In recent years, much has been learned about how the highly proinflammatory cytokines of the interleukin-1β (IL-1β) cytokine family are regulated. These cytokines differ from other cytokines in that they are produced as biologically inactive proforms in the cytosol upon transcriptional induction. In a second step, the protease caspase-1 mediates the cleavage and release of the IL-1β family of cytokines. Caspase-1 itself is produced as an inactive proform and also requires proteolytic activation, which is mediated by cytosolic multimolecular protein complexes that are termed inflammasomes. Among these, the NLRP3 inflammasome appears to be especially important as it is activated by a large variety of danger-associated signals including high concentrations of extracellular adenosine triphosphate, microbial pore-forming toxins, and aggregated materials.1 NLRP3 has been implicated in the pathogenesis of many inflammatory diseases; however, little is known about how the NLRP3 inflammasome can sense such a broad range of stimuli (see figure). The work by Meissner and colleagues contributes to our understanding of how caspase-1 is regulated and identifies IL-1 cytokines as potential therapeutic targets for inflammatory complications that are associated with chronic granulomatous disease (CGD).2
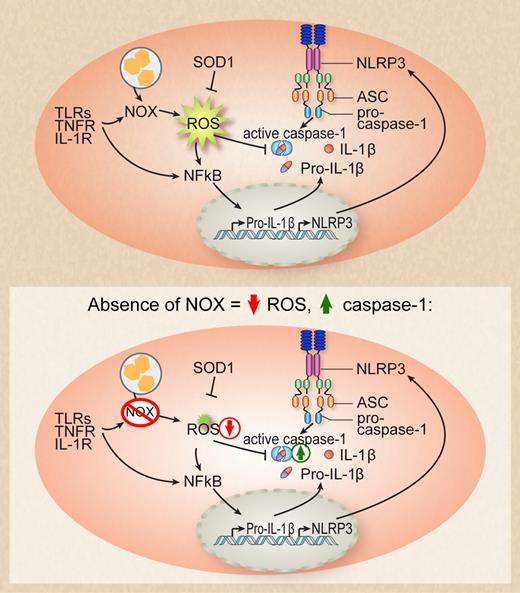
Activation of Toll-like receptor (TLR), TNF receptor, or IL-1 receptor as well as phagocytosis of particles activates phagosomal NOX enzymes that leads to the production of reactive oxygen species (ROS). ROS inhibits caspase-1 by mediating the covalent modification of redox-sensitive cysteine residues. In the absence of NOX enzymes, ROS production is diminished and inhibition of caspase-1 is relieved, leading to enhanced IL-1β processing. Professional illustration by Debra T. Dartez.
Activation of Toll-like receptor (TLR), TNF receptor, or IL-1 receptor as well as phagocytosis of particles activates phagosomal NOX enzymes that leads to the production of reactive oxygen species (ROS). ROS inhibits caspase-1 by mediating the covalent modification of redox-sensitive cysteine residues. In the absence of NOX enzymes, ROS production is diminished and inhibition of caspase-1 is relieved, leading to enhanced IL-1β processing. Professional illustration by Debra T. Dartez.
The primary immunodeficiency disease CGD is based on mutations in any of the components of the nicotinamide dinucleotide phosphate (NADPH) oxidase complex, which include the membrane-bound gp91phox and p22phox glycoproteins and the cytoplasmic components p47phox and p67phox.3 The functional outcome, and a feature that aids in the diagnosis of CGD, is that phagocytes of affected persons fail to generate the reactive oxidant superoxide anion and its metabolites, hydrogen peroxide, hydroxyl anion, and hypohalous acid. Because these highly reactive molecules can function in the phagosome to attack ingested microbes, it is not too surprising that CGD patients are hypersusceptible to certain bacteria and fungi and suffer from recurrent life-threatening infections. A second, etiologically less obvious hallmark of CGD is the frequent appearance of inflammatory lesions, such as chronic colitis or lupus-like symptoms.4 It is known that the inflammatory symptoms in CGD are of noninfectious origin and—in most cases—respond to immunomodulatory therapy such as glucocorticoids. However, the molecular basis for this hyperinflammatory state in CGD patients remains to be elucidated.
The current study by Meissner2 together with 2 other recent reports5,6 identify a likely mechanism that could explain why CGD patients frequently suffer from sterile inflammation. Meissner et al demonstrate that active caspase-1 is elevated in immune cells from asymptomatic CGD patients leading to increased secretion of biologically active IL-1β. Furthermore, immune cells derived from CGD patients presenting with hyperinflammatory conditions released copious amounts of IL-1β. This response as well as clinical symptoms of hyperinflammation could be counteracted with the IL-1 receptor antagonist anakinra.2 These results indicate that a functional phagocyte oxidase is not essential in human cells for caspase-1 activation and IL-1β secretion and that reactive oxygen species (ROS) down-modulate IL-1β rather than activating it in these cells.
In the past, the role of NADPH oxidases and ROS in the activation of caspase-1 has been quite controversial. In a previous, technically very elegant study, the same group reported that SOD1-deficient macrophages, which fail to detoxify reactive superoxide species and thus have much higher ROS levels, secrete much less active IL-1β upon inflammasome stimulation. The reason for this defective IL-1β cytokine response is that constitutively elevated ROS levels in SOD-1–deficient cells decrease the cellular redox potential and reversibly oxidize and glutathionylate cellular components including caspase-1. In vivo, SOD1-deficient mice are—similar to caspase-1–deficient mice—profoundly resistant to endotoxic shock.7 Consistent with these studies, a hyperinflammatory response is observed in mice that lack functional NADPH oxidases and caspase-1 activation is not impaired.7,8 Together, these studies provide persuasive evidence that ROS have an inhibitory effect on inflammasome activation.
Yet, in sharp contrast, it was also suggested that NADPH oxidases and ROS are critical for the activation of caspase-1 by danger signals, placing reactive oxygen species upstream of the activation of the NLRP3 inflammasome.9 The hypothesis that NADPH oxidase activity and ROS enable, rather than block, the activation of caspase-1 rests on experiments using small hairpin RNA knockdown of p22phox in a human monocytic cell line and on the use of pharmacologic ROS inhibitors.9 The reasons for the observed harsh discrepancies regarding the role of NADPH oxidases and ROS for NLRP3 inflammasome activation are not clear, but they could be explained by fundamental species differences, differential regulation of monocytes and macrophages, or the existence of functionally redundant NADPH enzymes.3 In this regard, it would be interesting to test whether riboflavin kinase–deficient cells can produce active IL-1β. Riboflavin kinase catalyzes the phosphorylation of riboflavin to flavin mononucleotide and is rate-limiting in the synthesis of flavin adenine dinucleotide (FAD). Because FAD is an essential prosthetic group of all NADPH oxidases one could test whether functionally redundant NADPH oxidases could influence NLRP3 inflammasome activation. ROS inhibitors should not solely be used as a proof of principle for the involvement of ROS in inflammasome activation for a given stimulator, as an inflammasome-independent effect on the transcriptional activation of IL-1β and tumor necrosis factor was demonstrated.6 Similarly, ROS inhibitors will likely also inhibit NLRP3 transcription, which was previously shown to be essential for NLRP3 activation in macrophages.10 Consequently, ROS inhibition of the NLRP3 inflammasome could be influenced by the effect on transcriptional depression of NLRP3 and not by an effect upstream of NLRP3. Apparently, the human “knockout” remains to be the best study system for identifying molecular disease mechanisms.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal