by GM-CSF, hypoxia, or the combination. Therefore, of the 3 cell types with the machinery to produce sVEGFR-1, or to respond to GM-CSF, mononuclear phagocytes were the only cells influenced synergistically by the combination of GM-CSF and hypoxia.
Because hypoxia augmented GM-CSF–induced sVEGFR-1 production from mononuclear phagocytes and because GM-CSF signals via the JAK2/STAT5 pathway,21 we speculated that there may be crosstalk between the transcription factor STAT5 and one of the HIF proteins. Indeed, hypoxia has been shown to increase STAT5 phosphorylation and DNA binding in mammary epithelial cells.17 We found that, even though the JAK/STAT pathway inhibitor, AG490, suppressed sVEGFR-1 production from GM-CSF-treated mononuclear phagocytes (and rescued VEGF detection in those same supernatants), there was no contribution from hypoxia in the phosphorylation of STAT5, suggesting no direct interaction between these 2 signaling pathways in this model. GM-CSF stimulation has been shown to lead to stronger p-STAT5 DNA binding in monocytes compared with macrophages, despite equivalent expression of STAT5 and the GM-CSF receptor machinery,22 which may provide an explanation for our finding of increased production of sVEGFR-1 by GM-CSF–stimulated monocytes compared with mature macrophages.
With no apparent crosstalk between the HIF proteins and the JAK/STAT pathway in this paradigm, we are left with investigating other avenues for the mechanism of synergy between GM-CSF and hypoxia with respect to monocyte sVEGFR-1 production. One possibility is the action of microRNAs (miRs). It has been shown that hypoxia regulates miR profiles in various cell types,23 and it is conceivable that specific miRs target repressors of the GM-CSF signaling pathway. In particular, the Suppressors of Cytokine Signaling (SOCS) proteins negatively regulate JAK/STAT activity by inhibiting the phosphorylation of STAT by JAK.24 Stimulation of monocytes with GM-CSF at normoxia for 24 hours results in an approximately 20-fold increase in mRNA for SOCS1, which is known to inhibit the JAK2/STAT5 interaction; however, SOCS1 mRNA is not induced when monocytes are stimulated with GM-CSF at hypoxia (data not shown). In silico analysis of the promoter of SOCS1 indicates potential binding sites for more than 30 different miRs, several of which were found to be up-regulated by hypoxia in other cell types.23 Our laboratory is currently investigating the role of miRs in the regulation of GM-CSF activity by hypoxia in mononuclear phagocytes.
We next sought to delineate the individual contributions of HIF-1α and HIF-2α in this paradigm. The exact contribution of HIF-1α and HIF-2α to the regulation of hypoxic gene expression appears to vary between cell types. In human breast cancer cells, for example, HIF-1α mediates the induction of virtually all hypoxia-activated genes, whereas HIF-2α is preferentially up-regulated in hypoxic renal tumor cells.25 Although HIF-1α is the predominant regulator of VEGF production during hypoxia in almost all cell types, including macrophages,15 HIF-2α is thought to be the main form of HIF up-regulated by tumor-associated macrophages,26,27 and recent studies have revealed an important role for HIF-2α in the transcriptional response to hypoxia in macrophages.5 To determine the regulatory role of HIF-1α or HIF-2α in sVEGFR-1 production, we used mice containing loxp sequences flanking the HIF-1α gene and expressing Cre recombinase from the LysM promoter. These HIF-1αflox/flox/LysMcre mice contain a targeted deletion of HIF-1α in the myeloid lineage. Our data suggest that HIF-1α does not regulate sVEGFR-1 production from GM-CSF–stimulated macrophages exposed to hypoxia, as sVEGFR-1 mRNA and protein were unchanged in the HIF-1α−/− macrophages after stimulation. In contrast, the HIF-1α deficiency in macrophages resulted in a significant reduction of both VEGF mRNA and protein, confirming the role of HIF-1α in VEGF production at hypoxia. Because deletion of HIF-1α had no effect on sVEGFR-1 production, we next targeted HIF-2α. We transfected bone marrow–derived macrophages with an siRNA cocktail specific for HIF-2α and subjected them to hypoxia and GM-CSF. A 30% reduction in HIF-2α mRNA resulted in an approximately 2.8-fold reduction in sVEGFR-1 mRNA and approximately 4-fold reduction in sVEGFR-1 protein in response to hypoxia. Notably, VEGF mRNA was unaffected in these cells. Because such a modest depletion of HIF-2α mRNA significantly affected sVEGFR-1 protein production using siRNA targeting, we derived macrophages from the bone marrow of LysMcre control mice and HIF-2α+/flox/LysMcre mice, which produce only 50% of the HIF-2α. We observed an inhibition of sVEGFR-1 production without effects on the transcriptional activity of VEGF, similar to the siRNA study (supplemental Figure 1). Unfortunately, attempts to confirm the connection between HIF-2α expression and antiangiogenic potential in an in vitro angiogenesis tube formation assay were inconclusive, as the murine endothelial cells formed tube-like structures in negative control conditions (minimal media devoid of serum or growth factors). We are currently optimizing alternative methods to validate HIF-2α as a key antiangiogenic molecule in the murine system.
A link between HIF-α activation and sVEGFR-1 secretion has been previously reported. The hydroxylation of the HIF-α proteins at normoxia is carried out by a family of prolyl hydroxylases known as the prolyl hydroxylase domain (PHD) proteins. Mazzone et al illustrated that a haplodeficiency in PHD2 stabilized HIF-1α and HIF-2α and induced sVEGFR-1 production within the tumor microenvironment.28 In this report, the growth of the primary tumor was unaffected, but sVEGFR-1 acted to normalize the leaky and misshaped vasculature normally associated with high levels of VEGF, inhibiting tumor cell intravasation and metastasis. Importantly, the levels of sVEGFR-1 produced in this model were far less than we observed in response to administration of superphysiologic doses of GM-CSF,19 which may explain the failure of PHD2 haplodeficiency to impede the growth of the primary tumor.
High numbers of mononuclear phagocytes within the hypoxic tumor microenvironment are well known to contribute to angiogenesis and tumor progression through the production of the proangiogenic factor VEGF. This study highlights the possibility that hypoxia, canonically thought to promote angiogenesis, can stimulate antiangiogenic molecules from macrophages within a GM-CSF-rich environment. To our knowledge, these data are the first to demonstrate an antiangiogenic role for mononuclear phagocytes under hypoxic conditions. Furthermore, these results demonstrate opposing roles for the HIFs in tumor angiogenesis, with HIF-1α exhibiting proangiogenic behavior via its effects on VEGF secretion, whereas HIF-2α exhibits antiangiogenic behavior by inducing the production of the endogenous angiogenesis inhibitor, sVEGFR-1.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the National Institutes of Health (R01 HL067167, C.B.M.; T32 HL07946-06, J.M.R.) and the National Cancer Institute (K99 CA131552-01, T.D.E.).
National Institutes of Health
Authorship
Contribution: T.D.E., J.M.R., and C.B.M. designed research and wrote the manuscript; T.D.E., J.M.R., H.L., and T.O. performed research; and all authors reviewed the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Clay B. Marsh, Dorothy M. Davis Heart and Lung Research Institute, Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Internal Medicine, Ohio State University College of Medicine, 370 W 9th Ave, Columbus, OH 43210; e-mail: Clay.Marsh@osumc.edu.
References
Author notes
T.D.E. and J.M.R. contributed equally to this study.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal