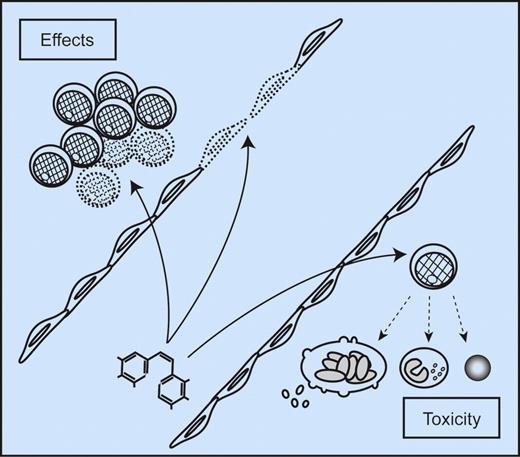
Bone marrow effects of antivascular agents for leukemia treatment. Vascular disrupting agents (basic structure of OXi4503 shown at the bottom left) can mediate their antileukemic effects through multiple mechanisms. First, the agents directly target the endothelial cells and destroy vasculature; this represents an indirect antileukemic activity by decreasing the supply of oxygen and nutrients and abrogating the supportive function of endothelial cells on leukemia cells. The influence of endothelial cells on vascular stem cell niches will then be particularly important. Second, the agents may have additional and direct effects on the AML cells. Finally, the antileukemic efficiency must be weighted against the risk and severity of bone marrow toxicity including suppression of remaining normal hematopoietic cells.
Bone marrow effects of antivascular agents for leukemia treatment. Vascular disrupting agents (basic structure of OXi4503 shown at the bottom left) can mediate their antileukemic effects through multiple mechanisms. First, the agents directly target the endothelial cells and destroy vasculature; this represents an indirect antileukemic activity by decreasing the supply of oxygen and nutrients and abrogating the supportive function of endothelial cells on leukemia cells. The influence of endothelial cells on vascular stem cell niches will then be particularly important. Second, the agents may have additional and direct effects on the AML cells. Finally, the antileukemic efficiency must be weighted against the risk and severity of bone marrow toxicity including suppression of remaining normal hematopoietic cells.
Angiogenesis in patients with acute myelogenous leukemia (AML) is evident by increased bone marrow microvessel density. The local cytokine network is probably an important regulator of the angiogenic process.2 However, the contribution of a single cytokine is difficult to predict and most likely varies between individual patients, as illustrated by a few examples: (1) the release of proangiogenic and antiangiogenic mediators—for example, vascular endothelial growth factor (VEGF) and CXCL8-11 chemokines—by primary human AML cells shows a wide variation between patients.3,4 (2) The role of the angiopoietin-1 (Ang-1)/Ang-2/Tie2 receptor system in leukemogenesis and chemosensitivity is still controversial. High pretreatment bone marrow levels of Ang-2 are associated with chemosensitivity and an adverse prognosis in AML in one study,5 and the reverse (associated with a favorable prognosis6 ) in another. The prognostic significance of Ang-2 has been reported to be influenced by VEGF expression.5,6
Despite the molecular and biologic heterogeneity of AML patients, the final effect of targeting intracellular signaling events in endothelial cells instead of the heterogeneous extracellular network is anticipated to be more predictable.1,7 The present study by Madlambayan et al combines the strategy of targeting endothelial cells with neutralization of proangiogenic VEGF.1 The vascular disrupting agent OXi4503 was used alone and in combination with the antiangiogenic agent bevacizumab in animal models of AML.1 This combined treatment was more effective than single-agent therapy; OXi4503 led to regression of human AML chloromas, though a thin layer of viable cells was left at the tumor periphery, which could thereupon almost be eliminated by bevacizumab. Furthermore, the tubulin-binding agent OXi4503 has both an indirect antileukemic effect through vascular disruption, and an additional cytotoxic effect directly on the AML cells. Vascular damage does not merely result in reduction of oxygen supply and nutrients, it also affects the leukemia-supportive function of endothelial cells, including support to leukemic stem cells in the vascular stem cell niches.8 The study by Madlambayan et al thereby suggests different therapeutic principles that should also be considered in the design of antiangiogenic treatment in human leukemia (see figure)1 : (1) direct targeting of microvascular endothelial cells is effective and the efficiency can be further increased by inhibition of key angioregulatory cytokines and (2) antiangiogenic drugs may also have direct antileukemic effects that contribute to the final therapeutic effect. In addition, VEGF seems to be a growth factor for primary human AML cells9 ; thus, even bevacizumab may represent an agent with both direct and indirect antileukemic effects at least for certain patients.
The question of systemic toxicity due to general endothelial cell damage or endothelial dysfunction remains. One method of decreasing systemic toxicity and increasing antileukemic efficiency could involve more selective targeted drug delivery to bone marrow. Microvascular endothelial cells in various organs have distinct gene expression profiles10 ; a detailed characterization of microvascular endothelial cells in leukemic bone marrow may open up the possibility of targeting the delivery of antiangiogenic agents to the bone marrow endothelium. By using this strategy, the antileukemic effect may be increased, but the consequence might then be enhanced toxicity toward normal hematopoietic cells (see figure).
Although the paper by Madlambayan et al presents promising results, several questions are still unresolved. First, although an antileukemic effect was demonstrated for primary AML cells for all patients included in the study, AML is a diverse disease. A larger follow-up study needs to examine whether resistant patients are present. This is especially important when targeting AML cells in their natural bone marrow environment. Second, this therapeutic strategy should be addressed in other experimental models including in vitro cocultures of primary human AML cells and endothelial cells.7 Finally, as outlined above, future studies should address the question of toxicity in more detail. Despite these unanswered questions, the findings by Madlambayan et al suggest that future studies of antiangiogenic/antivascular therapy in human AML should include direct targeting of endothelial cells,1 possibly in combination with cytokine-directed therapy. Identifying novel pharmacologic agents—that possess indirect antileukemic effects through inhibition of angiogenesis or vascular disruption as well as additional direct proapoptotic effects on the leukemic cells—should be a focus.
In summary, the study by Madlambayan et al strongly supports the hypothesis that antivascular therapy should be part of a treatment regimen that includes direct targeting of both endothelial and leukemic cells; future therapy should ensure targeting of different steps in angioregulation. Several therapeutic agents directed against different components of the angioregulatory network are now being developed, including antibodies against cytokine or cytokine receptors as well as other small molecule inhibitors directed against the downstream receptor signaling pathways. The paper by Madlambayan et al thus supports the use of these promising agents as a future therapeutic strategy for the treatment of AML, but optimal combination regimens in patients require further biologic as well as clinical studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■