Abstract
Mice with a severe genetic deficiency of protein C (PC), PC−/−PC(tg4), display enhanced susceptibility to lethal effects of gram-negative endotoxemia induced by lipopolysaccharide (LPS), whereas mice severely deficient in tissue factor (TF), TF−/−hTF(tg), are protected from LPS-mediated lethality. In this study, we show that a simultaneous severe deficiency of TF protected low-PC mice from LPS-induced death, resulting in a survival profile similar to that experienced by wild-type (WT) mice. Plasma and whole blood coagulation assays, the latter measured by thromboelastography, demonstrated development of coagulopathies in LPS-treated mice, which were more severe in the case of the doubly deficient TF−/−hTF(tg)/PC−/−PC(tg4) mice, mainly reflecting earlier signs of disseminated intravascular coagulation in this latter cohort. Markers of inflammation were also elevated in response to LPS in both groups of mice at times just preceding death. We conclude that whereas coagulopathies are more exacerbated in LPS-treated TF−/−hTF(tg)/PC−/−PC(tg4) mice, the lowering of TF levels in mice with an accompanying severe PC deficiency confers protection against death compared with mice with a single severe PC deficiency. This suggests that proteases generated as a result of factor VIIa/TF–mediated thrombin generation play a mechanistic role in the enhanced lethality seen under very low PC conditions in an endotoxemia model in mice.
Introduction
The hemostasis system is intricately involved in the innate immune response, in part, via linkages between coagulation and inflammation. The interactions between these systems are profoundly displayed in sepsis/endotoxemia, wherein pathogenic challenge up-regulates tissue factor (TF) on monocytes1 and endothelial cells,2 that not only leads to a hypercoagulation potential, but also provides several proteases (eg, thrombin) that are capable of signaling the inflammatory response through interaction with cellular receptors.3 The resulting inflammatory mediators down-regulate anticoagulant activated protein C (aPC) generation and inhibit fibrinolysis, thus enhancing the hypercoagulable state. In severe cases of sepsis, lethal disseminated intravascular coagulation (DIC) occurs. Some success in treatment of severe sepsis has been achieved with aPC administration,4 which is grounded in the anticoagulant, profibrinolytic, anti-inflammatory, endothelial barrier protective, and antiapoptotic activities of aPC.5,6
A deficiency of PC is symptomatic in humans, resulting in conditions such as purpura fulminans,7 and in experimental animals in a variety of challenge models.6 It has also been previously shown that embryos with total deficiencies of PC (PC−/−) do not live beyond the early neonatal stage,8 but embryos can survive to adulthood with PC levels greater than approximately 1% of wild-type (WT).9 In addition, maternal plasma PC levels are critical to embryonic survival. In that regard, we found that minimal maternal PC concentrations, approximating 20% of WT values, are needed for successful maintenance of pregnancy.9 However, simultaneous genetic attenuation of both PC and TF expression corrects the unfavorable pregnancy outcomes found in mothers with very low PC levels.10 Similarly, the early embryonic lethalities of mice with inactivations of the genes for endothelial cell PC receptor (EPCR−/−)11 or thrombomodulin (TM−/−)12 are also corrected when combined with mouse lines displaying a severe deficiency of TF, allowing survival to adulthood of EPCR−/−/TF−/−hTF(tg)13 or TM−/−/TF−/−hTF(tg)14 mice. The presence of TF, TM, and EPCR on giant trophoblast cells and the requirement for maternal plasma PC for favorable pregnancy outcomes indicate that lack of control of TF-mediated thrombin formation at the feto-maternal interface can result in defective embryogenesis.
Based on the protection toward low PC-mediated pregnancy failure afforded by a simultaneous very low expression of TF, we undertook a study to determine whether greatly attenuated TF expression would also correct the severe response to lipopolysaccharide (LPS)–induced endotoxemia found in very low PC expressing mice, since many of the same thrombotic and inflammatory consequences of uncontrolled TF-mediated thrombin formation occur in both conditions. The results of this study are presented herein.
Methods
Mice
Lines of mice that express PC and TF from nontargeted single allelic transgenic (tg) insertions of murine PC and human TF cDNAs, respectively, with the endogenous genes encoding these proteins totally inactivated through breeding strategies with mice carrying null alleles for these genes, have been described previously.9 Singly deficient PC−/−PC(tg4) and PC−/−PC(tg785) mice each express PC at 1% and 3% of WT levels, respectively,9 and TF−/−hTF(tg) mice (provided by Dr Nigel Mackman, University of North Carolina) express TF at approximately 1% of WT adult values.15 These lines of mice, fully backcrossed in the C57Bl/6 strain, were crossbred as PC−/−PC(tg4)/TF−/−hTF(tg) males and females to yield the desired offspring of the same genotype. Although PC−/−PC(tg4) female mice are not able to maintain pregnancies beyond early embryogenesis, normal reproduction was obtained with the doubly deficient lines,10 thus providing the final mice in a single intermating step.
C57Bl/6 mice with targeted heterozygous (Fg+/−) and homozygous (Fg−/−) inactivation of the Fg γ-chain have been described previously.16
Endotoxemia
Male WT and PC−/−(tg4)/TF−/−hTF(tg) mice, at 8-12 weeks of age, were injected intraperitoneally with a bolus of LPS (Escherichia coli endotoxin, serotype 0111:B4) at 10 μg LPS/g body weight. Survival was monitored in one set of mice and individuals of another group were killed at 0 and 12 hours after LPS administration for blood collection. All experimental protocols were approved by the University of Notre Dame Institutional Animal Care and Use Committee.
Plasma assays
Mice were anesthetized with rodent cocktail (0.075 mg ketamine, 0.015 mg xylazine, and 0.0025 mg aceprozamine per gram weight of mouse) and blood taken from the inferior vena cava in 4% sodium citrate. Platelets were counted using a VetScan HMT Hematology Analyzer (ABAXIS). Whole blood was then centrifuged for 10 minutes at 4000 rpm and plasma removed. Prothrombin times (PTs), activated partial thromboplastin times (aPTTs), and fibrinogen (Fg) assays were performed by clotting assays with the STart 8 Coagulation Analyzer using STA-neoplastine, STA-PTT, and Fibriprest kits (Diagnostica Stago), respectively. The activities of Factor (F)VIII, FIX, and FXI were determined by clotting assays with the STA-Deficient FVIII, FIX, and FXI kits, respectively (Diagnostica Stago). The D-dimer enzyme-linked immunosorbent assay (ELISA) kit was purchased from Diagnostica Stago. Thrombin-antithrombin-III (TAT) levels were determined with antibodies from ERL. Plasma PC was quantitated by an ELISA using in-house antibodies specific for murine PC. Plasma levels of macrophage-inflammatory protein-2 (MIP-2) and interleukin (IL)-6 were measured by ELISA using commercial kits (R&D Systems).
Thromboelastography
Thromboelastography (TEG) measurements were made on 4% citrated whole blood using an automated TEG Thromboelastograph 5000 Hemostasis System (a gift from Haemoscope Corp). Data analyses were performed with TAS Version 4.3 Software (Haemoscope). After first running the control samples provided by the manufacturer (level 1 and level 2) to ascertain that instrument performance was satisfactory, 20 μL of 0.2M CaCl2 was added to the cup followed by 360 μL of citrated whole blood. Measurements were then made for 1.5-2.0 hours. The automated parameters obtained were: R, the time from the start of the reaction until a measurable clot was detected, corresponding to clot initiation; K, the time from R until a specific clot firmness (a 20-mm signal) was achieved, indicating amplification of clot formation; A°, the angle in degrees, which reflects the rate and propagation of clot formation; and MA, the maximum amplitude (mm) of clot shear elasticity, which depends upon the contributions of cross-linked fibrin and platelets to clot strength.
Statistical analysis
Data are reported as mean ± SEM. Student t tests were performed to compare values for 2-tailed comparisons, using a value of P ≤ .05 to void the null hypothesis.
Results
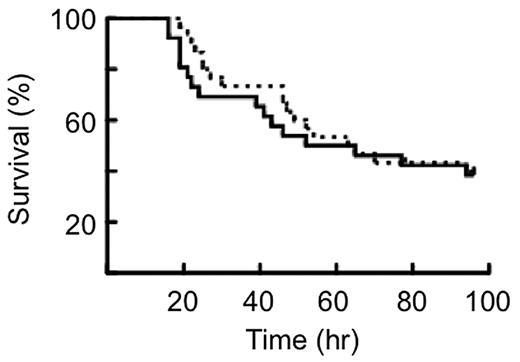
The survival characteristics of WT and PC−/−PC(tg4)/TF−/−TF(tg) mice after administration of a lethal dose of 10 μg/g LPS are illustrated by the Kaplan-Meier plots in Figure 1. No significant differences were found between the 2 groups of mice using the log-rank and Wilcoxon tests.
Kaplan-Meier plot of the survival of mice to LPS. Survival curves of WT mice (solid line, n = 30) and PC−/−PC(tg4)/TF−/−hTF(tg) mice (dashed line, n = 24) treated with 10 μg LPS/g body weight. No significant difference in survival was found between the 2 strains of mice.
Kaplan-Meier plot of the survival of mice to LPS. Survival curves of WT mice (solid line, n = 30) and PC−/−PC(tg4)/TF−/−hTF(tg) mice (dashed line, n = 24) treated with 10 μg LPS/g body weight. No significant difference in survival was found between the 2 strains of mice.
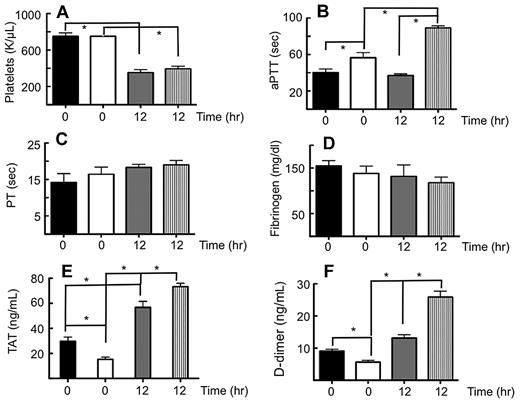
Coagulation assays were performed on resting WT and PC−/−PC(tg4)/TF−/−TF(tg) mouse blood and plasma, as well as on these same groups of mice immediately preceding the death spiral after administration of a lethal dose of LPS (12 hours after 10 μg LPS/g body weight). Platelets were at normal levels in both groups of resting mice, but were significantly reduced to approximately one-half of the resting levels post-LPS in both groups of mice (Figure 2A). The aPTT of resting PC−/−PC(tg4)/TF−/−TF(tg) mice was slightly elevated compared with WT mice. The aPTTs increased in both sets of mice at 12 hours post-LPS, compared with their resting counterparts, and that of PC−/−PC(tg4)/TF−/−TF(tg) mice showed a greater increase under these conditions than that of WT mice (Figure 2B). Thus, in addition to the expected coagulopathy from the very low TF, PC−/−PC(tg4)/TF−/−TF(tg) mice also possessed a significant coagulation dysfunction from deficiencies in the intrinsic system. In agreement with this, whereas FXI values did not change from WT levels at 12 hours LPS treatment, FVIII levels were diminished by more than 50% at 12 hours post-LPS compared with resting PC−/−PC(tg4)/TF−/−TF(tg) mice, which were approximately equal to resting and LPS-treated WT mice (not shown). FIX levels were also diminished by this same amount in both WT and PC−/−PC(tg4)/TF−/−TF(tg) mice at 12 hours after LPS administration, and to a greater extent in the latter group of mice (not shown). On the other hand, upon addition of a source of TF to the plasma, clot times, as reflected by the PTs, did not markedly change in either group of mice, with or without LPS (Figure 2C), suggesting that the extrinsic system is otherwise intact.
Plasma coagulation parameters from mice administered a lethal dose of LPS. WT mice and PC−/−PC(tg4)/TF−/−hTF(tg) mice were administered 10 μg LPS/g body weight. (A). Blood was collected at t = 0 and t = 12 hours in citrate and platelet counts determined. Plasma was then obtained for determinations of the (B) aPTT, (C) PT after addition of thromboplastin, (D) clottable Fg levels, (E) TAT levels, and (F) D-dimer levels. Black bars, resting WT mice; white bars, resting PC−/−PC(tg4)/TF−/−hTF(tg) mice; gray bars, WT mice at 12 hours post-LPS; vertical striped bars, PC(tg4)/TF−/−hTF(tg) mice at 12 hours post-LPS. *n = 8-10, P < .05.
Plasma coagulation parameters from mice administered a lethal dose of LPS. WT mice and PC−/−PC(tg4)/TF−/−hTF(tg) mice were administered 10 μg LPS/g body weight. (A). Blood was collected at t = 0 and t = 12 hours in citrate and platelet counts determined. Plasma was then obtained for determinations of the (B) aPTT, (C) PT after addition of thromboplastin, (D) clottable Fg levels, (E) TAT levels, and (F) D-dimer levels. Black bars, resting WT mice; white bars, resting PC−/−PC(tg4)/TF−/−hTF(tg) mice; gray bars, WT mice at 12 hours post-LPS; vertical striped bars, PC(tg4)/TF−/−hTF(tg) mice at 12 hours post-LPS. *n = 8-10, P < .05.
The coagulopathy reflected by the elevated aPTT is not due to diminishment of Fg levels, which become depleted at advanced stages of sepsis/endotoxemia. The data of Figure 2D, which show only a slight decrease in Fg at 12 hours post-LPS in PC−/−PC(tg4)/TF−/−TF(tg) mice, is not sufficient, in itself, to result in such elevated aPTTs, and do not reach significance compared with resting PC−/−PC(tg4)/TF−/−TF(tg) mice. Whereas resting PC−/−PC(tg4)/TF−/−TF(tg) mice possess lower TAT (Figure 2E) and D-dimer (Figure 2F) levels than WT mice, these parameters are significantly higher at 12 hours post-LPS than those of their resting controls. Furthermore, after LPS challenge, both TAT and D-dimer levels of PC−/−PC(tg4)/TF−/−TF(tg) mice are more elevated than those of challenged WT mice. This suggests that progression toward DIC after LPS administration is more rapid in the PC−/−PC(tg4)/TF−/−TF(tg) group.
The expected decline of PC in WT mice occurs at 12 hours post-LPS, and the very low level of PC in resting PC−/−PC(tg4)/TF−/−TF(tg) mice is further decreased below detection under these conditions (Figure 3A). Two markers of inflammation, MIP-2 and IL-6, are highly elevated in both groups of mice at 12 hours after LPS administration (Figure 3B-C), compared with the untreated controls. In the case of MIP-2, the increases for WT and PC−/−PC(tg4)/TF−/−TF(tg) mice are similar to each other (Figure 3B), whereas for IL-6, there is a somewhat greater elevation for WT mice (Figure 3C). This is likely due to the single 12-hour time point sampled, since cytokine responses would be expected to be time-dependent, but, more importantly, inflammation is up-regulated in both of these groups upon LPS administration.
The inflammatory response of mice to a lethal dose of LPS. Plasma was obtained from WT mice and mice at 12 hours post-LPS (10 μg LPS/g body weight). The levels of (A) PC, (B) MIP-2, and (C) IL-6 were determined by ELISA. Black bars, resting WT mice; white bars, resting PC−/−PC(tg4)/TF−/−hTF(tg) mice; gray bars, WT mice at 12 hours post-LPS; vertical striped bars, PC(tg4)/TF−/−hTF(tg) mice at 12 hours post-LPS. *n = 6-8, P < .05.
The inflammatory response of mice to a lethal dose of LPS. Plasma was obtained from WT mice and mice at 12 hours post-LPS (10 μg LPS/g body weight). The levels of (A) PC, (B) MIP-2, and (C) IL-6 were determined by ELISA. Black bars, resting WT mice; white bars, resting PC−/−PC(tg4)/TF−/−hTF(tg) mice; gray bars, WT mice at 12 hours post-LPS; vertical striped bars, PC(tg4)/TF−/−hTF(tg) mice at 12 hours post-LPS. *n = 6-8, P < .05.
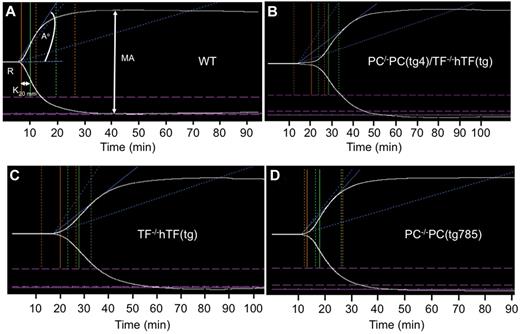
Whole blood coagulation responses in these mouse groups were evaluated by TEG analyses in order to assess the overall impact of LPS administration on platelet cross-linked fibrin interactions that stabilize the clot. The TEG results show greatly prolonged R, K, and A° values with resting PC−/−PC(tg4)/TF−/−TF(tg) mice (Figure 4B, Table 1), compared with WT mice (Figure 4A), demonstrating the importance of low TF to clotting time of whole blood. The importance of TF to the TEG results was confirmed by the similar data obtained with singly deficient TF−/−TF(tg) mice (Figure 4C, Table 1). We were unable to successfully breed sufficient numbers of single deficient PC−/−PC(tg4) mice for similar analyses, but did perform these measurements on blood of PC−/−PC(tg785) mice, which had slightly higher levels of PC (∼3% of WT) than PC−/−PC(tg4) mice (Figure 4D). The TEG values for PC−/−PC(tg785) blood were not greatly different from those of WT mice, except for a tendency toward longer clot formation, perhaps due to a beginning of consumption of intrinsic factors, as has been seen earlier with this mouse line.17 MA values for these resting mice are not greatly different from WT mice (Table 1), demonstrating that platelet-fibrin interactions are nearly normal.
Thromboelastograms of recalcified mouse whole blood samples. (A) Resting WT blood. (B) Resting PC−/−PC(tg4)/TF−/−hTF(tg) blood. (C) Resting TF−/−hTF(tg) blood. (D) Resting PC−/−PC(tg785) blood. The parameters measured are shown in (A) and include R (min), K (min), A° (degrees), and MA (mm). *P < .05.
Thromboelastograms of recalcified mouse whole blood samples. (A) Resting WT blood. (B) Resting PC−/−PC(tg4)/TF−/−hTF(tg) blood. (C) Resting TF−/−hTF(tg) blood. (D) Resting PC−/−PC(tg785) blood. The parameters measured are shown in (A) and include R (min), K (min), A° (degrees), and MA (mm). *P < .05.
TEG-derived values for global whole blood coagulation parameters of transgenic mouse lines
| . | R (minutes) . | K (minutes) . | A (°) . | MA (mm) . | n . |
|---|---|---|---|---|---|
| WT | |||||
| 0 | 7.3 ± 2.1 | 3.3 ± 1.2 | 52.4 ± 3.4 | 64.5 ± 2.1 | 9 |
| 12 hours | 19.8 ± 3.5* | 11.7 ± 2.5* | 18.6 ± 3.7* | 30.8 ± 4.1* | 6 |
| PC−/−PC(tg4)/TF−/−hTF(tg) | |||||
| 0 | 21.3 ± 1.9* | 8.9 ± 1.2* | 25.3 ± 2.2* | 56.3 ± 3.0* | 6 |
| 12 hours | > 120† | 0† | 0† | 0† | 6 |
| PC−/−PC(tg785) | 13.0 ± 3.2* | 4.8 ± 2.0 | 48.3 ± 4.7 | 65.0 ± 3.2 | 8 |
| TF−/−hTF(tg) | 19.9 ± 2.1* | 7.4 ± 1.1* | 31.5 ± 3.2* | 63.8 ± 4.6 | 7 |
| Fg−/− | > 120‡ | 0‡ | 0‡ | 0‡ | 6 |
| Fg+/− | 4.7 ± 0.9 | 1.8 ± 0.3 | 64.4 ± 3.3* | 61.3 ± 1.5 | 5 |
| . | R (minutes) . | K (minutes) . | A (°) . | MA (mm) . | n . |
|---|---|---|---|---|---|
| WT | |||||
| 0 | 7.3 ± 2.1 | 3.3 ± 1.2 | 52.4 ± 3.4 | 64.5 ± 2.1 | 9 |
| 12 hours | 19.8 ± 3.5* | 11.7 ± 2.5* | 18.6 ± 3.7* | 30.8 ± 4.1* | 6 |
| PC−/−PC(tg4)/TF−/−hTF(tg) | |||||
| 0 | 21.3 ± 1.9* | 8.9 ± 1.2* | 25.3 ± 2.2* | 56.3 ± 3.0* | 6 |
| 12 hours | > 120† | 0† | 0† | 0† | 6 |
| PC−/−PC(tg785) | 13.0 ± 3.2* | 4.8 ± 2.0 | 48.3 ± 4.7 | 65.0 ± 3.2 | 8 |
| TF−/−hTF(tg) | 19.9 ± 2.1* | 7.4 ± 1.1* | 31.5 ± 3.2* | 63.8 ± 4.6 | 7 |
| Fg−/− | > 120‡ | 0‡ | 0‡ | 0‡ | 6 |
| Fg+/− | 4.7 ± 0.9 | 1.8 ± 0.3 | 64.4 ± 3.3* | 61.3 ± 1.5 | 5 |
P < .05 from WT mice.
P < .05 from all mice tested, except when compared with Fg−/−.
P < .05 from all mice tested, except when compared with PC−/−(tg4)/hTF−/−(tg) at 12 hours post-LPS.
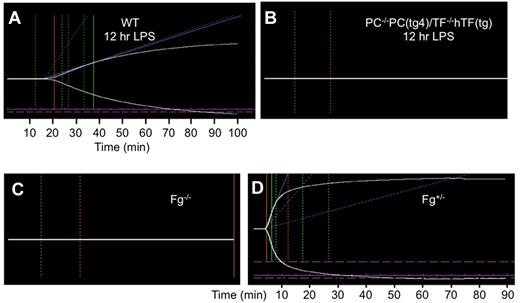
At 12 hours post-LPS, significant changes are seen in whole blood clotting of WT (Figure 5A) and PC−/−PC(tg4)/TF−/−TF(tg) (Figure 5B) mice. Under these conditions, WT mouse blood possesses greatly prolonged R and K values, a lower A°, and a concomitantly reduced MA, compared with resting mice (Table 1). With regard to PC−/−PC(tg4)/TF−/−TF(tg) mice, the R value was delayed beyond 120 minutes. This latter effect would obviously occur were Fg levels depleted (Figure 5C), as occurs with DIC in severe sepsis/endotoxemia, but this was not the case to any large extent with these mice (Figure 2C). Even if Fg was diminished by 50% (Fg+/−) as a result of LPS treatment, the TEG results would have been similar to WT mice (Figure 5D, Table 1). On the other hand, previous TEG studies on FVIII−/− mouse blood have shown abnormal R and K values,18 which is consistent with our data demonstrating large effects on whole blood clotting with consumption of intrinsic factors. In the case of LPS-treated PC−/−PC(tg4)/TF−/−TF(tg) mice, both the FVIII and FIX levels are reduced, exacerbating the abnormal clotting of whole blood.
Thromboelastograms of recalcified mouse whole blood samples. (A). Blood from WT mice treated with LPS (10 μg LPS/g body weight) for 12 hours. (B). Blood from PC−/−PC(tg4)/TF−/−hTF(tg) mice treated with LPS (10 μg LPS/g body weight) for 12 hours. (C). Blood from resting Fg−/− mice. (D). Blood from resting Fg+/− mice. The parameters measured are shown in (Figure 4A) and include R (min), K (min), A° (degrees), and MA (mm).
Thromboelastograms of recalcified mouse whole blood samples. (A). Blood from WT mice treated with LPS (10 μg LPS/g body weight) for 12 hours. (B). Blood from PC−/−PC(tg4)/TF−/−hTF(tg) mice treated with LPS (10 μg LPS/g body weight) for 12 hours. (C). Blood from resting Fg−/− mice. (D). Blood from resting Fg+/− mice. The parameters measured are shown in (Figure 4A) and include R (min), K (min), A° (degrees), and MA (mm).
Discussion
It is generally agreed that a major mechanism in Gram-negative septic coagulopathy is initiated by interaction of the bacterial endotoxin, LPS, with toll-like receptor 4 on monocytes, thus elevating TF and establishing a hypercoagulable state. The importance of enhancement of TF in the septic response is emphasized by findings that mice with diminished TF19 or FVII20 are protected from LPS-mediated lethality, as are baboons with a TF blockade after E coli–induced sepsis.21 Proteases that accrue during stimulation of the coagulation cascade interact with cellular protease activated receptors, enhancing the inflammatory response via cell signaling. As a result, PC levels are diminished, further enhancing inflammation and the hypercoagulable state (for a review, see6 ). Many complex innate immune sequelae result from these processes, such as loss of the barrier function of the endothelium, that aggravate the condition, which, under compromised controlled conditions, leads to a storm of coagulation, inflammatory, and hemodynamic systemic responses that result in fatal septicemia/endotoxemia.
To isolate the roles of PC and TF in lethal endotoxemia, mouse models have been used wherein the effects of low PC and low TF on various endpoints of the disease were examined. It has been shown that genetically induced attenuation of PC levels in mice resulted in severe hypersensitivity of the mice to lethal, as well as coagulopathic and inflammatory responses, of LPS.17 Further, genetic diminishment of TF in similar mouse models protected against LPS-induced lethality, and related elevated hypercoagulation and inflammation markers.19 Thus, we hypothesized that lowering of TF would protect PC-depleted mice from lethal effects of LPS, and we examined this hypothesis via simultaneous genetic attenuation of TF and PC in mice. Support for this hypothesis was present in studies that showed that TF-mediated thrombin generation was a dominant factor in pregnancy loss in PC-pathway deficient mice, due to poorly controlled inflammation and coagulation.13,14 Thus, we felt that similar effects would influence LPS-induced lethality in a well-utilized mouse model for lethal endotoxemia. Indeed, we show herein that the doubly deficient mouse line, PC−/−PC(tg4)/TF−/−hTF(tg), presented with LPS-induced lethality similar to WT mice and that a simultaneous diminishment of TF substantially protected PC-deficient mice from LPS-induced death. This suggested that the adverse effects of low PC were counterbalanced by the favorable effects of low TF in this model of endotoxemia.
The coagulation and inflammation parameters that influence the progression of acute endotoxemia in both WT and PC−/−PC(tg4)/TF−/−hTF(tg) mice were next examined. With regard to coagulation parameters, platelet numbers were similar in both groups of resting mice and were reduced similarly at 12 hours post-LPS. This is a normal thrombocytopenic response of the animals to LPS. The aPTT of resting PC−/−PC(tg4)/TF−/−hTF(tg) mice was slightly elevated compared with their WT counterparts, demonstrating that the expected lowering of the aPTT and elevated thrombin in PC-deficient mice was countered by the expected elevation of the aPTT by lowered thrombin levels in TF−/−hTF(tg) mice. At 12 hours post-LPS, the aPTT of WT mice was greater than that of resting WT mice, and that of PC−/−PC(tg4)/TF−/−hTF(tg) mice was more significantly increased over that of resting PC−/−PC(tg4)/TF−/−hTF(tg) mice. This suggests that in addition to extrinsic system abnormalities, due to very low TF in the PC−/−PC(tg4)/TF−/−hTF(tg) mice, intrinsic system dysfunction is also occurring in these groups at 12 hours post-LPS, due to consumption of intrinsic factor(s), thus elevating the aPTT. This was confirmed by the decreases observed in the FVIII and FIX levels as assessed by clotting assays post-LPS treatment. The increase seen in the TAT complex at 12 hours post-LPS in PC−/−PC(tg4)/TF−/−hTF(tg) mice, and of the fibrin split product D-dimer levels under these same conditions, also shows that thrombin was produced at times < 12 hours post-LPS, exacerbated by the low PC levels, and that fibrinolysis had also occurred. These increases in TAT and D-dimer levels are also consistent with the early emergence of DIC in these double-deficient mice with consumption of intrinsic clotting factors.
The above data suggest that measureable consumptive coagulopathy is occurring at a more rapid pace in PC−/−PC(tg4)/TF−/−hTF(tg) mice post-LPS, especially as related to intrinsic coagulation factors. In agreement with this view, clotting times, as measured by the PT, are not as severely affected by LPS under these conditions in either group of mice, since a source of exogenous TF (thromboplastin) is used in this assay. This shows that FVII, FX, FV, prothrombin, and Fg levels of the extrinsic/common pathways are functional at significant levels under these conditions. That the coagulopathies in PC−/−PC(tg4)/TF−/−hTF(tg) mice are not due to consumption of Fg is confirmed by measurement of plasma Fg concentrations that show only slightly diminished levels at 12 hours post-LPS. PC levels, which are expectedly very low in PC−/−PC(tg4)/TF−/−hTF(tg) mice, are undetectable in these mice at 12 hours post-LPS. In addition, as anticipated, the PC concentration decreases in WT mice at 12 hours after LPS administration, as has been found previously.
Low PC alone does not significantly elevate the levels of the inflammatory markers MIP-2 and IL-6 in resting mice, when accompanied by a simultaneous TF deficiency, perhaps due to the lower levels of thrombin present in resting PC−/−PC(tg4)/TF−/−hTF(tg) mice. However, when treated with LPS for 12 hours, both WT and PC−/−PC(tg4)/TF−/−hTF(tg) mice displayed greatly elevated levels of these cytokines, and concomitantly high levels of the TAT complex. This rise in thrombin, coupled with the lower PC levels, suggests an inflammatory state, as reflected in higher cytokine levels, in the LPS-treated groups.
To obtain a global perspective on the interaction of the different coagulation parameters, viz., coagulation factors, Fg, and platelets, in clot formation and stability, we used whole blood TEG analyses of these mouse groups. TEG is a viscoelastic bedside point of care assay of whole blood that determines the integrity of whole blood hemostasis and allows for the quantification of initiation, amplification, propagation, termination, and lysis of global whole blood clotting. The TEG has been used to predict blood component use in cardiac and transplant surgery, as well as in the trauma population requiring massive transfusion.22 In addition, the test has been used to determine prognosis in patients who have a septic coagulopathy.23 As a result, TEG analyses have been used as a goal-directed therapy guide to the use of blood products in trauma to maintain normal hemostasis,24 and has been similarly used in other maladies (eg, to monitor the effects of administration of rFVIIa in hemophilia A patients).25 A valuable extension of this type of work is to apply TEG to highly genetically controlled animal models of disease with various specific endpoints to establish the groundwork for testable principles of patient care during various disease states that involve coaglopathies.
Compared with WT plasma, R and K values are delayed in resting PC−/−PC(tg4)/TF−/−hTF(tg) mice, most likely due to the reduced initiation of extrinsic coagulation due to very low TF. K is also attenuated in these doubly mutated resting mice, thus slowing the kinetics of fibrin formation and cross-linking (A°). Platelet-fibrin interactions are only slightly inhibited, as the MA is not dramatically different in the resting PC−/−PC(tg4)/TF−/−hTF(tg) mice. Since platelet counts are approximately the same in resting WT and PC−/−PC(tg4)/TF−/−hTF(tg) mice, weakened fibrin formation, and possible cross-linking abnormalities, are likely responsible for the reduced MA value in this latter group of mice.
At 12 hours post-LPS treatment of WT mice, R and K are increased, again likely due to very low TF levels. This results in a lowered A° value. The MA value was significantly reduced in this mouse cohort, a result of the diminished platelet counts in the LPS-treated WT group and/or by a possible acquired platelet dysfunction known to occur with sepsis.26 A very profound effect of LPS administration on whole blood clotting of LPS-treated PC−/−PC(tg4)/TF−/−hTF(tg) mice was observed, wherein no coagulation was observed up to 120 hours after recalcification. This result is consistent with the prolonged aPTT data (Figure 2B), compounded by reduced platelets inhibiting a stable clot from forming.
In summary, whereas a large survival benefit for mice with a severe deficiency of PC is afforded by a simultaneous lowering of TF, a more severe coagulopathy, with earlier onset DIC, is found in these doubly deficient mice at times before death, compared with similarly challenged WT mice. However, this enhanced hypercoagulation is not predictive of earlier death for the PC−/−PC(tg4)/TF−/−hTF(tg) mice. Platelet reductions and a cytokine marker of inflammation are similar in the 2 groups of mice, showing that very low PC in itself does not exacerbate inflammation when thrombin formation via the extrinsic pathway is also inhibited. Thus, while a PC deficiency alone would enhance thrombin formation, ultimately leading to spontaneous phenotypes, such as premature pregnancy termination, DIC, and lowered survival rates of neonates,8 as well as challenge phenotypes, such as accelerated death from sepsis/endotoxemia,10,17 a simultaneous severe TF deficiency reverses these phenotypes and protects against low PC-mediated mortalities. While coagulopathies exist in these complex deficient mice, this malady is insufficient to terminate life. This may be due to the attenuation of coagulation proteases that interact with cellular receptors in the low TF animals. Lastly, these data provide insight into the mechanisms of the involvement of the coagulation cascade in sepsis/endotoxemia and suggest approaches on the clinical management of blood products in the treatment of inflammatory diseases, such as sepsis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Sarah van Houten and Dr Denise Smith for performing mouse plasma assays, the staff of the Freimann Life Sciences Center for animal breeding and husbandry services, and Ms Juan Fu for genotyping the mice.
This work was supported by grants HL01982 and HL073750 from the National Institutes of Health (to F.J.C.) and a grant from the Memorial Hospital Foundation of South Bend.
National Institutes of Health
Authorship
Contribution: F.J.C. managed the study, wrote the final paper, and prepared the table and figures; D.L.D. performed experiments and reduced the data; and V.A.P., R.M.N., and M.W. discussed the results, assisted in design of studies, and read and contributed to the drafts.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francis J. Castellino, W.M. Keck Center for Transgene Research, 230 Raclin-Carmichael Hall, University of Notre Dame, Notre Dame, IN 46556; e-mail: fcastell@nd.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal