Abstract
Activated platelets become procoagulant and efficiently promote the conversion of prothrombin to thrombin. A role of the GPIb-V-IX complex has long been postulated in view of the decreased prothrombin consumption in Bernard-Soulier patients. We evaluated the impact of GPIb-V-IX deficiency and the requirement for the GPIbα extracellular domain. In GPIbβ−/− mice, thrombin generation was profoundly decreased in tissue factor– or collagen-related peptide (CRP)–activated platelet-rich plasma and in washed platelets supplemented with normal plasma or with FVa, FXa, and prothrombin. Phosphatidylserine (PS) exposure was similarly decreased in response to thrombin, CRP, or CRP + PAR4 peptide despite a normal platelet phospholipid composition. The hypothesis that these defects originate from lack of the GPIbα N-terminal domain was evaluated after its removal from normal mouse and human platelets with Nk protease or O-sialoglycoprotein endopeptidase. Unexpectedly, the treated platelets exhibited normal thrombin generation and PS exposure, indicating that GPIb-V-IX regulates procoagulant activity independently of its GPIbα-binding region. These results suggested a more general structuring role through intracellular cytoskeleton-anchoring portions regulating responses leading to PS exposure. This hypothesis was supported by the decreased calcium mobilization observed in GPIbβ−/− platelets in response to several agonists, some acting independently of GPIb, in contrast to the normal calcium responses in Nk protease–treated platelets.
Introduction
Platelets activated by strong agonists such as thrombin or collagen become procoagulant and efficiently promote the conversion of prothrombin to thrombin.1,2 This activity requires the surface exposure of negatively charged phospholipids, especially phosphatidylserine (PS).3-5 PS exposure occurs through inversion of the membrane phospholipid asymmetry after a sustained agonist-induced rise in intracellular calcium and through mechanisms independent of platelet activation.3,5,6 The surface-exposed PS promotes the assembly of vitamin K–dependent factors and cofactors of the prothrombinase (FXa and FVa) and tenase (FIXa and FVIIIa) complexes, resulting in a potent enhancement of thrombin generation. Strong platelet activation also mobilizes FV/FVa from granular stores, making it available for incorporation into the prothrombinase complex.7
Other molecules have been proposed to contribute to platelet procoagulant activity, in particular surface receptors such as integrin αIIbβ3, glycoprotein VI (GPVI), and the GPIb-V-IX complex. The involvement of αIIbβ3 was suggested by the observation of decreased procoagulant activity in Glanzmann thrombasthenia8 and in the presence of αIIbβ3 inhibitors.9 A role of GPVI was proposed on the basis of the capacity of GPVI-specific agonists like convulxin or collagen-related peptide (CRP) to increase thrombin generation,10,11 a response that was absent in mice lacking GPVI signaling12 and in monkeys treated with anti-GPVI antibodies.13
A contribution of GPIb-V-IX has long been postulated on the grounds of the decreased prothrombin consumption observed in Bernard-Soulier patients.14-17 The evaluation of procoagulant activity in Bernard-Soulier platelets initially led to divergent conclusions.16,18-23 Whereas decreased thrombin generation was reported by some authors,18,23 increased thrombin generation19 and PS exposure was observed by others in activated and resting platelets.24 However, a consensus now prevails in the literature in favor of a positive contribution of GPIb-V-IX to platelet procoagulant activity.
Such a role is also supported by studies in which authors implicate the N-terminal extracellular region of GPIbα.25 One potential mechanism is determined by the thrombin binding capacity of this subunit, as demonstrated by the increased prothrombinase activity of thrombin-treated platelets and reversal of this effect by the addition of antibodies blocking GPIbα or the addition of glycocalicin as a competitor.26,27 Another model has proposed a role of fibrin in enhancing prothrombinase activity through its interaction with von Willebrand factor (VWF) and GPIbα, an effect that was absent in Bernard-Soulier platelets, in the presence of antibodies blocking GPIbα and in VWF-deficient plasma.21,22 Finally, it has been suggested that GPIbα binds and concentrates FXII and FXI and thereby increases thrombin generation,28-30 but no formal functional evidence has yet been provided for such a mechanism.31
All the proposed mechanisms implicate binding domains carried by the 45-kDa N-terminal region of GPIbα within the first approximately 290 amino acids. This region comprises a Leu-rich repeat domain flanked by disulfide loops and ends in a negatively charged sulfated tyrosine C tail.32 In biochemical and crystallographic analyses, researchers have identified thrombin binding sites in the C-flanking and sulfated tyrosine segments33-35 and VWF A1 domain binding sites in the Leu-rich N- and C-flanking regions.36,37 Binding sites for high molecular weight kininogen, FXII, and FXI appear to overlap with the thrombin binding domain but have not been precisely located.28-30
Using a mouse model of Bernard-Soulier syndrome, we have evaluated in more detail the impact of GPIb-V-IX deficiency on platelet procoagulant activity by measuring thrombin generation and PS externalization. In addition, the involvement of the GPIbα N-terminal binding domain in procoagulant responses was reassessed in normal platelets after its specific enzymatic removal.
Methods
Reagents and mice
CRP was kindly provided by Richard Farndale (University of Cambridge) and recombinant hirudin by Transgène. Human prothrombin, α-thrombin, FXa, and VWF were purified from human plasma at the EFS-Alsace. Human FVa was from American Diagnostica. Biotinylated PPACK-thrombin was obtained by addition of a 20M excess of biotinylated D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (Calbiochem) to thrombin, followed by dialysis. Calcium ionophore A23187 was from Calbiochem, and O-sialoglycoprotein endopeptidase (OSE) purified from Pasteurella haemolytica was obtained from Cerdalane. Nk protease is a cobra protease purified from the venom of Naja kaouthia as described by Wijeyewickrema et al.38 Rat monoclonal antibodies (mAbs) against mouse GPIbα (XiaG5), GPV (GonC2), GPIX (XiaB4), GPVI (JAQ1), and integrin α2β1 (SAMC1) were obtained from emfret Analytics. Rat mAbs against mouse integrin αIIbβ3 (RAM.2) and GPIbβ (RAM.1) were produced in our laboratory.39 Mouse mAbs against human GPIbα (SZ2) and integrin α2β1 (Gi9) were purchased from Beckmann Coulter, and a mouse mAb against human GPIX (FMC25) was from Santa Cruz Biotechnology Inc.
Other mouse mAbs against human GPIbα (LJ1B10 and WM23) were generous gifts from Zaverio Ruggeri (Scripps Research Institute, La Jolla, CA) and Michael Berndt (University College, Cork, Ireland), respectively. A mouse mAb against human integrin αIIbβ3 (AP2) was a generous gift from Thomas Kunicki (Scripps Research Institute, La Jolla, CA). A mouse mAb against human integrin α2β1 (3J24) was a generous gift from Martine Jandrot-Perrus (Inserm U698, Hôpital Bichat, University Paris 7, Paris, France). Fluorescein isothiocyanate (FITC)–conjugated annexin V was from Roche Diagnostics and tetramethylrhodamine isothiocyanate-phalloidin from Sigma-Aldrich. Echicetin and botrocetin were from PENTAPHARM. Recombinant human tissue factor (TF; Innovin) was from Dade Behring, and the fluorogenic substrate Z-GGR-AMC from Thrombinoscope BV. The chromogenic thrombin substrate S2238 was obtained from Chromogenix. Flow cytometry experiments were performed in a Becton Dickinson FACScalibur flow cytometer and analyzed with CellQuest Pro software.
Wild-type (WT; GPIbβ+/+) and GPIbβ−/− mice40 were backcrossed for 7 generations on a pure C57BL/6 genetic background and maintained in the animal facilities of the Etablissement Français du Sang-Alsace. Ethical approval for animal experiments was received from the French Ministry of Research in accordance with the European Union guidelines. GPIbβ−/− platelets express little GPIb-IX complex (3% of WT) but normally express integrin αIIbβ3, α2β1, and GPVI. Normal aggregation and secretion responses are observed in response to adenosine diphosphate (ADP; 5μM), collagen (10 μg/mL), CRP (35 μg/mL), and thrombin (0.4-10nM). The platelet volume is increased 4 to 5 times in GPIbβ−/− compared with WT.
Platelet preparation
Washed mouse platelets were prepared from acid citrate dextrose (ACD)–anticoagulated blood by separation of the platelet fraction on a Ficoll gradient and sequential centrifugation.40 The cells were resuspended (3 × 108/mL) in Tyrode buffer containing 0.35% human serum albumin (Tyrode Albumin buffer [TA]) and 0.02 U/mL potato apyrase. Washed human platelets were prepared exactly as described previously.41
Treatment of platelets with Nk protease or OSE
Washed platelets from GPIbβ+/+ mice were incubated at 37°C for 30 minutes with Nk protease (10 μg/mL) in Tyrode buffer without albumin or with OSE (48 μg/mL) in TA. The cells were then washed once in TA containing 7.5nM PGI2 and finally resuspended (4 × 108/mL) in TA. Washed human platelets were treated with OSE or Nk protease in the same manner and finally resuspended (3 × 108/mL) in TA.
Control of the integrity of GPIbα by flow cytometry
GPIbα cleavage in the mouse was evaluated in 2 μL of a platelet suspension in TA by adding FITC-conjugated XiaG5 (5 μg/mL) for 15 minutes at room temperature. The VWF binding capacity was determined by incubating the cells with Cy2-conjugated human VWF (10 μg/mL) in the presence of botrocetin (5 U/mL) for 15 minutes. The thrombin binding capacity was evaluated by addition of biotinylated PPACK-thrombin (600nM) for 30 minutes, followed by washing and incubation for 15 minutes with phycoerythrin (PE)–conjugated streptavidin. The integrity of other surface glycoproteins was checked by incubation with the following rat mAbs (5 μg/mL): GonC2 (GPV), XiaB4 (GPIX), JAQ1 (GPVI), SAMC1 (integrin α2β1), RAM.2 (αIIbβ3), and RAM.1 (GPIbβ). The treated cells were analyzed by flow cytometry, and results were expressed as the percentage mean fluorescence intensity compared with untreated platelets (Table 1).
Ligand binding and expression of the main surface glycoproteins in normal mouse platelets treated with Nk protease or OSE
| mAb or ligand . | Nk treatment (% ± SEM) . | OSE treatment (% ± SEM) . |
|---|---|---|
| GPIbα (XiaG5–45kDa N-ter) | 0.01 ± 0.01 (n = 6) | 0.6 ± 0.2 (n = 9) |
| GPIbβ (RAM.1) | 100.8 (n = 2) | 97.4 ± 2.7 (n = 3) |
| GPIX (XiaB4) | 99.7 (n = 2) | 100.9 (n = 2) |
| GPV (GonC2) | 110.2 ± 11.2 (n = 3) | 95.4 (n = 2) |
| Integrin αIIbβ3 (RAM.2) | 104.9 ± 2.3 (n = 3) | 100.9 (n = 2) |
| Integrin α2β1 (SAMC1) | 109.5 ± 6.3 (n = 3) | 101.4 ± 3.9 (n = 8) |
| GPVI (JAQ1) | 101.4 ± 2.7 (n = 5) | 60.0 ± 2.9 (n = 9) |
| VWF | 0.1 (n = 2) | 0 (n = 2) |
| Thrombin (biot PPACK) | No binding in CTRL | No binding in CTRL |
| mAb or ligand . | Nk treatment (% ± SEM) . | OSE treatment (% ± SEM) . |
|---|---|---|
| GPIbα (XiaG5–45kDa N-ter) | 0.01 ± 0.01 (n = 6) | 0.6 ± 0.2 (n = 9) |
| GPIbβ (RAM.1) | 100.8 (n = 2) | 97.4 ± 2.7 (n = 3) |
| GPIX (XiaB4) | 99.7 (n = 2) | 100.9 (n = 2) |
| GPV (GonC2) | 110.2 ± 11.2 (n = 3) | 95.4 (n = 2) |
| Integrin αIIbβ3 (RAM.2) | 104.9 ± 2.3 (n = 3) | 100.9 (n = 2) |
| Integrin α2β1 (SAMC1) | 109.5 ± 6.3 (n = 3) | 101.4 ± 3.9 (n = 8) |
| GPVI (JAQ1) | 101.4 ± 2.7 (n = 5) | 60.0 ± 2.9 (n = 9) |
| VWF | 0.1 (n = 2) | 0 (n = 2) |
| Thrombin (biot PPACK) | No binding in CTRL | No binding in CTRL |
Washed platelets from wild-type mice were treated for 30 minutes with Nk protease (10 μg/mL) or OSE (48 μg/mL) and analyzed by flow cytometry for binding of mAbs against specific glycoproteins or binding of GPIbα ligands (VWF, thrombin). Results are from separate experiments as indicated and are expressed as mean percentage values (± SEM) relative to the binding in control (CTRL) untreated platelets after correction for nonspecific binding.
GP indicates glycoprotein; mAb, monoclonal antibody; OSE, O-sialoglycoprotein endopeptidase; PPACK, D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone; and VWF, von Willebrand factor.
Cleavage of the thrombin binding site on human platelets was controlled by incubation with the following mouse mAbs: SZ2 (GPIbα-sulfated tyrosine motif) and LJ1B10 (GPIbα-thrombin binding site). The integrity of the GPIbα macroglycopeptide was checked with the mAb WM23. The thrombin binding capacity was evaluated with biotinylated PPACK-thrombin and the VWF binding capacity by incubating the cells with Cy2-conjugated VWF in the presence of botrocetin, as described for mouse platelets. The integrity of other surface glycoproteins was checked by incubation with the following mAbs (5 μg/mL): V.1 (GPV), FMC25 (GPIX), RAM.1 (GPIbβ), AP2 (αIIbβ3), Gi9 (integrin α2β1), and GPVI (3J24; Table 2).
Ligand binding and expression of the main surface glycoproteins in human platelets treated with Nk protease or OSE
| mAb or ligand . | Nk treatment (% ± SEM) . | OSE treatment (% ± SEM) . |
|---|---|---|
| GPIbα (SZ2-sulfated Tyr) | 0.3 ± 0.03 (n = 3) | 0.2 ± 0.4 (n = 3) |
| GPIbα (LJ1B10-Thrombin binding site) | 0.2 ± 0.5 (n = 4) | 0.1 ± 1.1 (n = 4) |
| GPIbα (WM23-macroglycopeptide) | 103.6 ± 13.1 (n = 4) | 82.0 ± 8.9 (n = 3) |
| GPIX (FMC25) | 102.7 ± 4.4 (n = 3) | 100.0 ± 13.8 (n = 3) |
| GPIbβ (RAM.1) | 104.3 ± 6.5 (n = 3) | 118.2 ± 4.9 (n = 3) |
| GPV (V.1) | 94.6 ± 6.4 (n = 4) | 147.9 ± 18 (n = 4) |
| Integrin αIIbβ3 (AP2) | 102.1 ± 15.6 (n = 4) | 119.4 ± 12.2 (n = 4) |
| Integrin α2β1 (Gi9) | 98.7 ± 4.5 (n = 5) | 139.6 ± 5.7 (n = 6) |
| GPVI (3J24) | 101.9 ± 6.5 (n = 5) | 68.2 ± 5.8 (n = 3) |
| VWF | 0.6 ± 0.2 (n = 4) | 0.5 ± 0.5 (n = 3) |
| Thrombin (biot PPACK) | 5.6 ± 0.9 (n = 6) | 5.9 ± 2.8 (n = 6) |
| Echicetin (GPIbα-Thrombin site) | 0.6 ± 0.08 (n = 4) | 0.8 ± 0.1 (n = 4) |
| mAb or ligand . | Nk treatment (% ± SEM) . | OSE treatment (% ± SEM) . |
|---|---|---|
| GPIbα (SZ2-sulfated Tyr) | 0.3 ± 0.03 (n = 3) | 0.2 ± 0.4 (n = 3) |
| GPIbα (LJ1B10-Thrombin binding site) | 0.2 ± 0.5 (n = 4) | 0.1 ± 1.1 (n = 4) |
| GPIbα (WM23-macroglycopeptide) | 103.6 ± 13.1 (n = 4) | 82.0 ± 8.9 (n = 3) |
| GPIX (FMC25) | 102.7 ± 4.4 (n = 3) | 100.0 ± 13.8 (n = 3) |
| GPIbβ (RAM.1) | 104.3 ± 6.5 (n = 3) | 118.2 ± 4.9 (n = 3) |
| GPV (V.1) | 94.6 ± 6.4 (n = 4) | 147.9 ± 18 (n = 4) |
| Integrin αIIbβ3 (AP2) | 102.1 ± 15.6 (n = 4) | 119.4 ± 12.2 (n = 4) |
| Integrin α2β1 (Gi9) | 98.7 ± 4.5 (n = 5) | 139.6 ± 5.7 (n = 6) |
| GPVI (3J24) | 101.9 ± 6.5 (n = 5) | 68.2 ± 5.8 (n = 3) |
| VWF | 0.6 ± 0.2 (n = 4) | 0.5 ± 0.5 (n = 3) |
| Thrombin (biot PPACK) | 5.6 ± 0.9 (n = 6) | 5.9 ± 2.8 (n = 6) |
| Echicetin (GPIbα-Thrombin site) | 0.6 ± 0.08 (n = 4) | 0.8 ± 0.1 (n = 4) |
Washed human platelets were treated for 30 minutes with Nk protease (10 μg/mL) or OSE (48 μg/mL) and analyzed by flow cytometry for binding of mAbs against the main surface glycoproteins or binding of GPIbα ligands (VWF, thrombin, echicetin). The epitopes of the mAbs are indicated in parentheses. Results are from separate experiments as indicated and are expressed as mean percentage values (± SEM) relative to the binding in control untreated platelets after correction for nonspecific binding.
GP indicates glycoprotein; mAb, monoclonal antibody; OSE, O-sialoglycoprotein endopeptidase; PPACK, D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone; and VWF, von Willebrand factor.
Calibrated automated thrombin generation analyses
Platelet-rich plasma (PRP) was obtained by centrifugation of fresh citrated (3.15%) mouse blood at 250g. The platelet count was adjusted to 2 × 108/mL with autologous platelet-poor plasma prepared by centrifugation of PRP at 3300g and then 9300g for 5 minutes at 4°C. When experiments were performed with protease-pretreated washed platelets, the suspension was mixed with an equivalent volume of autologous citrated platelet-poor plasma.
Calibrated automated thrombin (CAT) analyses were performed by the thrombogram method21 in a fluorescence plate reader (Fluoroskan Ascent; ThermoLabsystems). Aliquots (20 μL) of TF (0.5pM Innovin 1/12 000 final dilution) or CRP (10 μg/mL final concentration) were added in triplicate to 80 μL of PRP (1.3 × 108 platelets/mL final concentration) in a 96-well plate (Immulon 2 Dynex; Stago) maintained at 37°C. Thrombin generation was started by automated addition of the fluorogenic thrombin substrate Z-GGR-AMC (41μM) and CaCl2 (1.7mM). The accumulation of fluorescence from cleaved Z-GGR-AMC was measured continuously at excitation and emission wavelengths of 390 and 460 nm, respectively. First derivative curves of the fluorescence accumulation were converted into thrombin concentrations by the use of a human thrombin calibrator and Thrombinoscope BV Version 3.0.0.29 software (Stago). The area under the curve, also called the endogenous thrombin potential (ETP), corresponds to the total amount of thrombin formed in the sample. Peak corresponds to the maximal concentration of thrombin generated. Control and protease-pretreated human platelets were tested in the same way as mouse platelets except that the final cell count was 1 × 108/mL in CAT experiments.
Chromogenic prothrombinase assay
Washed mouse platelets were adjusted to 5 × 107 cells/mL in TA buffer. Aliquots (80 μL) were activated with 10 μL of various agonists: CRP (50 μg/mL), A23187 (100μM), or a mixture of CRP (50 μg/mL) and either thrombin (1nM) or the PAR4 agonist peptide AYPGKF (1mM). After incubation for 10 minutes at 37°C, human prothrombin (4μM) and human FVa (2.5nM) were added. At 3 minutes, thrombin generation was started by addition of FXa (150pM). Aliquots (25μL) were removed after 15 minutes and diluted in 50 μL of 50mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4) containing 20mM EDTA (ethylenediaminetetraacetic acid). The thrombin formed was quantified by adding 25 μL of the chromogenic substrate S-2238 (500μM) and measuring the rate of hydrolysis at 405 nm in a Vmax microplate reader (Molecular Devices). Thrombin concentrations were calculated from the rate of change in absorbance by comparison with a standard curve generated with purified human thrombin.
Measurement of annexin V binding
Washed mouse or human platelets in TA buffer were activated with thrombin (10nM), CRP (10 μg/mL), A23187 (100μM), or a combination of CRP (50 μg/mL) and either thrombin (1nM) or the PAR4 agonist peptide AYPGKF (1mM), for 10 minutes at 37°C without stirring. A 2-μL aliquot of activated or resting platelets was then incubated with FITC-conjugated annexin V (10 μg/mL) for 20 minutes in the dark, diluted, and analyzed by flow cytometry. The light scattering and fluorescence intensity from 10 000 cells were collected with a logarithmic gain. Flow cytometric analyses that used a forward scatter/FL1 dot blot were performed to determine the ratio of activated to nonactivated platelets. Activated annexin V–positive platelets were detected in the upper right quadrant and quantified as the percentage of total platelets.
[Ca2+]i measurements
Increases in intracellular calcium were measured as previously described.42 In brief, washed mouse platelets were incubated at 37°C for 30 minutes with (GPIbβ+/+ cells) or without (GPIbβ+/+ and GPIbβ−/− cells) Nk protease (10 μg/mL) in Tyrode buffer without albumin. The platelets were then washed, resuspended (75 × 104 cells/μL) in Tyrode buffer containing no calcium and 0.02 U/mL apyrase, and loaded with fura-2/AM (15μM). Finally, the cells were washed and resuspended (2 × 105 cells/μL) in Tyrode buffer containing apyrase, 2mM calcium, and 0.1% essentially fatty acid free human serum albumin. After addition of thrombin (10nM), ADP (5μM), the thromboxane A2 analog U46619 (1μM), CRP (35 μg/mL), a mixture of CRP (35 μg/mL) and the PAR4 agonist peptide AYPGKF (0.5mM), or a mixture of CRP (35 μg/mL) and U46619 (1μM), the rise in intracellular calcium was measured in a PTI Deltascan spectrofluorimeter (Photon Technology International Inc), by the use of excitation wavelengths of 340 and 380 nm and fluorescence emission detection at 510 nm.
Lipid analyses
Lipids were extracted from a pellet of 3 × 108 washed mouse platelets prepared as described40 by the use of the acidified Bligh and Dyer method. Sphingomyelin, phosphatidylcholine, PS plus phosphatidylinositol, and PE were separated by thin-layer chromatography in CHCl3/CH3OH/CH3COOH/H2O (75/45/12/6 vol/vol), identified by iodine vapor staining with reference to lipid standards and scraped off the chromatogram. The lipids were quantified according to their phosphorus content as previously described.43
Measurement of F-actin in mouse platelets
Washed platelets (10 μL), resting or activated with thrombin (10nM) or CRP (10 μg/mL), were incubated for 15 minutes at room temperature in TA containing 3% paraformaldehyde and 100 U/mL hirudin. The fixed platelets were diluted in 1 mL of TA and centrifuged for 5 minutes at 620g. The pellet was resuspended in 50 μL of Triton X-100 0.1% during 5 minutes, and then tetramethylrhodamine isothiocyanate-phalloidin (2 μg/mL) in TA was added during 10 minutes at room temperature and diluted in 250 μL of TA before flow cytometric analysis. Results were expressed as the ratio of the fluorescence intensity of activated compared with resting platelets.
Statistical analyses
Results were expressed as the mean (± SEM), and statistical comparisons were performed by the use of an unpaired, 2-tailed Student t test (Prism 3; GraphPad Software Inc). P values less than .05 were considered to be statistically significant.
Results
Decreased thrombin generation in platelets lacking the GPIb-V-IX complex
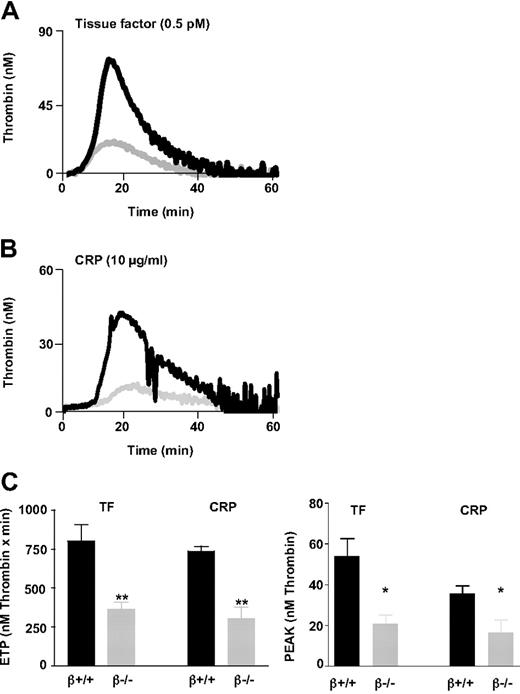
The thrombin generation capacity of WT (GPIbβ+/+) and GPIbβ−/− mouse platelets was measured in a CAT assay by use of citrated PRP (1.3 × 108 platelets/mL). The reaction was initiated by addition of TF (0.5pM) or the GPVI agonist CRP (10 μg/mL). The thrombograms in GPIbβ−/− PRP displayed an important decrease in peak and total thrombin generation under TF- (Figure 1A) or CRP-induced (Figure 1B) conditions but a normal lag phase. Total thrombin (Figure 1C) amounted to 46% of that formed in the WT in response to TF (365 ± 45nM vs 802 ± 101nM thrombin × minutes, n = 7, P < .01) and 42% of that formed in the WT in response to CRP (306 ± 75nM vs 736 ± 32nM thrombin × minutes, n = 4, P < .01). A similar decrease was observed when analyzing the thrombin peak levels in TF- and CRP-induced samples, which amounted to 39% and 46% of WT, respectively (Figure 1C). Because GPIbβ−/− platelets have an increased size, PRP from GPIbβ+/+ mice was also assayed at greater platelet counts (3 × 108 platelets/mL) to compensate for the smaller cell surface area. Under these conditions, the total thrombin generated in GPIbβ−/− PRP represented only 25% of that generated in GPIbβ+/+ PRP (data not shown).
Decreased thrombin generation in platelets lacking the GPIb-V-IX complex. Calibrated automated thrombin (CAT) experiments were performed in citrated platelet-rich plasma (PRP; final platelet count 1.3 × 108 cells/mL). The reaction was triggered by addition of tissue factor (TF; 0.5pM) or collagen-related peptide (CRP; 10 μg/mL) in the presence of calcium and the fluorogenic substrate Z-GGR-AMC. Thrombin activity was determined from the accumulation of the fluorescent product and was calculated relative to a thrombin calibrator. (A-B) Representative thrombin generation curves in PRP from GPIbβ−/− ( ) and GPIbβ+/+ mice (■). (C) Endogenous thrombin potential (ETP) values, representing the total amount of thrombin generated. Peak values represent the maximal thrombin concentration attained. The results are presented as the mean ± SEM in 7 (TF) or 4 (CRP) independent experiments. **P < .01, *P < .05 versus wild-type (WT) mice.
) and GPIbβ+/+ mice (■). (C) Endogenous thrombin potential (ETP) values, representing the total amount of thrombin generated. Peak values represent the maximal thrombin concentration attained. The results are presented as the mean ± SEM in 7 (TF) or 4 (CRP) independent experiments. **P < .01, *P < .05 versus wild-type (WT) mice.
Decreased thrombin generation in platelets lacking the GPIb-V-IX complex. Calibrated automated thrombin (CAT) experiments were performed in citrated platelet-rich plasma (PRP; final platelet count 1.3 × 108 cells/mL). The reaction was triggered by addition of tissue factor (TF; 0.5pM) or collagen-related peptide (CRP; 10 μg/mL) in the presence of calcium and the fluorogenic substrate Z-GGR-AMC. Thrombin activity was determined from the accumulation of the fluorescent product and was calculated relative to a thrombin calibrator. (A-B) Representative thrombin generation curves in PRP from GPIbβ−/− ( ) and GPIbβ+/+ mice (■). (C) Endogenous thrombin potential (ETP) values, representing the total amount of thrombin generated. Peak values represent the maximal thrombin concentration attained. The results are presented as the mean ± SEM in 7 (TF) or 4 (CRP) independent experiments. **P < .01, *P < .05 versus wild-type (WT) mice.
) and GPIbβ+/+ mice (■). (C) Endogenous thrombin potential (ETP) values, representing the total amount of thrombin generated. Peak values represent the maximal thrombin concentration attained. The results are presented as the mean ± SEM in 7 (TF) or 4 (CRP) independent experiments. **P < .01, *P < .05 versus wild-type (WT) mice.
To eliminate the possibility of a plasmatic defect, thrombin production was measured in platelet-plasma cross-mixing experiments. GPIbβ−/− plasma added to WT platelets did not modify their capacity to generate thrombin, whereas a 60% decrease in thrombin generation was observed in the reverse experiment (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In addition, the activated partial thromboplastin time and prothrombin time were normal in GPIbβ−/− plasma (ie, activated partial thromboplastin time: 30.4 ± 0.8 seconds and 29.4 ± 0.4 seconds; prothrombin time: 12.5 ± 0.2 seconds and 13.0 ± 0.1 seconds in GPIbβ+/+ and GPIbβ−/−, respectively, n = 3), further indicating that the defect was not attributable to an abnormal plasma content of coagulation factors or cofactors. Hence, the decreased prothrombinase activity in GPIbβ−/− animals was essentially linked to platelets.
Decreased PS exposure in platelets lacking the GPIb-V-IX complex
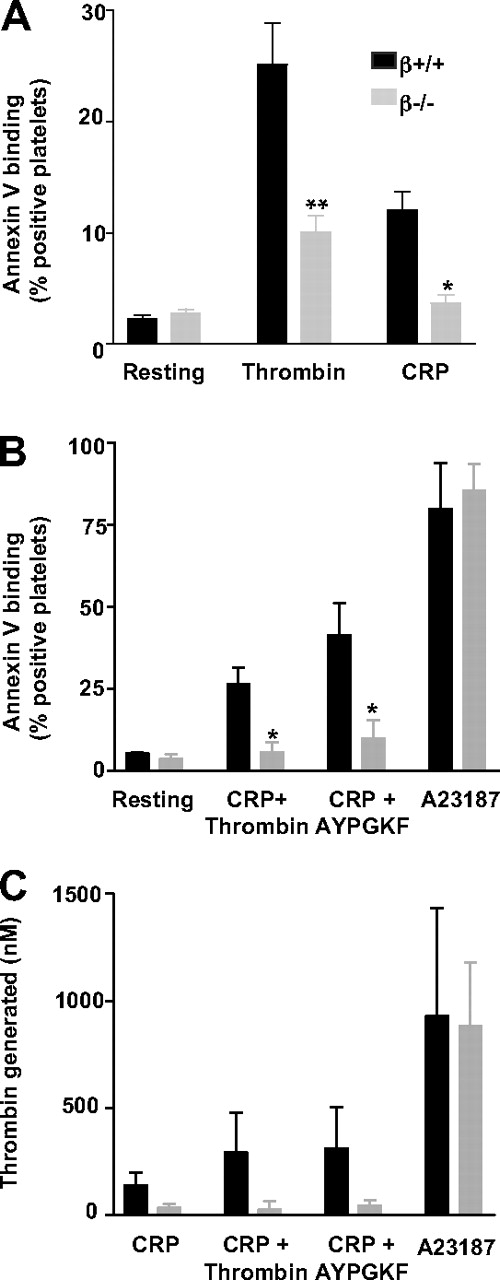
PS surface exposure, an essential component of platelet procoagulant activity, was quantified by FITC-annexin V binding. The proportion of annexin V–positive platelets was decreased by 60% and 70% in GPIbβ−/− mice compared with the WT after activation with thrombin (10nM) or CRP (10 μg/mL), respectively (Figure 2A). Reduced PS exposure was likewise observed after a stimulus combining CRP (50 μg/mL) with thrombin (1nM) or the PAR4 agonist peptide AYPGKF (1mM; Figure 2B). In contrast, no difference was observed after activation with the calcium ionophore A23187 (100μM), which potently externalized PS in WT and GPIbβ−/− platelets (80% ± 14% and 86% ± 8% positivity, respectively; Figure 2B). In the resting state, WT and GPIbβ−/− platelets exhibited a minimal positivity of 2.2% (± 0.3%) and 2.7% (± 0.4%), respectively.
Decreased PS exposure in GPIb-V-IX–deficient platelets after stimulation by single or dual agonists. Binding of annexin V to GPIbβ+/+ and GPIbβ−/− platelets was measured by flow cytometry in resting platelets or after stimulation with (A) thrombin (10nM; n = 9) or CRP (10 μg/mL; n = 3), (B) CRP (50 μg/mL) in combination with thrombin (1nM; n = 3) or PAR4 agonist peptide (AYPGKF, 1mM; n = 3), or the calcium ionophore A23187 (100μM; n = 3). Values represent the mean percentage (± SEM) of annexin V-positive cells. *P < .05 versus WT mice. (C) Prothrombinase activity generated by GPIbβ+/+ and GPIbβ−/− platelets after stimulation with CRP (10 μg/mL; n = 3), CRP (50 μg/mL) in combination with thrombin (1nM; n = 3) or the PAR4 agonist AYPGKF peptide (1mM; n = 3), or A23187 (100μM; n = 3). Thrombin generation, expressed as the total amount of thrombin formed after 15 minutes, was measured by the use of a chromogenic substrate after the addition of FXa, FVa, and prothrombin. The thrombin generated by resting platelets was subtracted, and values are the mean ± SEM.
Decreased PS exposure in GPIb-V-IX–deficient platelets after stimulation by single or dual agonists. Binding of annexin V to GPIbβ+/+ and GPIbβ−/− platelets was measured by flow cytometry in resting platelets or after stimulation with (A) thrombin (10nM; n = 9) or CRP (10 μg/mL; n = 3), (B) CRP (50 μg/mL) in combination with thrombin (1nM; n = 3) or PAR4 agonist peptide (AYPGKF, 1mM; n = 3), or the calcium ionophore A23187 (100μM; n = 3). Values represent the mean percentage (± SEM) of annexin V-positive cells. *P < .05 versus WT mice. (C) Prothrombinase activity generated by GPIbβ+/+ and GPIbβ−/− platelets after stimulation with CRP (10 μg/mL; n = 3), CRP (50 μg/mL) in combination with thrombin (1nM; n = 3) or the PAR4 agonist AYPGKF peptide (1mM; n = 3), or A23187 (100μM; n = 3). Thrombin generation, expressed as the total amount of thrombin formed after 15 minutes, was measured by the use of a chromogenic substrate after the addition of FXa, FVa, and prothrombin. The thrombin generated by resting platelets was subtracted, and values are the mean ± SEM.
To find out whether reduced PS externalization was the direct cause of defective thrombin generation, the latter was assayed under conditions where PS availability represents the limiting factor. Washed platelets preactivated with CRP, alone or in combination with thrombin or AYPGKF, were supplemented with optimal concentrations of prothrombin, FVa, and FXa, and thrombin production was measured with a chromogenic substrate (Figure 2C). Thrombin generation was decreased by 75% to 90% in GPIbβ−/− platelets. However, GPIbβ−/− platelets exposed to A23187 displayed a full response similar to that of the WT, in agreement with the annexin V binding results.
To determine whether GPIbβ−/− cells had an abnormal phospholipid composition or content, as has been suggested for BSS patients, the major phospholipid species were quantified in platelet extracts.43 Thin-layer chromatography showed a normal distribution of PS plus phosphatidylinositol, PE, sphingomyelin, and phosphatidylcholine in GPIbβ−/− extracts (supplemental Figure 2). Thus, the defective procoagulant activity was the result of reduced phospholipid exposure rather than an abnormal membrane composition. It is noteworthy that the total amount of phospholipid per platelet was increased rather than decreased in GPIbβ−/− platelets, representing approximately twice the value in WT platelets. This finding is in line with the enlarged size of the cells, indicating once again that the deficient thrombin generation was not the result of a lower PS content.
Normal thrombin production and PS exposure in platelets lacking the GPIbα N-terminal domain
In view of the proposed role of the N-terminal domain of GPIbα in procoagulant activity, we investigated whether its removal would reproduce the defects observed in the absence of the whole receptor. Normal mouse and human platelets were treated with Nk protease, which is known to selectively cleave this region in human GPIbα,38 or with OSE, which eliminates the GPIbα N-terminal domain in human and mouse platelets.44
Mouse platelets treated with OSE or Nk protease lost reactivity to mAbs against the 45-kDa N-terminal region of GPIbα and no longer bound VWF in the presence of botrocetin (Table 1). An effect on thrombin binding could not be documented in the mouse because untreated platelets did not bind PPACK-thrombin (Table 1). The GPIbα selectivity of Nk protease was confirmed by the normal capacity of treated platelets to bind mAbs against GPIbβ, GPIX, GPV, integrin α2β1, integrin αIIbβ3, and GPVI (Table 1). OSE was less selective because it also decreased GPVI reactivity by 40%. OSE did not affect other glycoproteins in the mouse but, surprisingly, increased reactivity by 18% to 48% against GPIbβ, GPV, αIIbβ3, and α2β1 in human platelets (Table 2). In human platelets, Nk protease and OSE similarly abolished binding of VWF and mAbs against the GPIbα N-terminal domain. Furthermore, the thrombin binding region was removed as documented by the loss of PPACK-thrombin, echicetin,45 and mAb LJ1B10 binding (Table 2).23 Thrombin-induced aggregation was not affected by Nk protease or OSE treatment despite GPIbα cleavage (supplemental Figure 3). Collagen-induced aggregation was also normal after Nk protease treatment but was decreased in OSE-treated platelets in line with its capacity to cleave GPVI (Tables 1–2).
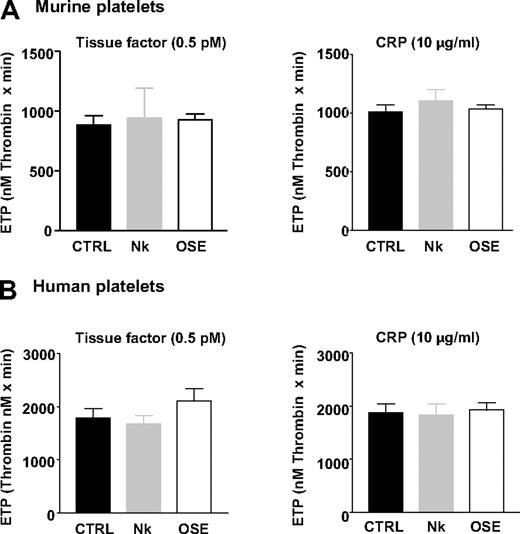
Unexpectedly, in Nk protease– and OSE-treated mouse platelets reconstituted in normal plasma, total thrombin generation (ETP) was completely normal in the CAT assay, after activation with TF or CRP (Figure 3A). Similarly, Nk protease– and OSE-treated human platelets generated thrombin equally as untreated cells (Figure 3B). Similar results were obtained when analyzing the thrombin peak levels in TF- and CRP-induced samples (supplemental Table 1).
Normal thrombin production in platelets lacking the GPIbα N-terminal domain. Washed platelets from WT mice (A) or washed human platelets (B) were left untreated (CTRL) or treated with Nk protease (Nk) or OSE until complete release of the GPIbα N-terminal domain (Tables 1–2). Thrombin generation was measured by the CAT method in platelets reconstituted in normal mouse plasma at 1.3 × 108 cells/mL (A) or normal human plasma at 1 × 108 cells/mL (B), after activation with TF (0.5pM) or CRP (10 μg/mL). ETP values are expressed as the mean ± SEM in 3 to 6 independent experiments.
Normal thrombin production in platelets lacking the GPIbα N-terminal domain. Washed platelets from WT mice (A) or washed human platelets (B) were left untreated (CTRL) or treated with Nk protease (Nk) or OSE until complete release of the GPIbα N-terminal domain (Tables 1–2). Thrombin generation was measured by the CAT method in platelets reconstituted in normal mouse plasma at 1.3 × 108 cells/mL (A) or normal human plasma at 1 × 108 cells/mL (B), after activation with TF (0.5pM) or CRP (10 μg/mL). ETP values are expressed as the mean ± SEM in 3 to 6 independent experiments.
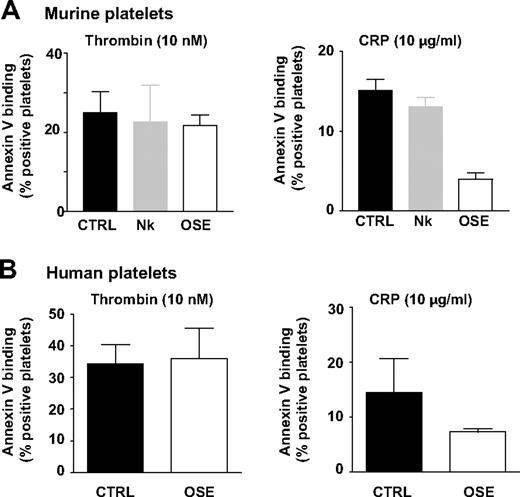
Moreover, normal PS exposure was observed in Nk protease–treated mouse platelets compared with untreated cells, after thrombin (22.6% ± 9.3% vs 26.9% ± 10.6% annexin V positivity) or CRP activation (13.1% ± 1.2% vs 15.1% ± 1.4% annexin V positivity; Figure 4A). Annexin V binding in response to thrombin (10nM) was likewise not affected by OSE treatment in murine or human platelets. This finding was also observed for lower thrombin concentrations (1.25-5nM; data not shown). However, decreased PS exposure was observed in response to CRP, possibly because of cleavage of GPVI by this enzyme (Figure 4A-B). The effect of OSE was also evaluated in the absence of stimulation on PS, active αIIbβ3 and P-selectin exposure and thrombin generation. Minimal differences were observed in comparison with untreated platelets excluding the possibility of preactivation by OSE (supplemental Tables 2-3). In addition, OSE-treated platelets exhibited a resting smooth disc morphology in scanning electron microscopy (supplemental Figure 4). Overall, these results indicated that the reduced procoagulant activity in platelets lacking GPIb-V-IX was not directly caused by the absence of the GPIbα N-terminal binding region.
Normal PS exposure in platelets lacking the GPIbα N-terminal domain. Washed platelets from WT mice (A) or washed human platelets (B) were treated as in Figure 3 and assayed for annexin V binding by flow cytometry after stimulation with thrombin (10nM) or CRP (10 μg/mL). Results are expressed as the mean percentage (± SEM) of annexin V-positive platelets and are from 3 to 5 independent experiments.
Normal PS exposure in platelets lacking the GPIbα N-terminal domain. Washed platelets from WT mice (A) or washed human platelets (B) were treated as in Figure 3 and assayed for annexin V binding by flow cytometry after stimulation with thrombin (10nM) or CRP (10 μg/mL). Results are expressed as the mean percentage (± SEM) of annexin V-positive platelets and are from 3 to 5 independent experiments.
Decreased calcium mobilization in GPIb-V-IX–deficient platelets but normal response in GPIbα-cleaved cells
In view of the calcium dependency of PS externalization, which was defective in GPIb-V-IX–deficient (Figure 2) but not in GPIbα-cleaved platelets (Figure 4), calcium mobilization was evaluated under these 2 conditions. Compared with normal cells, GPIbβ−/− platelets exhibited smaller calcium increases in response to thrombin (10nM) or a combination of CRP (35 μg/mL) and AYPGKF (500μM), with values representing 53.2% (± 14.0%) and 33.0% (± 3.1%) of the WT, whereas the response to ADP appeared to be unchanged (Figure 5A). In contrast, cleavage of the GPIbα terminus by Nk protease did not affect calcium mobilization in response to thrombin, CRP + AYPGKF, or ADP.
Decreased calcium mobilization in GPIb-V-IX–deficient platelets but normal responses in the absence of the GPIbα N-terminal domain. (A) Washed platelets from GPIbβ+/+ (β+/+) or GPIbβ−/− (β−/−) mice and GPIbβ+/+ platelets treated with Nk protease (WT + Nk) were loaded with Fura-2. Intracellular calcium levels were quantified by spectrofluorometry after stimulation with ADP (5μM), thrombin (10nM), or a mixture of CRP (35 μg/mL) and the PAR4 agonist peptide AYPGKF (0.5mM). (B) Intracellular calcium levels were quantified in platelets from GPIbβ+/+ or GPIbβ−/− mice after stimulation with U46619 (1μM), CRP (35 μg/mL), or a mixture of CRP (35 μg/mL) and U46619 (1μM). Use of a different batch of CRP likely explains the increased calcium levels compared with panel A. Results are expressed as the mean calcium concentration in nanomolars (± SEM) and are from 3 to 6 independent experiments (*P < .05 vs WT).
Decreased calcium mobilization in GPIb-V-IX–deficient platelets but normal responses in the absence of the GPIbα N-terminal domain. (A) Washed platelets from GPIbβ+/+ (β+/+) or GPIbβ−/− (β−/−) mice and GPIbβ+/+ platelets treated with Nk protease (WT + Nk) were loaded with Fura-2. Intracellular calcium levels were quantified by spectrofluorometry after stimulation with ADP (5μM), thrombin (10nM), or a mixture of CRP (35 μg/mL) and the PAR4 agonist peptide AYPGKF (0.5mM). (B) Intracellular calcium levels were quantified in platelets from GPIbβ+/+ or GPIbβ−/− mice after stimulation with U46619 (1μM), CRP (35 μg/mL), or a mixture of CRP (35 μg/mL) and U46619 (1μM). Use of a different batch of CRP likely explains the increased calcium levels compared with panel A. Results are expressed as the mean calcium concentration in nanomolars (± SEM) and are from 3 to 6 independent experiments (*P < .05 vs WT).
Decreased calcium signaling was also observed in GPIbβ−/− platelets in response to the thromboxane analog U46619 (1μM), CRP (35 μg/mL), or a combination of U46619 and CRP (Figure 5B). These results indicate a general signaling defect linked to GPIb that is not explained by PAR receptors alterations.
Discussion
The involvement of the GPIb-V-IX complex in platelet procoagulant activity is now well accepted, but questions remain as to its exact role and the underlying mechanisms. The present study of GPIb-V-IX–deficient mice clearly establishes an important positive role of this receptor in prothrombinase activity and PS exposure in response to several agonists. More importantly, we demonstrate that GPIb-V-IX regulates procoagulant activity independently of the N-terminal binding region of the GPIbα subunit, which suggests an alternative structuring function.
Despite existing data supporting a role of the N-terminal domain of GPIbα,25-29 its enzymatic removal did not lead to decreased thrombin generation or PS exposure in mouse or human platelets. In human cells, binding of VWF and thrombin could be clearly eliminated, ruling out the involvement of these 2 ligands. An earlier observation of decreased prothrombinase activity in the presence of antibodies against the GPIbα thrombin binding region could possibly be explained by the experimental design. This required the preactivation of platelets by thrombin, which is itself blocked by these antibodies.27 In another study implicating the GPIbα VWF binding domain, the fact that procoagulant activity and GPIb dependency required the addition of preformed fibrin makes comparison with the present work difficult.21,22 Recently, another GPIb-dependent mechanism contributing to thrombin generation and implicating FVIIa was proposed.46 Despite demonstration that the extracellular domain of GPIbα interacts with rFVIIa, prevention of this interaction by OSE cleavage appeared to have only a minimal impact on thrombin generation.
If the GPIbα N-terminus is dispensable for platelet procoagulant activity, then other regions of the GPIb-V-IX complex are likely to be involved. A role of GPV can be eliminated because GPV-deficient platelets, which have normal levels of GPIb-IX,47 display a normal procoagulant response (C.R. and F.L., unpublished data, 2005). Other domains of GPIbα could play a part, including the macroglycopeptide, transmembrane, and intracellular portions. It is tempting to implicate the intracellular side of the receptor in view of the abnormal calcium signaling observed only in GPIb-deficient platelets (Figure 5), which would suggest a more general defect of platelet signaling possibly caused by the absence of proper anchoring of the membrane to the cytoskeleton. Several intracellular partners may be considered, in particular filamin, which links the receptor to cortical actin, and other adaptor and signaling proteins, such as 14-3-3, phosphatidylinositol 3-kinase, and calmodulin.25 Studies of platelets with intracellular modifications will be required to evaluate these candidate domains and effectors. Another argument for a role of the intracellular side of the receptor is the decreased ability to generate filamentous actin after activation by thrombin or CRP in GPIb-deficient compared with WT platelets (supplemental Figure 5). The abnormal actin polymerization is in line with the reported effect of actin on procoagulant activity48 and consistent with a structuring function of GPIb through the filamin linkage. A general role of GPIb-V-IX, independent of a GPIb-restricted pathway, is also indicated by the decreased thrombin generation and PS exposure of GPIbβ−/− platelets in response to a series of agonists not acting through GPIb, such as the CRP and TRAP agonist peptides.
Concerning CRP, questions remain on the mechanisms of its procoagulant effect. They likely involve GPVI-dependent PS exposure, but other events could take place, such as a contact activation process, suggested by the slight effect of corn trypsin inhibitor on thrombin generation (supplemental Table 4), and implication of blood-borne TF is also possible. GPVI-independent events can also take place after the first traces of thrombin are generated through platelet endogenous agonists. This could explain why OSE treatment, which reduces GPVI expression, inhibited annexin V binding triggered by CRP but did not prevent normal thrombin generation (Figures 3–4). OSE treatment did not affect other glycoproteins in the mouse. Surprisingly, increased GPIbβ, GPV, αIIbβ3, and α2β1 levels were observed in human platelets. These do not appear to result from externalization of intracellular pools because these OSE-treated platelets did not express P-selectin or active αIIbβ3 and exhibited a discoid resting morphology. One possibility is that OSE cleavage could facilitate mAb access to certain glycoproteins.
In GPIb-V-IX–deficient platelets, inefficient externalization of negatively charged phospholipids was observed in response to all agonists and appears to be a likely explanation for their decreased prothrombinase activity. The latter was not caused by a limited content of membrane phospholipids or an abnormal proportion of PS. PS is exposed through an activation dependent process triggered by a rise in intracellular calcium and also through mechanisms independent of platelet activation.1-6 Although GPIb-V-IX–deficient platelets exhibited normal aggregation and secretion after stimulation by thrombin or a combination of PAR4 peptide and CRP, calcium mobilization was reduced in response to these stimuli and also in response to U46619 alone or in combination with CRP. These results support the hypothesis of a general signaling defect that is not explained by PAR receptors alterations. GPIb-V-IX deficiency could also have consequences on calcium-independent PS exposure, which will require further investigation. The authors of a previous study of a Bernard-Soulier patient49 also reported decreased calcium mobilization in response to thrombin and the PAR1 agonist peptide. These results point once again to a general role of the GPIb-V-IX complex in the intracellular responses regulating PS redistribution. Several investigators have pointed to differential signaling pathways leading to platelet aggregation and the formation of procoagulant platelets.50 How these findings fit in with our present results will be a matter for future investigations.
In conclusion, this study further establishes a central role of the GPIb-V-IX complex in platelet procoagulant responses and agrees with the original description of abnormal prothrombin consumption in patients. The observation of normal thrombin generation after removal of the VWF and thrombin binding sites indicates that other domains of this multisubunit receptor play a major part. Intracellular signaling functions and the capacity of GPIb-V-IX to structure the actin cytoskeleton might be involved in view of the decreased PS exposure in response to several agonists.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Claire Petitjean and Dominique Cassel (UMR_S 949 Inserm, Etablissement Français du Sang-Alsace, Strasbourg, France) for their technical assistance. Monique Freund is gratefully acknowledged for animal care, Robert Andrews for generously providing NK protease, Martine Jandrot-Perrus for her helpful advice, and Juliette Mulvihill (Scientific Translations, Salzburg, Austria) for reviewing the manuscript for proper English usage.
Authorship
Contribution: C.R., C.S., C.G., and F.L. designed the research; C.R. and C.S. performed the research; B.H. supervised the calcium measurement experiments; S.S. assisted C.R. in all experiments; G.C. and B.P. performed lipid analyses, C.R., C.S., B.H., C.G., B.P., and F.L. analyzed the data; and C.R., C.S., C.G., and F.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr François Lanza, Inserm U.949, EFS-Alsace, 10, rue Spielmann, BP No 36, 67065 Strasbourg Cedex, France; e-mail: francois.lanza@efs-alsace.fr.