Abstract
The antiphospholipid syndrome is defined by the presence of antiphospholipid antibodies in blood of patients with thrombosis or fetal loss. There is ample evidence that β2-glycoprotein I (β2GPI) is the major antigen for antiphospholipid antibodies. The autoantibodies recognize β2GPI when bound to anionic surfaces and not in solution. We showed that β2GPI can exist in at least 2 different conformations: a circular plasma conformation and an “activated” open conformation. We also showed that the closed, circular conformation is maintained by interaction between the first and fifth domain of β2GPI. By changing pH and salt concentration, we were able to convert the conformation of β2GPI from the closed to the open conformation and back. In the activated open conformation, a cryptic epitope in the first domain becomes exposed that enables patient antibodies to bind and form an antibody-β2GPI complex. We also demonstrate that the open conformation of β2GPI prolonged the activated partial thromboplastin time when added to normal plasma, whereas the activated partial thromboplastin time is further prolonged by addition of anti-β2GPI antibodies. The conformational change of β2GPI, and the influence of the autoantibodies may have important consequences for our understanding of the antiphospholipid syndrome.
Introduction
The antiphospholipid syndrome (APS) is defined as the presence of antiphospholipid antibodies in the blood of patients with thrombosis or fetal loss. The APS is one of the most common causes of acquired thrombophilia,1 especially at younger age. In 1990, it was shown that the so-called antiphospholipid antibodies do not recognize phospholipids directly but they interact with phospholipids via the plasma protein β2-glycoprotein I (β2GPI).2-4 However, the discovery of β2GPI as target for the autoantibodies did not provide a more in-depth knowledge on the underlying cause of the syndrome. It was unclear which metabolic pathway was disturbed by the autoantibodies because no physiologic function has convincingly been ascribed to β2GPI to date. Nevertheless, as antibodies against β2GPI can induce thrombosis in animal models,5-7 the protein β2GPI must hold an important functional clue to our understanding of the syndrome.
β2GPI is a highly abundant 43-kDa protein that circulates at a concentration of approximately 200 μg/mL. β2GPI consists of 326 amino acids arranged in 5 short consensus repeat domains.8,9 The first 4 domains contain 60 amino acids each, whereas the fifth domain has a 6-residue insertion and an additional 19-amino acid C-terminal extension. The extra amino acids are responsible for the formation of a large positively charged patch within the fifth domain of β2GPI10 that forms the binding site for anionic phospholipids. The anti-β2GPI antibodies that recognize an epitope located in the first domain correlate better with the thrombotic complications than antibodies directed against other domains.11-13 Antibodies directed against β2GPI have become one of the serologic markers characterizing the APS.14
After binding to anionic surfaces, β2GPI exposes a cryptic epitope that is recognized by the autoantibodies present in the APS.11,12,15,16 These antibodies only recognize β2GPI when it is bound to a surface and do not recognize β2GPI in solution. Moreover, no circulating immune complexes between antibodies and β2GPI have been detected in patient plasmas.17 This seems not to be the result of clearance of these complexes from plasma because plasma levels of β2GPI in antiphospholipid patients are the same as plasma levels of β2GPI in healthy persons.18
The crystal structure of β2GPI revealed a fishhook-like shape of the molecule.9,19 Part of the epitope that is recognized by autoantibodies consists of amino acids Arg39 and Arg43 in the first domain.11,12 The crystal structure indicated that these amino acids are expressed on the surface of domain I of β2GPI and are thus accessible for autoantibodies. This observation clearly contradicts with the absence of circulating β2GPI-antibody immune complexes in APS patients. The lack of binding of antibodies to β2GPI in solution fits better with a circular structure of β2GPI, a structure that was originally proposed by Koike et al.20 In this hypothetical structure, this potential epitope for the autoantibodies was not exposed on the outside of the molecule. The aim of our study was to investigate the structure of β2GPI as it occurs in the circulation and to elucidate the changes that occur within the protein when antiphospholipid antibodies interact with the protein.
Methods
Purification of human plasma β2GPI
Plasma β2GPI was isolated from fresh citrated human plasma as described previously.21 Purity of β2GPI was determined with sodium dodecyl sulfate (SDS)–polyacrylamide (PAA) gel electrophoresis (GE Healthcare). Purified plasma β2GPI showed a single band with a molecular mass of approximately 43 kDa under nonreducing conditions. The concentration of the protein was determined with the bicinchoninic acid protein assay (Thermo Fisher Scientific LSR). Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) analysis of the purified protein showed that it was more than 99.9% pure.
Negative staining transmission electron microscopy
β2GPI, in 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer, pH 7.4, was analyzed by negative staining electron microscopy as described previously.22 Solutions of β2GPI (5-10nM) with or without preincubation with mouse monoclonal antibody 3B7 against domain I of β2GPI were placed on a carbon-coated copper grid and negatively stained with uranyl formate (UF). A 0.75% UF solution was obtained by dissolving 37.5 mg of UF (BDH Chemicals) in 5 mL of boiling water, and stabilized with 5 μL of 5M NaOH. Grids were rinsed for 45 seconds with 100 μL of 20mM Tris, 150mM NaCl, pH 7.4, and blotted on filter paper. A total of 5 μL of sample was added to the grid, left for 45 seconds, and blotted off with a filter paper. The sample was washed twice with 100 μL of H2O drops and blotted off after each wash with a filter paper. Subsequently, the sample was stained for 3 seconds with 100 μL of 0.75% UF, transferred to another 100-μL drop of 0.75% UF, and then stained for an additional 15 to 20 seconds. Samples were visualized using a Jeol JEM 1230 transmission electron microscope operated at 60 kV accelerating voltage and recorded with a Gatan Multiscan 791 CCD camera using the software provided by the manufacturer (magnification 200 000×). Figures were prepared in Adobe Photoshop CS.
Conformational conversion of β2GPI
Conversion from the closed circular conformation of β2GPI to the open conformation was performed in a Slide-A-Lyzer 3.5K MWCO dialysis cassette (Thermo Fisher) by dialysis against 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid containing 1.15M NaCl, pH 11.5, for 48 hours at 4°C followed by dialysis with 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 150mM NaCl, pH 7.4. Conversion from the open conformation of β2GPI to the closed conformation was achieved by dialysis against 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 150mM NaCl, pH 3.4, for 48 hours at 4°C followed by dialysis against 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 150mM NaCl, pH 7.4. Samples were concentrated to a final concentration of 1.0 mg/mL β2GPI with an Ultrafree-0.5 Centrifugal Filter Unit (Millipore). Samples were snap-frozen in liquid nitrogen and stored at −80°C for analysis. After subjection to electron microscopy, 300 randomly selected β2GPI molecules per treatment were scored for their conformation.
Purification of anti-β2GPI antibodies from APS patients' sera
Anti-β2GPI antibodies from APS patients' sera were purified by applying sera, diluted 1:4 in phosphate-buffered saline (PBS), to a HiTrap Protein G column (GE Healthcare). Subsequently, the column was washed with 25 mL of PBS and eluted with 25 mL of 0.5M acetic acid, pH 2.8. Eluted samples were dialyzed against PBS and stored at −20°C for analysis. The patient plasmas used were positive for both lupus anticoagulant and anti-β2GPI antibodies and were not selected for anti–domain I positivity. The presence of lupus anticoagulant and anti-β2GPI antibodies was detected as described.23 Patient samples were collected with approval of the University of Utrecht ethics committee. Informed consent was obtained in accordance with the Declaration of Helsinki.
Immunosorbent assay of β2GPI
NUNC MaxiSorp High Protein-Binding Capacity ELISA plates (Nalge Nunc International) were coated with 1 μg/mL mouse monoclonal antidomain IV of β2GPI (21B2) or with 1 μg/mL purified APS patient IgG antibodies by incubation in 50mM carbonate buffer, pH 9.6, 100 μL in each well for 1 hour at room temperature (RT). After washing with 20mM Tris, 150mM NaCl, and 0.1% Tween-20, pH 7.4 (wash buffer), the plates were blocked by the addition of 200 μL per well of 3% bovine serum albumin (Sigma-Aldrich) in 20mM Tris, 150mM NaCl, pH 7.4 (blocking buffer) for 1 hour at RT. After washing the wells 3 times with wash buffer, 100 μL of circular or open β2GPI (0-1 μg/mL in blocking buffer) or pooled normal plasma was added to the wells and incubated for 1 hour at RT. Subsequently, after washing 3 times with wash buffer, 100 μL of peroxidase-conjugated anti-β2GPI antibodies (Affinity Biologicals; 1 μg/mL in blocking buffer) was added to the wells and incubated for 1 hour at RT. After the removal of unbound antibodies by washing with wash buffer, peroxidase activity of the bound antibody was measured by addition of 100 μL per well of TMB substrate (Tebu-Bio Laboratories). After 20 minutes, color development was stopped by adding an equal volume of 1.0M sulfuric acid. The optical density was measured at 450 nm with a spectrophotometer (Molecular Devices).
Cardiolipin and β2GPI binding assay
A total of 50 μL of a solution of cardiolipin (20μM; Sigma-Aldrich) in Tris-buffered saline (TBS), pH 7.4, was added to 96-well polyvinyl microtiter plates (Corning Life Sciences) and incubated overnight at 4°C. The plate was blocked by addition of 150 μL per well of 4% bovine serum albumin (Sigma-Aldrich) in TBS (blocking buffer) for 2 hours at 37°C. After washing the wells 3 times with TBS, 50-μL serial dilutions of closed and open β2GPI (0.4-50 μg/mL) were added to the wells and incubated for 2 hours at 37°C. Subsequently, after washing 3 times with TBS, 50 μL of purified APS patient IgG antibodies (5 μg/mL in blocking buffer) was added to the wells and incubated for 1 hour at 37°C. After removal of the unbound patient antibodies, 50 μL of goat anti–human IgG alkaline phosphatase-conjugated antibodies (Invitrogen), diluted 1:4000 in TBS, was added to the wells and incubated for 1 hour at 37°C. After washing 3 times with TBS, 50 μL per well of phosphatase substrate (Sigma-Aldrich) was added, and color development was stopped after 30 minutes by addition of 50 μL per well of 1.0M sulfuric acid. The optical density was measured at 405 nm.
Analysis of anticoagulant activity of β2GPI by aPTT
A diluted activated partial thromboplastin time (aPTT) clotting assay was used to analyze the anticoagulant activity of β2GPI. The aPTT was measured with Pathromtin SL and calcium chloride reagents (Siemens Healthcare Diagnostics). All coagulation measurements were carried out in a coagulometer (KC 10, Amelung). First, 80 μL of human pooled plasma (pool from > 200 healthy volunteers) and 20 μL of β2GPI (final concentration of 15 μg/mL) were incubated for 1 minute at 37°C. Subsequently, 100 μL of Pathromtin SL reagent (5× diluted in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer, pH 7.4) was added to the mixture and incubated for 3 minutes at 37°C. After the incubation, 100 μL of 25mM CaCl2 was added and the clotting time was recorded.
Protein digestion and MALDI-TOF MS
For detection of protected residues, equal amounts of open and circular β2GPI in 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 150mM NaCl, pH 7.4, were incubated for 12 hours at 37°C with 1:10 unmodified, sequence-grade trypsin (Roche Diagnostics), followed by reduction with 10mM dithiothreitol (30 minutes at 37°C) and alkylation of cysteines with 25mM iodoacetamide (30 minutes at RT). For optimal peptide sequence coverage, the proteins were first incubated with 0.1% Rapigest (Waters) before digestion. This mass spectrometry (MS)–compatible, acid-cleavable detergent was then removed by acidification (0.5% trifluoroacetic acid), followed by incubation at 37°C for 30 minutes and centrifugation for 10 minutes at 13 000g.
For MALDI analysis, the resulting peptide mixtures were dried in a vacuum centrifuge and dissolved in 1% formic acid and 60% acetonitrile. Subsequently, peptides were mixed 1:1 (vol/vol) with a solution containing 52mM α-cyano-4-hydroxycinnamic acid (Sigma-Aldrich) in 49% ethanol/49% acetonitrile/2% trifluoroacetic acid and 1mM ammonium acetate. Before dissolving, the α-cyano-4-hydroxycinnamic acid was washed briefly with chilled acetone. The mixture was spotted on a MALDI target plate and allowed to dry at room temperature. Reflectron MALDI-TOF MS spectra were acquired on a Micromass M@LDI (Wythenshawe). The acquired peptide spectra were analyzed with Masslynx 3.5 (Micromass).
Peptide and sequence analysis by oMALDI- or LC-MS/MS
MALDI-TOF MS/MS peptide sequencing was performed using a QSTAR-XL equipped with an oMALDI interface (Applied Biosystems/MDS Sciex). The generated peptide mixtures were also analyzed by LC-MS/MS as described24 with an Agilent 1100 series LC system fitted with a nanoscale reversed-phase high performance liquid chromatography setup, coupled to a QSTAR-XL (Applied Biosystems/MDS Sciex). For peptide identification, MS/MS spectra were searched against the Swiss-Prot database with the online MASCOT (Matrixscience) search engine (both available at http://www.matrixscience.com). For relative comparison of peptide abundance, single MS spectra were acquired and deconvoluted using the BioAnalyst 1.1.5 extension to Analyst QS1.1 (Applied Biosystems).
Construction of individual domains of β2GPI
Human β2GPI cDNA (kindly provided by Dr T. Kristensen from the University of Aarhus, Aarhus, Denmark) was used for the construction of domains I, IV, and V of β2GPI. cDNA was subcloned into a PCR-Blunt II-TOPO vector (Invitrogen), and the separate domains were constructed with a set of 2 primers with BamHI and NotI restriction sites. For domain I, the primers GGATCCGGACGGACCTGTCCCAAGCC and GCGGCCGCTTATACTCTGGGTGTACATTTCAGAGTG were used. For domain IV, the primers GGATCCAGGGAAGTAAAATGCCCATTCC and GCGGCCGCAGATGCTTTACAACTTGGCATGG were used. For domain V, GATCCGCATCTTGTAAAGTACCTGTGAAAAAAGC and GCGGCCGCTTAGCATGGCTTTACATCGG were used. The PCR product was cloned into a PCR-Blunt II-TOPO vector, and sequence analysis was performed to confirm the sequence of domains I, IV, and V. From this vector, the PCR product was subcloned into the expression vector HisN-Tev (Promega).
Protein expression and purification
The individual domains were expressed in HEK293E cells and collected by elution from a Nickel-Sepharose column with a buffer containing 25mM Tris, 500mM NaCl, and 500mM imidazole, pH 8.2. Purification on size was performed with a Hi-Load Superdex 200 XK26 column (GE Healthcare). The different domains were more than 95% pure as was checked on a 4% to 15% SDS-PAA gel (GE Healthcare).
Surface plasmon resonance measurements
Surface plasmon resonance analysis was performed with a BIAcore 2000 (GE Healthcare). Purified domain I was immobilized (150 RU) on an activated CM-5 sensor chip according to the manufacturer's instructions. Specific binding to the individual domains was corrected for nonspecific binding to the deactivated control channel. The nonspecific binding was 6% to 20% of total binding depending on the concentration. Recombinant domains IV and V in various protein concentrations in a buffer containing 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 150mM NaCl, 20μM ZnCl2, 0.0005% Tween-20, pH 7.4 (flow buffer), was injected for 3 minutes at a flow rate of 30 μL/min. The dissociation was followed for a period of 5 minutes. Regeneration of the sensor chip was achieved by a 10 μL wash of 4mM ethylenediaminetetraacetic acid and subsequent equilibration with flow buffer.
Results
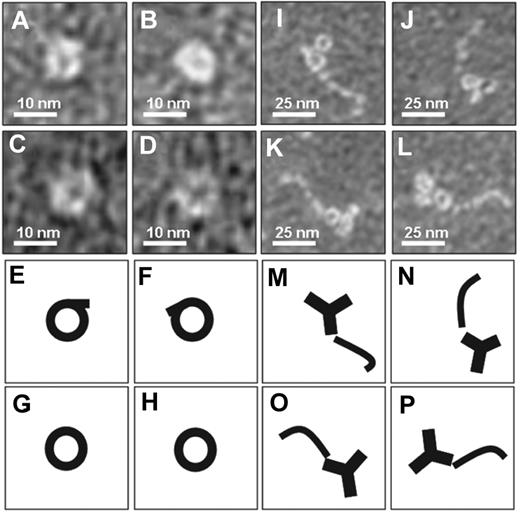
To investigate possible structural differences between β2GPI as it circulates in plasma and β2GPI in complex with antibodies, we performed negative staining electron microscopy studies of purified plasma β2GPI in the absence or presence of an anti–domain I β2GPI antibody (Figure 1). Purified β2GPI was visible as a circular structure, whereas β2GPI in complex with the antibody showed a fishhook-like structure comparable with the published crystal structures of β2GPI. These observations suggest that plasma β2GPI circulates in a circular (closed) conformation, whereas after interaction with antibodies β2GPI undergoes a major conformational change into a fishhook-like (open) structure.
Electron microscopy analysis of β2GPI. Purified human plasma β2GPI was visualized with electron microscopy. (A-D) Magnifications of purified plasma β2GPI show a circular conformation. (E-H) Representation of panels A through D, respectively. (I-L) Purified plasma β2GPI in the presence of antibodies directed against domain I of β2GPI shows on magnification an open fishhook-like shape of β2GPI. (M-P) Representation of panels I through L, respectively.
Electron microscopy analysis of β2GPI. Purified human plasma β2GPI was visualized with electron microscopy. (A-D) Magnifications of purified plasma β2GPI show a circular conformation. (E-H) Representation of panels A through D, respectively. (I-L) Purified plasma β2GPI in the presence of antibodies directed against domain I of β2GPI shows on magnification an open fishhook-like shape of β2GPI. (M-P) Representation of panels I through L, respectively.
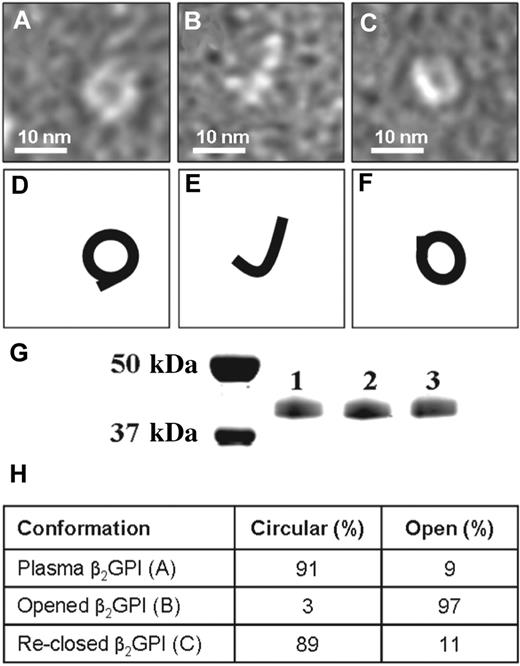
To achieve a better understanding of these observed conformational differences, we determined conditions that allowed transition of one conformation into the other. When we dialyzed purified plasma β2GPI at high pH and high salt conditions, the circular plasma conformation of β2GPI (Figure 2A) was converted into the open conformation (Figure 2B). The open conformation of β2GPI could be converted back into the circular conformation of β2GPI by dialysis at low pH, as was shown by electron microscopy (Figure 2C). The treatment of β2GPI at low and high pH did not induce an apparent modification of the protein as determined by SDS-PAA gel electrophoresis (Figure 2G) and MALDI-TOF MS/MS analysis (not shown). To quantify the number of open and closed β2GPI molecules present, we randomly counted 300 β2GPI molecules of plasma purified β2GPI and plasma purified β2GPI dialyzed against a high and low pH (Figure 2H). Plasma purified β2GPI contained 91% circular particles and 9% fishhook-like particles. Plasma purified β2GPI first dialyzed against high salt and pH 11.5 followed by dialysis against pH 7.4, contained 3% circular particles and 97% fishhook-like particles confirming the opening of plasma purified β2GPI. When this open fishhook-like conformation of β2GPI was dialyzed against pH 3.5 followed by a dialysis against pH 7.4, 89% circular particles and 11% fishhook-like particles were found, confirming the reclosing of opened β2GPI.
Conversion of different forms of β2GPI. Electron microscopy visualization of β2GPI treated at high and low pH. (A) Purified plasma β2GPI. (B) Plasma β2GPI after dialysis for 2 days at pH 11.5 with subsequent neutralization to pH 7.4. (C) Plasma β2GPI, first dialyzed against pH 11.5, then dialyzed for 2 days against pH 3.5 and subsequently neutralized to pH 7.4. (D-F) Representation of panels A through C, respectively. (G) SDS-PAA gel electrophoresis of purified plasma β2GPI (lane 1), β2GPI after dialysis against pH 11.5 (lane 2) and β2GPI first dialyzed against pH 11.5 followed by dialysis against pH 3.5 (lane 3). All 3 samples show a single band at approximately 43 kDa. (H) The percentages of circular and open conformation in purified plasma β2GPI (A), opened β2GPI (B), and reclosed β2GPI (C).
Conversion of different forms of β2GPI. Electron microscopy visualization of β2GPI treated at high and low pH. (A) Purified plasma β2GPI. (B) Plasma β2GPI after dialysis for 2 days at pH 11.5 with subsequent neutralization to pH 7.4. (C) Plasma β2GPI, first dialyzed against pH 11.5, then dialyzed for 2 days against pH 3.5 and subsequently neutralized to pH 7.4. (D-F) Representation of panels A through C, respectively. (G) SDS-PAA gel electrophoresis of purified plasma β2GPI (lane 1), β2GPI after dialysis against pH 11.5 (lane 2) and β2GPI first dialyzed against pH 11.5 followed by dialysis against pH 3.5 (lane 3). All 3 samples show a single band at approximately 43 kDa. (H) The percentages of circular and open conformation in purified plasma β2GPI (A), opened β2GPI (B), and reclosed β2GPI (C).
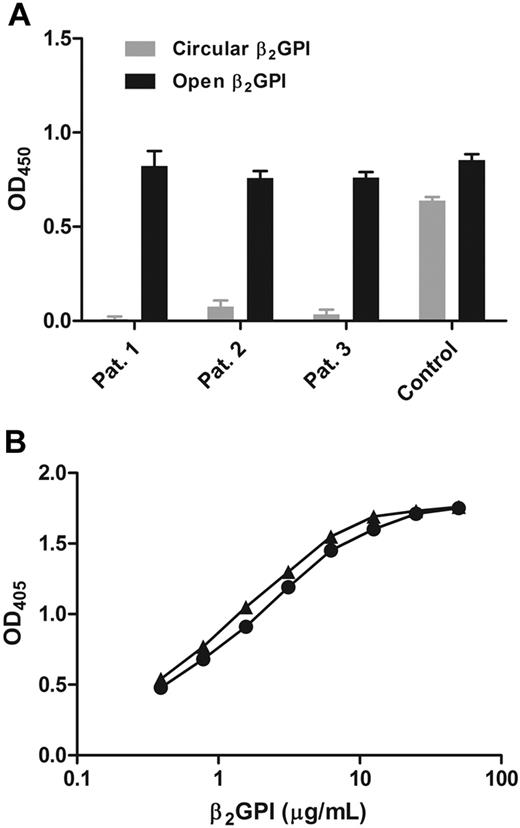
To confirm that only the open conformation of β2GPI was recognized by patient antibodies, a sandwich enzyme-linked immunosorbent assay (ELISA) was developed. Microtiter plates were coated with purified APS patient antibodies and incubated with open or circular β2GPI. The amount of β2GPI bound from the solution was detected with a peroxidase-conjugated anti–β2GPI antibody. Patient antibodies bound to open fishhook-like conformation of β2GPI, although they did not recognize the circular conformation (Figure 3A). When a mouse monoclonal antibody directed against domain IV of β2GPI was coated, both conformations of β2GPI were recognized. When cardiolipin was coated on the surface and incubated with open or circular β2GPI in the presence of purified APS patient antibodies, the patient antibodies recognized both conformations of β2GPI (Figure 3B). To determine the amount of open β2GPI in normal plasma, we also incubated different dilutions of normal plasma with the coated patient antibodies and compared the signal with open β2GPI dissolved in buffer. We found that less than 0.1% of the β2GPI in the circulation was in the open conformation (data not shown).
Detection of β2GPI with antibodies. (A) ELISA plates were coated with purified anti-β2GPI IgGs isolated from APS patients. Circular or open β2GPI was added, and binding of β2GPI was measured with a polyclonal anti–β2GPI antibody labeled with horseradish peroxidase. (▬) Open conformation of β2GPI. ( ) Circular conformation of β2GPI. As a control for β2GPI concentration, a mouse monoclonal anti–domain IV of β2GPI antibody was used. Bars represent mean ± SD (n = 3). (B) ELISA plates were coated with cardiolipin, and a serial dilution (0.4-50 μg/mL) of circular (●) or open (▴) β2GPI was added, subsequently followed by addition of purified APS patient IgG antibodies. Binding of β2GPI was measured with a goat anti-IgG alkaline phosphatase–conjugated antibody.
) Circular conformation of β2GPI. As a control for β2GPI concentration, a mouse monoclonal anti–domain IV of β2GPI antibody was used. Bars represent mean ± SD (n = 3). (B) ELISA plates were coated with cardiolipin, and a serial dilution (0.4-50 μg/mL) of circular (●) or open (▴) β2GPI was added, subsequently followed by addition of purified APS patient IgG antibodies. Binding of β2GPI was measured with a goat anti-IgG alkaline phosphatase–conjugated antibody.
Detection of β2GPI with antibodies. (A) ELISA plates were coated with purified anti-β2GPI IgGs isolated from APS patients. Circular or open β2GPI was added, and binding of β2GPI was measured with a polyclonal anti–β2GPI antibody labeled with horseradish peroxidase. (▬) Open conformation of β2GPI. ( ) Circular conformation of β2GPI. As a control for β2GPI concentration, a mouse monoclonal anti–domain IV of β2GPI antibody was used. Bars represent mean ± SD (n = 3). (B) ELISA plates were coated with cardiolipin, and a serial dilution (0.4-50 μg/mL) of circular (●) or open (▴) β2GPI was added, subsequently followed by addition of purified APS patient IgG antibodies. Binding of β2GPI was measured with a goat anti-IgG alkaline phosphatase–conjugated antibody.
) Circular conformation of β2GPI. As a control for β2GPI concentration, a mouse monoclonal anti–domain IV of β2GPI antibody was used. Bars represent mean ± SD (n = 3). (B) ELISA plates were coated with cardiolipin, and a serial dilution (0.4-50 μg/mL) of circular (●) or open (▴) β2GPI was added, subsequently followed by addition of purified APS patient IgG antibodies. Binding of β2GPI was measured with a goat anti-IgG alkaline phosphatase–conjugated antibody.
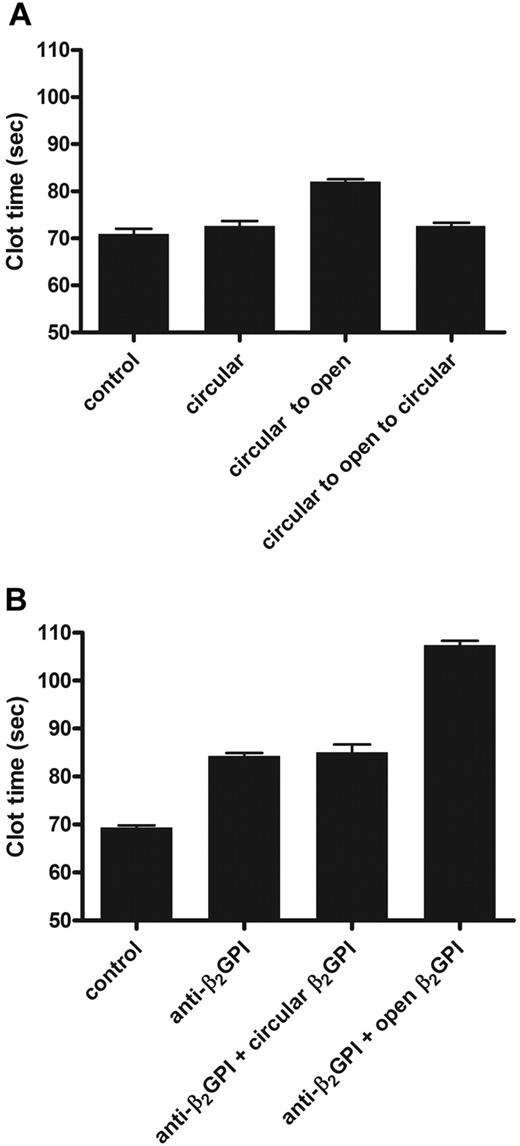
β2GPI is present in high concentrations in plasma, and depletion of β2GPI from normal plasma does not influence the results of coagulation assays.21,25 When antibodies toward β2GPI were added to plasma, clotting times prolonged in a β2GPI-dependent way. This effect of anti–β2GPI antibodies is known as lupus anticoagulant activity. We investigated whether the open and circular forms of β2GPI acted differently on coagulation. When circular plasma β2GPI was added to normal plasma (Figure 4A) or β2GPI-depleted plasma (data not shown), no effect on the dilute aPTT was observed. When 15 μg/mL open β2GPI was added to plasma or β2GPI-depleted plasma, the aPTT was prolonged for more than 10 seconds. To investigate whether, besides opening of β2GPI, there was an additional effect of anti-β2GPI antibodies, we studied whether addition of anti-β2GPI could further prolong the clotting time induced by open β2GPI. Addition of antibody and open β2GPI together to normal plasma gave an additional anticoagulant effect on top of the effect of open β2GPI alone (Figure 4B). The additional effect of anti–β2GPI antibodies on the aPTT was observed with every concentration of open β2GPI tested (data not shown).
Effect of β2GPI on coagulation. The effects of circular and open β2GPI on the aPTT were determined. Circular and open β2GPI was added to normal pool plasma (NPP). (A) Control indicates NPP; circular, NPP with addition of 15 μg/mL plasma β2GPI; circular to open, NPP to which 15 μg/mL plasma β2GPI treated at pH 11.5 was added; and circular to open to circular, NPP to which 15 μg/mL plasma β2GPI first incubated at pH 11.5 and subsequently treated at pH 3.5 was added. (B) Control indicates NPP; anti-β2GPI, NPP to which 15 μg/mL purified monoclonal anti-β2GPI antibody was added; anti-β2GPI + circular β2GPI, NPP with addition of preincubated (5 minutes) anti-β2GPI antibody and plasma β2GPI; and anti-β2GPI + open β2GPI, NPP with addition of preincubated (5 minutes) anti-β2GPI antibody and plasma β2GPI first incubated at pH 11.5 and subsequently treated at pH 3.5 (both 15 μg/mL). Bars represent mean ± SD (n = 3).
Effect of β2GPI on coagulation. The effects of circular and open β2GPI on the aPTT were determined. Circular and open β2GPI was added to normal pool plasma (NPP). (A) Control indicates NPP; circular, NPP with addition of 15 μg/mL plasma β2GPI; circular to open, NPP to which 15 μg/mL plasma β2GPI treated at pH 11.5 was added; and circular to open to circular, NPP to which 15 μg/mL plasma β2GPI first incubated at pH 11.5 and subsequently treated at pH 3.5 was added. (B) Control indicates NPP; anti-β2GPI, NPP to which 15 μg/mL purified monoclonal anti-β2GPI antibody was added; anti-β2GPI + circular β2GPI, NPP with addition of preincubated (5 minutes) anti-β2GPI antibody and plasma β2GPI; and anti-β2GPI + open β2GPI, NPP with addition of preincubated (5 minutes) anti-β2GPI antibody and plasma β2GPI first incubated at pH 11.5 and subsequently treated at pH 3.5 (both 15 μg/mL). Bars represent mean ± SD (n = 3).
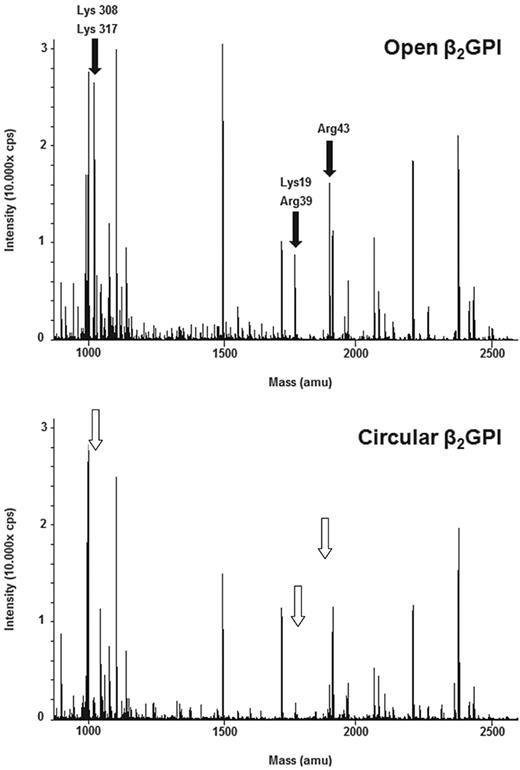
To better understand the conformational changes of β2GPI, we trypsinized both forms of β2GPI and analyzed the peptides formed with MALDI-TOF MS (data not shown) and LC-MS/MS. Under nondenaturing conditions, specific peptides were more abundantly formed from the open form than the circular form. In particular, the amino acids Lys19, Arg39, and Arg43 in domain I and Lys305 and Lys317 in domain V were not or less accessible to trypsin in the circular form, although they were accessible in the open form (Figure 5). When the digestion with trypsin was performed under denaturing conditions, in the presence of an MS-compatible detergent, an identical panel and abundance of peptides was formed from both conformations of β2GPI (data not shown).
Comparison of LC-MS surveys of β2GPI. LC-MS analyses of open and circular β2GPI. The molecular masses of detected tryptic peptides are indicated. Comparison of these 2 analyses shows that in the open conformation of β2GPI (top panel) cleavage has taken place of the amino acids Lys19, Arg39, Arg43 (domain I), Lys305, and Lys317 (domain V) indicated by black arrows, whereas they are not or less visible in the circular conformation of β2GPI (bottom panel) indicated by white arrows.
Comparison of LC-MS surveys of β2GPI. LC-MS analyses of open and circular β2GPI. The molecular masses of detected tryptic peptides are indicated. Comparison of these 2 analyses shows that in the open conformation of β2GPI (top panel) cleavage has taken place of the amino acids Lys19, Arg39, Arg43 (domain I), Lys305, and Lys317 (domain V) indicated by black arrows, whereas they are not or less visible in the circular conformation of β2GPI (bottom panel) indicated by white arrows.
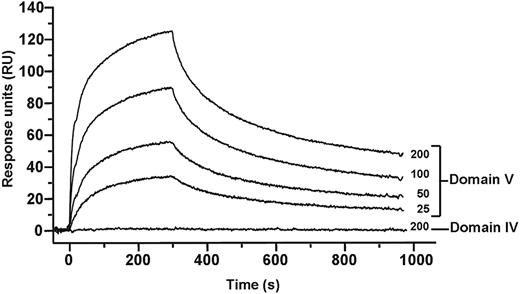
In the circular conformation, interaction between different domains of β2GPI is necessary and from the mass spectrometry data an interaction between domain I and V was suggested. To study this, we have cloned the individual domains of β2GPI and studied their mutual interactions with surface plasmon resonance. As shown in Figure 6, domain V interacted with domain I, whereas no interaction was found between domain I and domain IV of β2GPI. The interaction between domains I and V was found to be completely dependent on the presence of zinc ions. The dissociation constant between domain I and V of β2GPI was approximately 0.8 × 10−9M. These observations suggest that, to maintain the circular structure of β2GPI, domain V of β2GPI interacts with domain I (Figure 7).
Binding analysis of domains I, IV, and V of β2GPI. Binding of domain I to domains IV and V was investigated with surface plasmon resonance. Domain I of β2GPI (150 RU) was immobilized to a CM5 sensor chip, and increasing concentrations (25-200nM) of domain IV or domain V were applied to the chip. Binding of domain V to domain I in a concentration-dependent manner was observed. No detectable interaction could be observed between domain IV and domain I.
Binding analysis of domains I, IV, and V of β2GPI. Binding of domain I to domains IV and V was investigated with surface plasmon resonance. Domain I of β2GPI (150 RU) was immobilized to a CM5 sensor chip, and increasing concentrations (25-200nM) of domain IV or domain V were applied to the chip. Binding of domain V to domain I in a concentration-dependent manner was observed. No detectable interaction could be observed between domain IV and domain I.
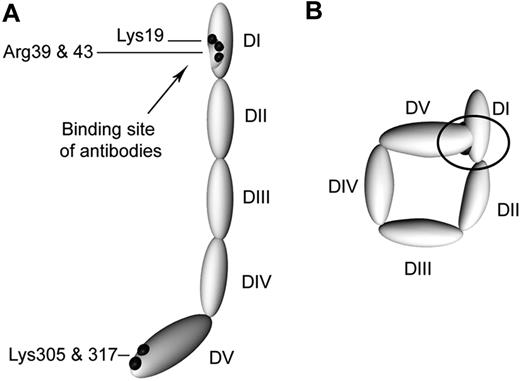
Schematic representation of circular and open β2GPI. (A) β2GPI with its 5 domains (DI-DV) as it is in complex with anti-β2GPI antibodies. (B) A proposed model of plasma β2GPI. Black dots represent the amino acids not accessible for trypsin; black arrow indicates the location of the amino acids involved in the recognition of anti-β2GPI antibodies. In the circular conformation, the black circle indicates the concealing of the amino acids involved in the binding site of anti-β2GPI antibodies as a result of interaction between domains I and V of β2GPI.
Schematic representation of circular and open β2GPI. (A) β2GPI with its 5 domains (DI-DV) as it is in complex with anti-β2GPI antibodies. (B) A proposed model of plasma β2GPI. Black dots represent the amino acids not accessible for trypsin; black arrow indicates the location of the amino acids involved in the recognition of anti-β2GPI antibodies. In the circular conformation, the black circle indicates the concealing of the amino acids involved in the binding site of anti-β2GPI antibodies as a result of interaction between domains I and V of β2GPI.
Discussion
Here we have shown that plasma β2GPI exists in 2 different conformations. Plasma-derived β2GPI is in a circular conformation, as was shown by electron microscopy observations. Analysis of the different conformations with MALDI-TOF MS, LC-MS/MS, and surface plasmon resonance confirmed an interaction between domains I and V. As a result of the interaction with domain V, the epitope on domain I for the autoantibodies that characterize the APS is not available for binding by the antibodies (Figure 3). This also explains why no circulating immune complexes between β2GPI and the antibodies are observed in APS. After exposure to anionic structures, such as negatively charged phospholipids, β2GPI binds, opens up, and exposes the epitope of the autoantibodies and the antibodies are able to recognize β2GPI.12 The interaction with antibodies probably stabilizes β2GPI in the open conformation.
There is a large difference between the (low) affinity of patient antibodies and the (high) affinity of murine monoclonal antibodies for β2GPI.26 The affinity of the murine monoclonal antibody 3B7 for domain I is probably higher than the affinity of domain V for domain I, resulting in opening of β2GPI. The affinity of the patient antibodies could be too low to compete with domain V for binding to domain I. Only when anionic phospholipids compete for the binding to domain V with domain I, the affinity of the patient antibodies is sufficient to bind to domain I. This explains why, after addition of a monoclonal antibody, we could observe the open form with electron microscopy.
We were not able to show an interaction between β2GPI and anionic phospholipid vesicles by electron microscopy because our efforts to prepare complexes between negatively charged vesicles and β2GPI resulted in agglutination of the vesicles, which made the samples inappropriate for electron microscopy. Hammel et al27 mentioned already that they observed increased turbidity of their solutions of liposomes and β2GPI, and that particle aggregation was responsible for the turbidity increase. Moreover, they observed an unexpected decrease in intensity of the fluorescent signal of the tryptophan residues, which could only be explained by agglutination of β2GPI.27 In addition, in their article they observed, with calorimetric and circular dichroism studies, conformational changes when β2GPI was incubated with cardiolipin vesicles. They assumed that a partial loss of tertiary structural elements had taken place on lipid association, which fits with the observations we have made in this manuscript.
Depletion of β2GPI from normal plasma does not influence coagulation.22 In contrast to the circular conformation, the open fishhook-like conformation of β2GPI has a profound effect on the aPTT. Therefore, we propose that the conformation of β2GPI in plasma is predominantly circular. When we analyzed β2GPI isolated from plasma, we found that less than 0.1% was in the open conformation. Analysis of purified β2GPI with electron microscopy suggested that 9% of the molecules were in the open conformation, but this high percentage was probably a technical problem because of adsorption of β2GPI to the copper grids. Addition of β2GPI in a fishhook-like conformation to normal plasma prolonged the aPTT, but addition in combination with anti-β2GPI antibodies prolonged the clotting time of normal plasma even more. This suggests that the change in conformation alone can cause prolongation of the aPTT, but on dimerization of β2GPI by the antibodies the clotting time is further prolonged.21 To express lupus anticoagulant activity, a conformational change within β2GPI is essential, whereas dimerization of β2GPI by the antibodies is necessary to express full lupus anticoagulant activity.
In 1998, Koike et al proposed a circular structure for β2GPI, which was constructed based on the nuclear magnetic resonance coordinates of short consensus repeat domains of human factor H.20 No attention has been paid to this proposed structure since publication of the fishhook-like conformation of β2GPI that was deduced from resolution of the crystal structure.9,19 Here we show that both conformations can exist and that both conformations can be converted into each other by changing pH and salt concentration. As this interaction can be influenced by changing pH and salt concentration, we speculate that there is a hydrophilic interaction between the 2 domains. The crystallization of β2GPI was performed under extreme buffer and salt conditions, circumstances that probably interfered with the hydrophilic interaction and favored the open form of β2GPI. The existence of 2 different conformations has important consequences for functional studies on β2GPI. We have shown that we can change one conformation into the other by changing the in vitro conditions. This means that researchers performing studies with β2GPI have to consider with which conformation of β2GPI their experiments were performed. The presence of β2GPI in a certain conformation is among others dependent on the presence of anionic surfaces but also on the method of purification of β2GPI. Our findings may have impact on the interpretation of research findings in the field of APS as the outcome of many laboratory experiments strongly depends on the conformation of β2GPI as we have shown for the effect of β2GPI on clotting assays.
Schousboe et al,28 Brighton et al,29 Mori et al,30 and Shi et al31 suggested that binding of β2GPI to either FXI or FXII results in inhibition of the intrinsic pathway of coagulation in in vitro systems. A counterargument against these in vitro observations was that the aPTT of normal plasma is independent of the plasma levels of β2GPI, which can vary significantly.32 Moreover, deficiency of β2GPI did not result in a prolongation of the aPTT.33 Here we can provide an explanation for these conflicting results. Apparently, the experiments were performed with β2GPI in an open conformation, whereas plasma β2GPI is in the inactive circular conformation. The open conformation is necessary to express the inhibitory activity on the contact activation of blood coagulation.
We performed direct binding experiments with domain I and domain V. We calculated a dissociation constant of approximately 0.8nM, but the fit of the binding curves in the surface plasmon resonance experiments was not optimal. There are theoretical and practical reasons for this suboptimal fit. In the circular conformation, the binding between domains I and V takes place between 2 domains present in the same molecule, which is incomparable with the interaction between 2 domains freely present in solution. The concentration of domain I in the vicinity of domain V will be increased because domain I is via domains II through IV covalently connected to domain V. This will enhance binding through mass action effects. There is also an entropy term that must be considered. Domains I and V present in one molecule have less mutual mobility than domains I and V in solution. This loss of freedom is entropy unfavorable. When domains I and V are part of the same molecule, the entropy of the system has decreased and the interaction between domains I and V becomes thermodynamically favorable. We had also technical problems measuring the kinetics of the interaction of domains I and V. At higher concentrations, we observed a mutual interaction between domains V, which interfered with the kinetic measurements. This mutual interaction between domains V might be of interest because it could explain the increase in binding affinity of β2GPI for anionic phospholipids when it forms a complex with the antibodies. It is possible that after dimerization of β2GPI, which takes place via binding of the antibodies, subsequently 2-dimensional array formation can occur34,35 on anionic phospholipids via domain V–domain V interactions. For annexin A5, the formation of these 2-dimensional aggregates is essential for its inhibitory potential on coagulation.36,37
To better understand the epitopes responsible for the maintenance of the circular conformation of β2GPI, we have performed enzymatic digestions on both forms of β2GPI under both native and denatured conditions and analyzed the peptides formed by LC-MS/MS. The amino acids Lys19, Arg39, and Arg43 in domain I and Lys305 and Lys317 in domain V were not accessible for trypsin in the circular form, although they were accessible in the open form. After addition of a detergent before digestion, the same panel and abundance of peptides were formed with trypsin from both conformations of β2GPI. These observations suggest that these particular amino acids are hidden from the solution in the circular form and exposed to the solution in the open form of β2GPI, indicating that epitopes within domains I and V are involved in the maintenance of the circular conformation of β2GPI.
Originally, we have suggested that patient antibodies are not able to bind to plasma β2GPI because the epitope Arg39-Arg43 in β2GPI for the antibodies is covered by a negatively charged carbohydrate side chain.12 The conformational change that is induced in β2GPI after binding to phospholipids should interfere with the intramolecular interaction of the carbohydrate side chain with the epitope for the antibodies, which subsequently results in the exposure of the epitope for the antibodies. This idea was further elaborated by Kondo et al38 who demonstrated increased sialylation of the glycan structures of β2GPI of APS patients, suggesting an altered intramolecular interaction and conformational instability of β2GPI in patients. At the moment, we have no information whether differences in sialylation of carbohydrate side chains of β2GPI facilitate the conversion between the open and circular conformation by anti-β2GPI antibodies.
In conclusion, the observations made here on the structural changes that can take place within β2GPI and the subsequent stabilization of this conformation by autoantibodies can have very important consequences for our understanding of the APS.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Johannes H. M. Levels and Valentina De Angelis for technical support and Arjan D. Barendrecht for the artistic impression of open and closed β2-glycoprotein I (Figure 7).
This study was supported in part by a grant from the Netherlands Organization for Scientific Research (ZonMW 91207002; J.C.M.M and P.G.D.G) and an Academic Medical Center stimulation grant (J.C.M.M.).
Authorship
Contribution: Ç.A., G.M.A.v.O., M.M., R.R.S., and J.A.M. performed experiments; Ç.A., G.M.A.v.O., M.M., R.R.S., J.A.M., R.T.U., and R.H.W.M.D. analyzed the results; Ç.A. made the figures; J.C.M.M. and P.G.d.G. designed the research and participated in the analysis, discussion, and interpretation of the data; Ç.A. drafted the first version of the paper; and all authors were involved in finalizing the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joost C. M. Meijers, Department of Experimental Vascular Medicine, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: j.c.meijers@amc.uva.nl.
References
Author notes
Ç.A., G.M.A.v.O., J.C.M.M., and P.G.d.G. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal