Abstract
The plasminogen activation system plays an integral role in the migration of macrophages in response to an inflammatory stimulus, and the binding of plasminogen to its cell-surface receptor initiates this process. Although previous studies from our laboratory have shown the importance of the plasminogen receptor S100A10 in cancer cell plasmin production, the potential role of this protein in macrophage migration has not been investigated. Using thioglycollate to induce a peritoneal inflammatory response, we demonstrate, for the first time, that compared with wild-type (WT) mice, macrophage migration across the peritoneal membrane into the peritoneal cavity in S100A10-deficient (S100A10−/−) mice was decreased by up to 53% at 24, 48, and 72 hours. Furthermore, the number of S100A10-deficient macrophages that infiltrated Matrigel plugs was reduced by 8-fold compared with their WT counterpart in vivo. Compared with WT macrophages, macrophages from S100A10−/− mice demonstrated a 50% reduction in plasmin-dependent invasion across a Matrigel barrier and a 45% reduction in plasmin generation in vitro. This loss in plasmin-dependent invasion was in part the result of a decreased generation of plasmin and a decreased activation of pro-MMP-9 by S100A10-deficient macrophages. This study establishes a direct involvement of S100A10 in macrophage recruitment in response to inflammatory stimuli.
Introduction
Monocytes/macrophages play a central role in pathogenic inflammatory responses associated with atherosclerosis, restenosis, tumor surveillance, and arthritis.1-3 In response to changes in the cellular environment, monocytes and monocytoid cells undergo extensive phenotypic alterations, including marked changes in their fibrinolytic properties. Synthesis and activation of matrix-degrading proteinases by monocytes and macrophages play an essential role in their migration through tissue. A key proteinase that participates in pericellular proteolysis is the serine proteinase plasmin. Plasmin is a broad substrate proteinase that is formed from the inactive zymogen plasminogen (Plg) by the Plg activators, tissue Plg activator (tPA) and urokinase-type Plg activator (uPA).4,5 The participation of plasmin in cell invasion and migration is dependent on the ability of plasmin not only to degrade extracellular matrix (ECM) proteins but to also activate other proteinases that have matrix-degrading activity. Plasmin can degrade a variety of matrix proteins, such as laminin and fibronectin, and appears to activate matrix metalloproteinase-1 (MMP-1), MMP-3, and MMP-13 directly, and to activate MMP-2 and MMP-9 indirectly, thereby facilitating cell migration through ECMs.6
The assembly of Plg and its activators on the cell surface is facilitated by the protein S100A10 (also referred to as p11). S100A10 is a member of the S100 family of calcium-binding proteins and is typically found in most cells bound to its annexin A2 (p36) ligand as the heterotetrameric (S100A10)2-(annexin A2)2 complex, annexin A2 heterotetramer (AIIt).7,8 The S100A10 subunit of AIIt possesses a carboxy-terminal lysine residue that binds tPA and Plg, resulting in the conversion of Plg to plasmin.9 The binding of plasmin to AIIt protects plasmin from inactivation by α2-antiplasmin.10 Removal of the carboxyl-terminal lysines from S100A10 by carboxypeptidase B (CpB) results in the loss of Plg binding and plasmin generation.11 Loss of S100A10 from the extracellular surface of cancer cells significantly reduces plasmin generation, thus dramatically impacting on the cells capacity to degrade ECM and infiltrate into surrounding tissue.12 In contrast, increased extracellular levels of S100A10 result in increased Plg binding and increased production of plasmin.12
The formation of a ternary complex between tPA, Plg, and S100A10 provides a mechanism to localize the proteolytic activity of plasmin to the cell surface. Previous studies have established the presence of S100A10 and its binding partner, annexin A2 (p36), on the surface of murine macrophages.13,14 These studies showed that knockdown of annexin A2 resulted in decreased plasmin generation, matrix remodeling, and a dramatic loss in directed migration.13,15 However, because annexin A2 knockdown results in concomitant loss of S100A10,16 it has been difficult to attribute these effects to annexin A2 or S100A10. Our laboratory has previously shown that loss of S100A10 from the extracellular surface of cancer cells, such as HT1080 fibrosarcoma cells, significantly reduces plasmin generation and also results in a loss in the ability of these cells to degrade ECM and also to infiltrate into surrounding tissues.12
In the current report, we investigated the potential role of S100A10 in the regulation of peritonitis-dependent macrophage migration using the recently developed S100A10-deficient (S100A10−/−) mouse. This study marks the first characterization of macrophage function in the S100A10−/− mouse and establishes that S100A10 plays a significant role in peritonitis-directed macrophage migration in vivo.
Methods
A detailed description of the routine methods is presented in the supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Only nonroutine procedures and specialized materials are described here.
Mice
A detailed description of the mice used in this study can be found in the Supplemental data. All mouse experiments were performed in accordance with protocols approved by the University Committee on Laboratory Animals at Dalhousie University.
Mouse leukocyte collection
Wild-type (WT) and S100A10−/− mice were injected intraperitoneally with 2.5 mL of a 4% Brewers thioglycollate (TG) solution (Sigma-Aldrich). Peritoneal leukocytes were collected each day for 6 days after injection of TG (n = 3-9) and cell viability determined by Trypan blue exclusion. Resident leukocytes were collected from uninjected mice (n = 6). Peritoneal cells were collected by lavage with 5 mL complete RPMI media. The lavage fluid was centrifuged, the supernatant aspirated, and the cell pellet was resuspended in complete RPMI. Total numbers of leukocytes were determined by cell counting with a hemocytometer. Cytospin slides were also prepared at each time point using a Shandon Cytospin 2 (Thermo Shandon). Slides were air dried, stained with May-Grünwald-Giemsa (Sigma-Aldrich), and cellular morphology examined under the light microscope. Cell differentials were obtained from morphologic analysis (n = 5), and values were expressed as mean plus or minus SD.
Mouse peritoneal macrophages
WT and S100A10−/− mice were injected intraperitoneally with 2.5 mL of a 4% Brewers TG solution (Sigma-Aldrich). After 4 days, when most recruited cells are macrophages,17 mice were killed and peritoneal cells were collected by lavage with 5 mL complete RPMI media. The lavage fluid was centrifuged, the supernatant aspirated, and the cell pellet resuspended in complete medium. Macrophages were further purified by adherence for 3 to 4 hours at 37°C, at which point all nonadherent (nonmacrophage) cells were eliminated. Cells were maintained in RPMI media.
Analysis of protein expression
Proteins were analyzed by Western immunoblot, flow cytometry, and cell-surface biotinylation as described in detail in the supplemental data. Proteolyzed annexin A2 was prepared according to Kwon et al.18
Matrigel invasion and cell migration
Thioglycollate-elicited macrophages were loaded (1 × 105 to 3 × 105 cells/well, where indicated) into the upper portion of Transwell chambers, coated with Matrigel (invasion assays) or uncoated (migration assays; BD Biosciences). Plg (0.5μM, American Diagnostica) was added in the absence or presence of ϵ-amino caproic acid (ϵ-ACA, 100mM; Sigma-Aldrich), CpB (5 U/mL; Sigma-Aldrich), aprotinin (2.2μM; Sigma-Aldrich), MMP inhibitor GM6001 (25μM; Millipore), the MMP-9 neutralizing antibody (20 μg/mL; Calbiochem), proMMP9 (2 μg/mL; R&D Systems), plasmin (0.3μM; Enzyme Research Laboratories), S100A10, annexin A2, or bovine serum albumin (2 μg/mL). As a chemoattractant, MCP-1 (10 ng/mL; R&D systems), C5a (10 ng/mL; R&D Systems), plasmin (0.43 catalytically active [CTA] U/mL), or thioglycollate (4% weight/volume; Sigma-Aldrich) was added to the lower chamber for both the invasion and migration assays. After 48 hours, cells on the underside of the membrane were stained with hematoxylin and eosin (Sigma-Aldrich) and counted. Recombinant S100A10 and annexin A2 were purified from bovine lung as described by Khanna et al.19
Plg activation
WT and S100A10−/− thioglycollate-elicited macrophages (2.5 × 105 cells) were plated in 96-well plates. Cells were incubated with or without uPA (50nM) for 10 minutes at room temperature. Cells were washed 3 times with incubation buffer (Hanks balanced salt solution [HBSS] containing 3mM CaCl2 and 1mM MgCl2) and incubated with 0.5μM glu-Plg with or without CpB (5 U/mL) for 10 minutes before the addition of 500μM plasmin substrate S2251 (Chromogenix, Diapharma Group). The rate of plasmin generation was measured using a spectrophotometer (405nM) taking readings every minute for 2 hours.
FITC-Plg preparation
Glu-Plg (2-5 mg/mL) was dialyzed against 0.1M carbonate buffer (pH 9), and a 50M excess of fluorescein isothiocyanate (FITC) was added after being dissolved in dimethyl sulfoxide. Plg and FITC were mixed for 16 hours in the dark and treated with 0.01% hydroxylamine to remove all labile FITC-Plg bonds. Unincorporated FITC was removed by gel filtration through an NAP-10 column using HBSS (20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 1mM CaCl2, and 1mM MgCl2; pH 7.4). Typically, 2 FITC molecules were bound to each Plg molecule.
Plg binding assays
WT and S100A10−/− thioglycollate-elicited macrophages were washed and cultured in the absence of serum for 2 hours before assay. Cells were incubated with 200nM FITC Glu-Plg, either with or without ϵ-ACA (100mM), for 1 hour at 4°C in HBSS (1mM MgCl2 and 3mM CaCl2). Plg binding was measured by fluorescence-activated cell sorter (FACS), excluding cells that were positive for propidium iodide (Sigma-Aldrich). Percentage binding refers to mean fluorescence intensity.
Zymography
Detailed zymographic techniques are described in the supplemental data.
Matrigel-plug assay
A total of 750 μL growth factor–reduced Matrigel with 200 ng/mL basic fibroblast growth factor (Invitrogen) and 60 U/mL heparin (Calbiochem) added was injected subcutaneously into WT and S100A10−/− C57BL/6 mice. After 7 days, the Matrigel plug was removed and processed for immunohistochemistry.
Histochemistry and immunohistochemistry
Matrigel plugs were fixed in 10% formalin and embedded in paraffin. Paraffin sections were deparaffinized, blocked with horse serum (1:20; Invitrogen) and incubated with an antibody against Mac-3 (55092; BD Biosciences), F4/80 (MCA497; Abd Serotec), or normal mouse IgG1 (as control; BD Biosciences) at room temperature overnight. Subsequently, a peroxidase diaminobenzidine detection system (Dako North America) was applied according to the manufacturer's instructions. Sections were counterstained with hematoxylin. Sections were mounted using Cytoseal 60 mounting media (Richard-Allen Scientific) and viewed using either a 40×/0.75 NA or 10×/0.3 NA objective lens. Images were captured by the Nikon Eclipse E600 microscope using a Nikon DXM1200F camera. Digital acquisition of the images was performed using ACT-1 v2.7 software (Nikon). Figures were generated using Adobe Photoshop CS3 v10 (Adobe Systems Incorporated). The data were quantified using Image J v1.42q software (National Institutes of Health).
Statistics
Statistical significance was determined by Student t test or one-way analysis of variance with Tukey multiple comparisons. Results were regarded as significant if 2-tailed P values were less than .05. Data are mean plus or minus SD.
Results
Leukocyte recruitment after induction of peritoneal inflammation with thioglycollate is impaired in S100A10−/− mice
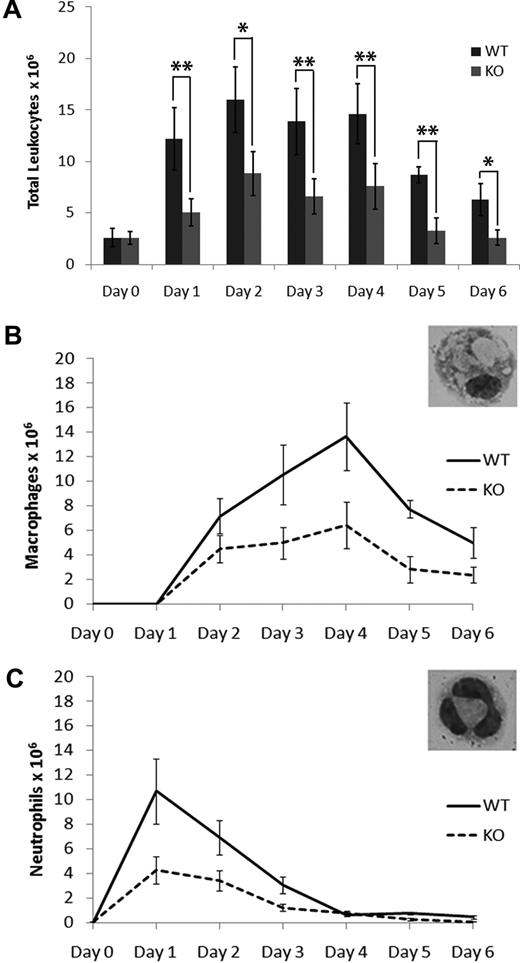
Recruitment of macrophages in response to thioglycollate-induced peritonitis is severely compromised in Plg-knockout mice, indicating that macrophages use the Plg activation system for migration to the site of inflammation.20-22 Although S100A10 has been shown to be an important Plg-binding protein in endothelial and cancer cells, the role of this protein in macrophage migration and invasion has not been examined.7,11 To study the in vivo recruitment of inflammatory cells in S100A10−/− (KO) mice, a sterile inflammatory response was induced by the intraperitoneal injection of thioglycollate. An analysis of the time course of total leukocyte recruitment was performed in both WT and S100A10−/− mice (Figure 1A). We observed that the number of resident leukocytes (day 0) was identical in WT and S100A10−/− mice. However, the number of leukocytes that had migrated into the peritoneal cavity was decreased by up to 60% in S100A10−/− mice compared with their WT counterparts.
Leukocyte recruitment in response to thioglycollate is impaired in S100A10−/− mice. (A) Mice were injected intraperitoneally with thioglycollate, and the total peritoneal leukocytes were collected at various time points by peritoneal lavage. Resident leukocytes were collected from unstimulated peritoneum and are represented as t = 0. Cell differentials of the total peritoneal leukocytes were determined by May-Grünwald-Giemsa staining and macrophage (B) and neutrophil (C) content determined. Statistical analysis was performed by Student t test: *P < .05; **P < .01. No significant difference was observed between resident leukocytes from WT and S100A10−/− (KO) mice (P < .124).
Leukocyte recruitment in response to thioglycollate is impaired in S100A10−/− mice. (A) Mice were injected intraperitoneally with thioglycollate, and the total peritoneal leukocytes were collected at various time points by peritoneal lavage. Resident leukocytes were collected from unstimulated peritoneum and are represented as t = 0. Cell differentials of the total peritoneal leukocytes were determined by May-Grünwald-Giemsa staining and macrophage (B) and neutrophil (C) content determined. Statistical analysis was performed by Student t test: *P < .05; **P < .01. No significant difference was observed between resident leukocytes from WT and S100A10−/− (KO) mice (P < .124).
To identify the inflammatory cells contributing to this response, blood differentials were performed (Figure 1B-C). Although the kinetics of leukocyte recruitment was identical in WT and S100A10−/− models, the total number of neutrophils and macrophages recruited in response to thioglycollate stimulation were dramatically decreased in the S100A10−/− mice. At day 1, neutrophil recruitment was maximal in WT and S100A10−/− mice, but the total number of cells was decreased by 60% in S100A10−/− mice compared with WT (10.7 ± 2.65 × 106, n = 6 in WT mice, and 4.28 ± 1.12 × 106, n = 6 in S100A10−/− mice; Figure 1C). The number of neutrophils that accumulated in the peritoneal cavity decreased after day 1 as expected.23 Macrophage recruitment peaked at 96 hours after thioglycollate stimulation; however, macrophage recruitment was severely compromised in S100A10−/− mice at that time point (13.7 ± 2.74 × 106, n = 9 in WT mice, and 6.43 ± 1.87 × 106, n = 6 in S100A10−/− mice; Figure 1B).
The diminished response in leukocyte recruitment into the peritoneum in response to thioglycollate-induced peritonitis cannot be explained by a diminished source of leukocytes in circulation in the S100A10−/− mice because the levels of blood cells in these mice were similar to WT mice (supplemental Table 1). These results suggest that, although the accumulation of leukocytes in the peripheral circulation is unaffected by S100A10 deficiency, the recruitment of neutrophils and macrophages into the peritoneal cavity in response to an inflammatory stimulus is reduced by S100A10 deficiency. Because the role of Plg in macrophage migration has been well documented, we focused our attention on characterizing the role of S100A10 in macrophage function.
Macrophage migration into a Matrigel plug is impaired in S100A10−/− mice
The simplest explanation for the decreased inflammatory response of S100A10−/− macrophages was that the loss of cell-surface S100A10 resulted in decreased plasmin generation and consequently an inability of the S100A10−/− macrophages to clear a passage through the basement membrane and ECM barrier of the peritoneal membrane. Therefore, a Matrigel plug assay was used to assess the ability of macrophages to infiltrate into an artificial basement membrane barrier in vivo. Matrigel is an extract of murine tumor cell-generated basement membrane, which is commonly used to access the role of proteinases in cellular migration. WT and S100A10−/− mice were implanted with Matrigel plugs, and the migration of macrophages into these plugs was visualized by immunohistochemical analysis (Figure 2A-B). Compared with the WT mice, the S100A10−/− mice demonstrated 5-fold more macrophages in the region of the tissue in proximity to the Matrigel plug and 2-fold more macrophages at the tissue-Matrigel barrier (Figure 2C-D). In contrast, we observed that sections obtained from the center of the Matrigel plug isolated from the S100A10−/− mice contained 8-fold less macrophages than Matrigel isolated from the WT mice. These results established that migration of S100A10−/− macrophages through Matrigel was impaired and therefore suggest that S100A10 is used by macrophages for movement through the basement membrane barrier.
Macrophage recruitment into a Matrigel plug is decreased in S100A10−/− mice. WT and S100A10−/− mice were implanted with a Matrigel plug containing 200 ng/mL basic fibroblast growth factor and 60 U/mL heparin. Plugs were removed after 7 days, fixed in formalin, and processed for immunohistochemical analysis. Sections were deparaffinized and incubated with anti-Mac-3 (A top panel, C) and anti-F4/80 antibody (A bottom panel). Microscopic fields containing the entire Matrigel plug (B), outer tissue surrounding the plug, Matrigel-tissue border, and center of Matrigel (D) were quantified by determining the percentage coverage of macrophages in each region using ImageJ software. Results are expressed as percentage of macrophages per field plus or minus SD of 3 independent experiments. Statistical analysis was performed by Student t test: ***P < .001. Arrows indicate the Matrigel-tissue border.
Macrophage recruitment into a Matrigel plug is decreased in S100A10−/− mice. WT and S100A10−/− mice were implanted with a Matrigel plug containing 200 ng/mL basic fibroblast growth factor and 60 U/mL heparin. Plugs were removed after 7 days, fixed in formalin, and processed for immunohistochemical analysis. Sections were deparaffinized and incubated with anti-Mac-3 (A top panel, C) and anti-F4/80 antibody (A bottom panel). Microscopic fields containing the entire Matrigel plug (B), outer tissue surrounding the plug, Matrigel-tissue border, and center of Matrigel (D) were quantified by determining the percentage coverage of macrophages in each region using ImageJ software. Results are expressed as percentage of macrophages per field plus or minus SD of 3 independent experiments. Statistical analysis was performed by Student t test: ***P < .001. Arrows indicate the Matrigel-tissue border.
Macrophages from S100A10−/− mice show impaired invasion through Matrigel
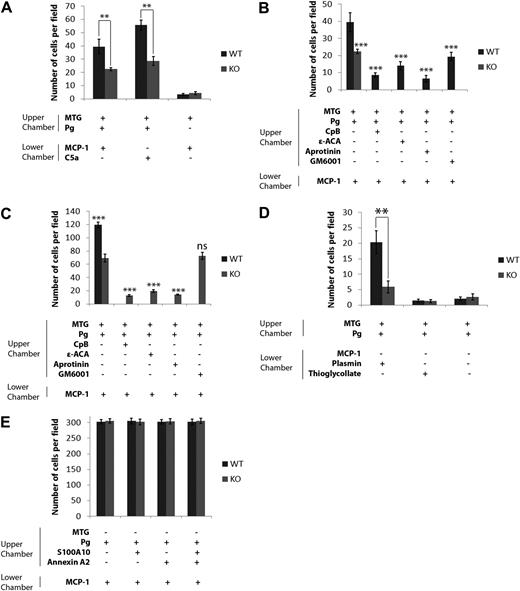
Next, we examined the mechanism by which macrophages isolated from WT and S100A10−/− mice migrate through the basement membrane. These experiments used Boyden chambers in which Plg and macrophages isolated from WT or S100A10−/− mice were placed in the upper chamber, the insert between chambers was coated with Matrigel, and a chemoattractant stimulus, MCP-1 or C5A, was added to the lower chamber. We observed that, in response to the chemoattractants, MCP or C5a, 50% fewer S100A10−/− macrophages migrated across the Matrigel barrier than WT macrophages (Figure 3A). Furthermore, as was previously observed by others,14 the migration of the macrophages was dependent on the presence of Plg in the upper chamber and the chemotactic agent in the lower chamber, suggesting that plasmin played an essential role in the migration of the macrophages through Matrigel in response to a chemotactic stimulus (Figure 3A; supplemental Figure 4). Consistent with this observation, we also observed that prior treatment of macrophages with the plasmin inhibitor aprotinin blocked Plg-dependent invasion (Figure 3B-C). S100A10 is known to bind plasmin.9 We also observed that invasion in response to plasmin was reduced almost by 75% by macrophages isolated from S100A10−/− mice compared with WT macrophages (Figure 3D; supplemental Figure 4), thus establishing the importance of S100A10 in plasmin-dependent invasion. When active plasmin was added to both WT and S100A10−/− macrophages, invasion was identical and higher than invasion in the absence of added plasmin, suggesting that activation of plasmin by S100A10 plays an important role in macrophage invasion (supplemental Figure 3). As expected, there was minimal invasion of macrophages when thioglycollate was added to the lower chamber, confirming that thioglycollate is not a chemoattractant (Figure 3D). These results suggested that S100A10-dependent plasmin generation played an important role in the invasion of macrophages across a Matrigel barrier.
Role of S100A10 in Matrigel invasion and cell migration. WT and S100A10−/− (KO) peritoneal macrophages (1 × 105 cells) were added to the upper chamber of Matrigel (MTG)-coated chambers (BD Biocoat chambers, 8-μm pore) and incubated in the presence or absence of Plg (0.5μM). The lower chamber contained MCP-1 or C5a (10 ng/mL). Cells were incubated at 37°C for 48 hours (A). Invading cells were quantified as described in “Matrigel invasion and cell migration.” Data are expressed as mean number of cells per 40× field plus or minus SD of 3 independent experiments. Statistical analysis was performed by Student t test: **P < .01. No significant difference in invasion was observed for WT and S100A10−/− macrophages in the absence of Plg (P < .053) by t test. WT (1 × 105 cells; B) and S100A10−/− macrophages (3 × 105 cells; C) were incubated with Plg, and aprotinin, ϵ-ACA, or CpB, and invasion determined in response to MCP-1 (10 ng/mL) in the lower chamber (B-C). The invading cells were quantified as described in panel A. Statistical analysis was performed by one-way analysis of variance comparison of untreated WT macrophage invasion (B) or untreated S100A10−/− macrophage invasion (C). WT and S100A10−/− macrophages (1 × 105 cells) were added with Plg to the upper chamber of Matrigel-coated chambers with plasmin (0.43 CTA U/mL) or thioglycollate (4%) as chemoattractant in the lower chamber (D). Invasion was quantified as described in panel A. Statistical analysis was performed by Student t test: **P < .01. No significant difference in invasion was observed between WT and S100A10−/− macrophages in the absence of a chemoattractant or when thioglycollate was used as a chemoattractant. WT and S100A10−/− peritoneal macrophages (1 × 105 cells) were added to uncoated invasion chambers in the presence of 0.5μM Plg (E). Cells were incubated at 37°C for 48 hours. Migrating cells were quantified as described in panel A. Statistical analysis was performed by Student t test: **P < .01. No significant difference was observed between WT and S100A10−/− macrophage migration in the absence of Matrigel: ***P < .001. ns indicates not significant.
Role of S100A10 in Matrigel invasion and cell migration. WT and S100A10−/− (KO) peritoneal macrophages (1 × 105 cells) were added to the upper chamber of Matrigel (MTG)-coated chambers (BD Biocoat chambers, 8-μm pore) and incubated in the presence or absence of Plg (0.5μM). The lower chamber contained MCP-1 or C5a (10 ng/mL). Cells were incubated at 37°C for 48 hours (A). Invading cells were quantified as described in “Matrigel invasion and cell migration.” Data are expressed as mean number of cells per 40× field plus or minus SD of 3 independent experiments. Statistical analysis was performed by Student t test: **P < .01. No significant difference in invasion was observed for WT and S100A10−/− macrophages in the absence of Plg (P < .053) by t test. WT (1 × 105 cells; B) and S100A10−/− macrophages (3 × 105 cells; C) were incubated with Plg, and aprotinin, ϵ-ACA, or CpB, and invasion determined in response to MCP-1 (10 ng/mL) in the lower chamber (B-C). The invading cells were quantified as described in panel A. Statistical analysis was performed by one-way analysis of variance comparison of untreated WT macrophage invasion (B) or untreated S100A10−/− macrophage invasion (C). WT and S100A10−/− macrophages (1 × 105 cells) were added with Plg to the upper chamber of Matrigel-coated chambers with plasmin (0.43 CTA U/mL) or thioglycollate (4%) as chemoattractant in the lower chamber (D). Invasion was quantified as described in panel A. Statistical analysis was performed by Student t test: **P < .01. No significant difference in invasion was observed between WT and S100A10−/− macrophages in the absence of a chemoattractant or when thioglycollate was used as a chemoattractant. WT and S100A10−/− peritoneal macrophages (1 × 105 cells) were added to uncoated invasion chambers in the presence of 0.5μM Plg (E). Cells were incubated at 37°C for 48 hours. Migrating cells were quantified as described in panel A. Statistical analysis was performed by Student t test: **P < .01. No significant difference was observed between WT and S100A10−/− macrophage migration in the absence of Matrigel: ***P < .001. ns indicates not significant.
When chemotaxis assays were repeated in the absence of a Matrigel barrier, the migration of macrophages from WT and S100A10−/− mice through the inserts was indistinguishable, even with addition of S100A10 and annexin A2 (Figure 3E). This established that the ability of the S100A10−/− macrophages to migrate in response to a chemotactic stimulus was unaffected by genetic ablation of S100A10.
Our laboratory has demonstrated that S100A10 binds Plg through the interaction of its carboxyl-terminal lysine with the kringle domains of Plg and that removal of the carboxyl-terminal lysine of S100A10 results in the loss of Plg binding and plasmin generation by the protein.7,11 When macrophages from WT or S100A10−/− mice were treated either with the lysine analog,24 ϵ-ACA, which blocks the interaction of carboxyl-terminal lysines with Plg, or with CpB, which cleaves carboxy-terminal lysines,11 we observed a drastic reduction in macrophage invasion through Matrigel (Figure 3B-C). The loss in invasion after treatment of S100A10−/− macrophages with ϵ-ACA or CpB suggested that, in addition to S100A10, other Plg receptors possessing a carboxyl-terminal lysine also participated in macrophage invasion.
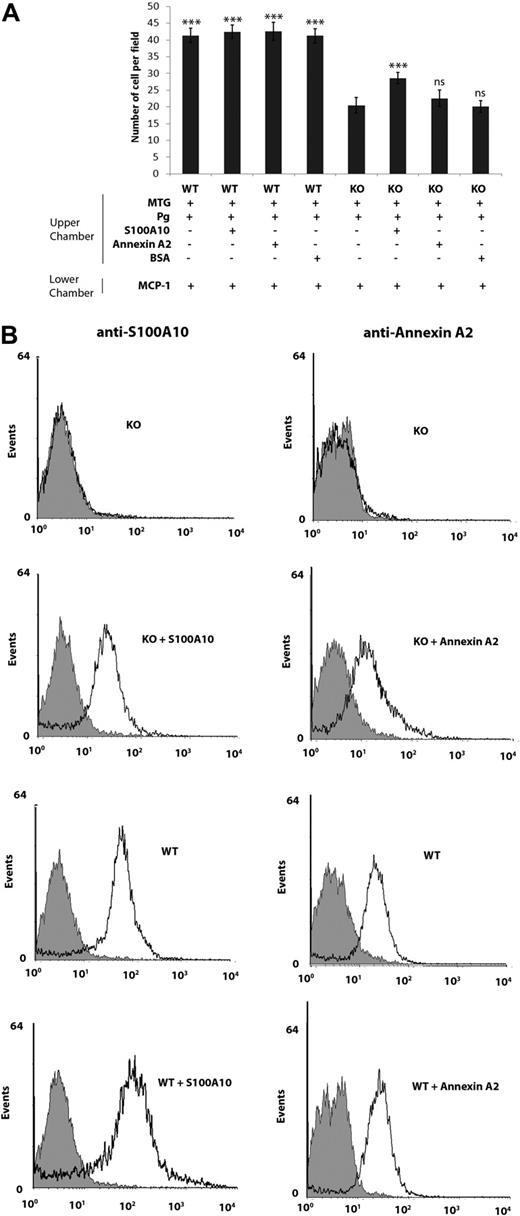
To determine whether readdition of S100A10 would affect the invasion of S100A10−/− macrophages, recombinant S100A10 was incubated with S100A10−/− and WT cells and invasion compared (Figure 4A). The addition of recombinant S100A10 to WT macrophages had no effect on their invasiveness; however, the invasiveness of S100A10−/− macrophages increased by 30%, compared with untreated S100A10−/− macrophages (20.4 ± 2.3 for untreated S100A10−/− cells vs 28.6 ± 1.7 for S100A10-treated S100A10−/− cells; Figure 4A). Importantly, addition of annexin A2 to both WT and S100A10−/− macrophages did not affect invasion. FACS analysis verified that addition of annexin A2 and S100A10 to S100A10−/− macrophages increased the extracellular levels of these proteins on the cell surface of the S100A10−/− macrophages (Figure 4B).
Addition of S100A10, but not annexin A2, increases invasion of S100A10−/− macrophages. (A) WT and S100A10−/− (KO) peritoneal macrophages (1 × 105 cells) were added to the upper chamber of Matrigel-coated chambers (BD Biocoat chambers, 8-μm pore), which contained Plg and S100A10, annexin A2, or bovine serum albumin (2 μg/mL). The lower chamber contained MCP-1 (10 ng/mL). Cells were incubated at 37°C for 48 hours. Invading cells were quantified as described in “Matrigel invasion and cell migration.” Data are expressed as mean number of cells per 40× field plus or minus SD of 3 independent experiments. Statistical analysis was performed by one-way analysis of variance (with Tukey multiple comparisons) compared with Plg-dependent S100A10−/− invasion: ***P < .001. ns indicates not significant. (B) Flow cytometric analysis of cell-surface annexin A2 and S100A10 levels in WT and S100A10−/− (KO) peritoneal macrophages. Macrophages were incubated in the presence or absence of annexin A2 (2 μg/mL) or S100A10 (2 μg/mL), washed, and incubated with antiannexin A2 and anti-S100A10 antibodies. Black line indicates staining with anti–annexin A2 or anti-S100A10 antibodies; and light gray area, the reaction with nonimmune rabbit IgG.
Addition of S100A10, but not annexin A2, increases invasion of S100A10−/− macrophages. (A) WT and S100A10−/− (KO) peritoneal macrophages (1 × 105 cells) were added to the upper chamber of Matrigel-coated chambers (BD Biocoat chambers, 8-μm pore), which contained Plg and S100A10, annexin A2, or bovine serum albumin (2 μg/mL). The lower chamber contained MCP-1 (10 ng/mL). Cells were incubated at 37°C for 48 hours. Invading cells were quantified as described in “Matrigel invasion and cell migration.” Data are expressed as mean number of cells per 40× field plus or minus SD of 3 independent experiments. Statistical analysis was performed by one-way analysis of variance (with Tukey multiple comparisons) compared with Plg-dependent S100A10−/− invasion: ***P < .001. ns indicates not significant. (B) Flow cytometric analysis of cell-surface annexin A2 and S100A10 levels in WT and S100A10−/− (KO) peritoneal macrophages. Macrophages were incubated in the presence or absence of annexin A2 (2 μg/mL) or S100A10 (2 μg/mL), washed, and incubated with antiannexin A2 and anti-S100A10 antibodies. Black line indicates staining with anti–annexin A2 or anti-S100A10 antibodies; and light gray area, the reaction with nonimmune rabbit IgG.
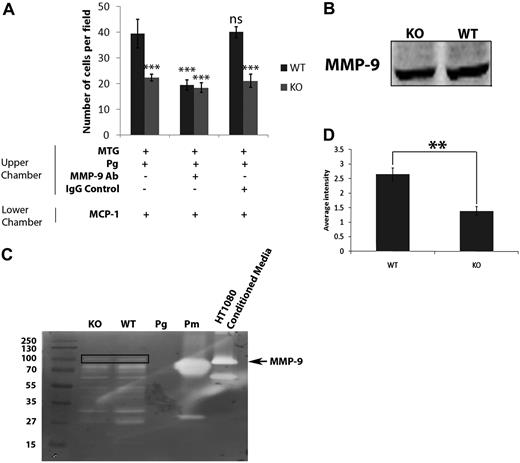
We also observed that treatment of WT macrophages with the general MMP inhibitor GM6001 reduced chemotaxis by approximately 50% (Figure 3B). Surprisingly, GM6001 did not affect the invasion of macrophages from S100A10−/− mice (Figure 3C). Plasmin activates several MMPs,25 and it has been shown that plasmin-dependent activation of MMP-9 plays a key role in macrophage migration through Matrigel.22 It was therefore possible that S100A10-directed plasmin generation might function to activate MMP-9. We tested this possibility by pretreating the macrophages with an MMP-9 inhibitory antibody or an isotypic antibody as a control. Although the MMP-9 inhibitory antibody reduced the migration of WT macrophages to a similar levels as was observed for the S100A10−/− macrophages, the MMP-9 inhibitory antibody did not affect the invasion of the S100A10−/− macrophages (Figure 5A). Furthermore, addition of proMMP-9 increased the invasion of both WT and S100A10−/− macrophages (supplemental Figure 3).
S100A10 mediates activation of MMP-9. WT and S100A10−/− (KO) peritoneal macrophages (1 × 105 cells) were added to the upper chamber of Matrigel-coated chambers, which also contained Plg (0.5μM) and, where indicated, MMP-9 inhibitory antibody (20 μg/mL). The lower chamber contained MCP-1 (10 ng/mL) as chemoattractant (A). Cells were incubated at 37°C for 48 hours. Invading cells were quantified as described in “Matrigel invasion and cell migration.” Data are expressed as mean number of cells per field per 40 × field plus or minus SD of 3 independent experiments. Statistical analysis was performed by one-way analysis of variance (with Tukey multiple comparisons) compared with WT untreated macrophages: ***P < .001. WT and S100A10−/− macrophages were stimulated with Plg (0.5μM) and MCP-1 (10 ng/mL) for 24 hours in serum-free media. Cell lysates were prepared, electrophoresed, and immunoblotted for MMP-9 (B). WT and S100A10−/− macrophages were treated as in panel B, except that cell-conditioned media was collected. Conditioned media was subjected to zymography for MMP-9 (C; box indicates MMP-9 band; Pg, Plg; and Pm, plasmin), and quantification of MMP-9 was performed by densitometry and analyzed by Student t test (D).
S100A10 mediates activation of MMP-9. WT and S100A10−/− (KO) peritoneal macrophages (1 × 105 cells) were added to the upper chamber of Matrigel-coated chambers, which also contained Plg (0.5μM) and, where indicated, MMP-9 inhibitory antibody (20 μg/mL). The lower chamber contained MCP-1 (10 ng/mL) as chemoattractant (A). Cells were incubated at 37°C for 48 hours. Invading cells were quantified as described in “Matrigel invasion and cell migration.” Data are expressed as mean number of cells per field per 40 × field plus or minus SD of 3 independent experiments. Statistical analysis was performed by one-way analysis of variance (with Tukey multiple comparisons) compared with WT untreated macrophages: ***P < .001. WT and S100A10−/− macrophages were stimulated with Plg (0.5μM) and MCP-1 (10 ng/mL) for 24 hours in serum-free media. Cell lysates were prepared, electrophoresed, and immunoblotted for MMP-9 (B). WT and S100A10−/− macrophages were treated as in panel B, except that cell-conditioned media was collected. Conditioned media was subjected to zymography for MMP-9 (C; box indicates MMP-9 band; Pg, Plg; and Pm, plasmin), and quantification of MMP-9 was performed by densitometry and analyzed by Student t test (D).
S100A10 deficiency results in decreased activation of MMP-9
Previous studies have shown that, in response to thioglycollate-induced peritonitis, macrophage movement across the peritoneal membrane into the peritoneal cavity or similar movement across an artificial Matrigel barrier, requires the secretion of MMP-9 from the macrophages followed by plasmin-dependent activation.22 The invasion of S100A10−/− macrophages through a Matrigel barrier was unaffected by treatment with the MMP inhibitor GM6001 or after treatment with an MMP-9 inhibitory antibody (Figures 3C, 5A), suggesting MMP-9-independent invasion by S100A10−/− macrophages. We tested the possibility that activation of MMP-9 by S100A10−/− macrophages might be reduced. We measured MMP-9 protein in cell lysates and MMP-9 activity in cell-conditioned media from thioglycollate-stimulated WT and S100A10−/− mice (Figure 5B-C). MMP-9 protein levels in macrophage lysates were unchanged (Figure 5B), but activation of MMP-9 in conditioned media was decreased more than 2-fold by S100A10−/− macrophages compared with WT macrophages (Figure 5C-D).
S100A10 promotes Plg activation on the surface of murine macrophages
S100A10 can account for the generation of as much as 90% of the cell-surface plasmin generation of some cells.26 Therefore, we compared the rates of plasmin generation by the macrophages isolated from WT and S100A10−/− mice. We observed a 45% reduction (0.0068 ± 0.000603 Δ405 nm/sec in WT cells, and 0.0037 ± 0.000058 Δ405 nm/sec in S100A10−/− macrophages, P < .001; Figure 6A) in plasmin generation by the macrophages isolated from the S100A10−/− mice compared with WT mice. The rate of plasmin generation was also dramatically reduced in the absence of exogenous uPA (0.0015 ± 0.000141 Δ405 nm/sec in WT cells, and 0.0010 ± 0.000205 Δ405 nm/sec in KO cells, P > .05; Figure 6A). Plasmin generation was negligible in the absence of Plg in both WT and KO cells. When WT and S100A10−/− macrophages were pretreated with CpB, the rate of plasmin generation was also reduced (Figure 6A).
Role of S100A10 in macrophage Plg activation and binding. (A) WT and S100A10−/− (KO) macrophages were incubated with or without uPA (50nM) for 10 minutes at room temperature. Cells were washed and incubated with Plg (0.5μM), in the presence or absence of CpB (5 U/mL), followed by addition of the plasmin substrate S2251. The rate of plasmin generation was measured at 405 nm. Statistical analysis was performed by one-way analysis of variance with Tukey multiple comparisons. Data are percentage of specific binding plus or minus SD of 3 independent experiments. (B) WT and KO thioglycollate-elicited macrophages were cultured in the absence of serum for 2 hours before assay. Cells were incubated with 200nM FITC Plg, either with or without ϵ-ACA (100mM), for 1 hour at 4°C in HBSS containing 1mM MgCl2 and 3mM CaCl2. Plg binding was measured by FACS, excluding cells that were positive for propidium iodide. Statistical analysis was performed by Student t test: ***P < .001; *P < .01. Data are Δ405 nm/sec plus or minus SD of 3 independent experiments.
Role of S100A10 in macrophage Plg activation and binding. (A) WT and S100A10−/− (KO) macrophages were incubated with or without uPA (50nM) for 10 minutes at room temperature. Cells were washed and incubated with Plg (0.5μM), in the presence or absence of CpB (5 U/mL), followed by addition of the plasmin substrate S2251. The rate of plasmin generation was measured at 405 nm. Statistical analysis was performed by one-way analysis of variance with Tukey multiple comparisons. Data are percentage of specific binding plus or minus SD of 3 independent experiments. (B) WT and KO thioglycollate-elicited macrophages were cultured in the absence of serum for 2 hours before assay. Cells were incubated with 200nM FITC Plg, either with or without ϵ-ACA (100mM), for 1 hour at 4°C in HBSS containing 1mM MgCl2 and 3mM CaCl2. Plg binding was measured by FACS, excluding cells that were positive for propidium iodide. Statistical analysis was performed by Student t test: ***P < .001; *P < .01. Data are Δ405 nm/sec plus or minus SD of 3 independent experiments.
S100A10−/− macrophages have decreased Plg binding capacity
S100A10 has been shown to bind Plg (dissociation constant = 1.8μM) via a mechanism involving the interaction of the carboxyl-terminal lysine residue of S100A10 with the kringle domains of Plg.11 The contribution of S100A10 to peritoneal macrophage Plg binding capacity was evaluated by FACS by comparing the amount of Plg bound to the surface of WT and S100A10−/− macrophages. S100A10 deficiency resulted in a 30% decrease (P < .001) in Plg binding (60.4% ± 2.4%, n = 3, by WT cells, and 41.6% ± 3.65%, n = 3, by S100A10−/− cells; Figure 6B). In the presence of ϵ-ACA, Plg binding was reduced 80% and 90% in WT and S100A10−/− macrophages, respectively (10.3% ± 2.1%, n = 3, in WT cells and 3.4% ± 0.6%, n = 3, in S100A10−/− cells), suggesting the presence of other lysine-dependent Plg binding proteins on the surface of the S100A10−/− macrophages.
Loss of S100A10 results in loss of its binding partner, annexin A2, from the cell surface
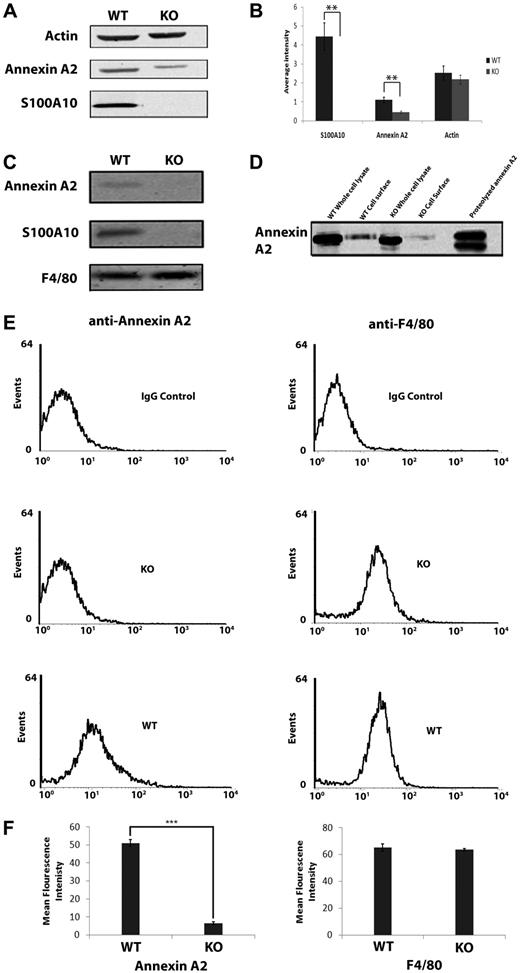
The levels of S100A10 have been shown to be reduced by as much as 90% in the tissues of annexin A2−/− mice.27 We observed unexpectedly that, compared with macrophages isolated from WT mice, the total cellular levels of annexin A2 of macrophages isolated from S100A10−/− mice were reduced by 60% (1.1 ± 0.14, n = 3, in WT cells, and 0.46 ± 0.06, n = 3, in S100A10−/− cells, P < .001; Figure 7A-B). Furthermore, cell-surface biotinylation and flow cytometric analysis revealed that cell-surface annexin A2 is also dramatically reduced in S100A10-deficient macrophages by approximately 8-fold (51.1% ± 2.0%, n = 3, on WT macrophages, and 6.6% ± 0.58%, n = 3, on S100A10−/− macrophages, P < .001; Figure 7C-F). Reverse-transcribed polymerase chain reaction analysis suggested that the annexin A2 mRNA levels were similar in WT and S100A10−/− macrophages (data not shown), suggesting increased turnover of annexin A2 in the S100A10−/− mice. Because annexin A2 is known to be ubiquitinated, we investigated the possibility that S100A10 stabilized annexin A2 protein levels by blocking its ubiquitination. However, we observed that the proteasomal inhibitors MG132 (supplemental Figure 1) or lactacystin (not shown) did not affect the annexin A2 levels, thereby eliminating proteasomal degradation as a possible mechanism.
Levels of S100A10 on thioglycollate-elicited macrophages. Peritoneal macrophages were collected 4 days after injection with 4% thioglycollate. Cell lysates were prepared, and total levels of S100A10 or annexin A2 were examined by Western blotting (A) using actin as a loading control and quantitated (B). (C) Cell-surface proteins of peritoneal macrophages were incubated with Sulfo-NHS-SS-biotin, lysed, and the biotinylated (cell surface) proteins were collected with streptavidin beads and subjected to SDS-PAGE and Western blotting for S100A10, annexin A2, and F4/80 (loading control). (D) Total and cell-surface annexin A2 obtained from WT and S100A10−/− peritoneal macrophages were compared with proteolyzed recombinant annexin A2. (E) Flow cytometric analysis of cell-surface annexin A2 and F4/80 levels in WT and S100A10−/− (KO) peritoneal macrophages. (F) Cells were incubated with antiannexin A2 and anti-F4/80 (control) antibodies. Quantification of flow cytometric analysis of cell-surface annexin A2 and F4/80 levels in WT and S100A10−/− macrophages, calculated using WinMDI v2.9 software (***P < .001).
Levels of S100A10 on thioglycollate-elicited macrophages. Peritoneal macrophages were collected 4 days after injection with 4% thioglycollate. Cell lysates were prepared, and total levels of S100A10 or annexin A2 were examined by Western blotting (A) using actin as a loading control and quantitated (B). (C) Cell-surface proteins of peritoneal macrophages were incubated with Sulfo-NHS-SS-biotin, lysed, and the biotinylated (cell surface) proteins were collected with streptavidin beads and subjected to SDS-PAGE and Western blotting for S100A10, annexin A2, and F4/80 (loading control). (D) Total and cell-surface annexin A2 obtained from WT and S100A10−/− peritoneal macrophages were compared with proteolyzed recombinant annexin A2. (E) Flow cytometric analysis of cell-surface annexin A2 and F4/80 levels in WT and S100A10−/− (KO) peritoneal macrophages. (F) Cells were incubated with antiannexin A2 and anti-F4/80 (control) antibodies. Quantification of flow cytometric analysis of cell-surface annexin A2 and F4/80 levels in WT and S100A10−/− macrophages, calculated using WinMDI v2.9 software (***P < .001).
S100A10 is anchored to the cell surface through its tight binding to annexin A2. Although controversial, annexin A2 has been suggested to contribute to plasmin generation at the macrophage cell surface.28 Although intact annexin A2 does not bind Plg,14,29 the proteolytic cleavage of annexin A2 at Lys-307 has been suggested to be required for the interaction of cell-surface annexin A2 with Plg.29-31 However, analysis of macrophage cell-surface annexin A2 by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) suggests that only intact annexin A2 is present at the cell surface of thioglycollate-stimulated macrophages, suggesting that macrophage annexin A2 does not participate in Plg binding (Figure 7D). Therefore, the loss of cell-surface annexin A2 exhibited by the S100A10−/− mice is doubtful to account for the loss of Plg binding or plasmin generation exhibited by S100A10-deficient macrophages.
Discussion
Previous studies have shown that the cell-surface generation of plasmin is required for macrophage recruitment and that macrophage recruitment is mediated in part through the plasmin-dependent activation of MMP-9.22 Therefore, macrophage-generated plasmin plays 2 roles in invasion: it directly hydrolyzes ECM proteins and also activated MMP-9, thus promoting further ECM degradation. It has remained a mystery as to the identity of the Plg receptor(s) that participates in macrophage invasion. In the current study, we investigated the mechanism of Plg-dependent inflammatory cell recruitment in 2 in vivo models: thioglycollate-induced peritonitis and the Matrigel plug assay. We observed that, in response to thioglycollate-induced peritonitis, macrophage migration through the peritoneal membrane into the peritoneal cavity of the S100A10−/− mouse was reduced by approximately 53%. Our results with the Matrigel plug assay demonstrated that S100A10−/− macrophages had a limited capability to infiltrate into the Matrigel plug. Analysis of the mechanism of macrophage migration through Matrigel suggested that S100A10-deficient macrophages had a reduced capacity to generate plasmin and activate MMP-9. Therefore, the simplest explanation of our results is that S100A10 and other carboxyl-terminal Plg receptors contribute to macrophage plasmin generation that is used for ECM hydrolysis and MMP-9 activation.
Four Plg receptors that play an important role in plasmin generation have been identified on the surface of the macrophage. These include α-enolase,32 histone 2B,14 annexin A2,13 and S100A10.14,33 S100A10 is a member of the S100 family of proteins. S100A10 typically exists in most cells together with annexin A2 in a heterotetrameric form referred to as the AIIt. Our laboratory has proposed the model that the annexin A2 subunits act as a scaffold, which anchors S100A10 to the cell surface, whereas S100A10 binds tPA and Plg through its carboxyl-terminal lysine residues.34 Binding of Plg to S100A10 converts Plg into the open, activation-susceptible confirmation, thereby resulting in enhanced Plg activator-dependent conversion of Plg to plasmin.35 CpB treatment of AIIt eliminates AIIt-dependent plasmin generation. Knockdown of S100A10 in fibrosarcoma and colorectal cells inhibits plasmin generation by approximately 75% to 90%.11,26,36 The results in the current report establish the importance of S100A10 in plasmin generation on the surface of macrophages. Furthermore, our observation that CpB treatment of macrophages from S100A10−/− mice results in a significant decrease in invasion suggests that, in addition to S100A10, other Plg receptors, which possess carboxyl-terminal lysines, such as α-enolase or histone 2B, also participate in macrophage invasion.
Interestingly, the Plg receptor enolase-1 has been shown to play an important role in the recruitment of monocytes to the acutely inflamed lung,37 whereas the Plg receptor histone H2B has been shown to play an important role in macrophage recruitment into the peritoneum.14 Analysis of Matrigel invasion with Boyden chambers has revealed that enolase-1 regulates both the invasion and migration of LPS-stimulated monocytes. In contrast, it has been shown that histone H2B regulates Matrigel invasion but not migration of the macrophage cell line, RAW 264.7.14 These previous studies and our current results highlight the critical function that Plg receptors play in the inflammatory process and establish the importance of enolase-1, histone H2B, and S100A10 as the key macrophage Plg receptors.
Of note was our observation that neutrophil recruitment to the peritoneal cavity in response to a thioglycollate-dependent inflammatory stimulus was also regulated by S100A10. It has been reported that neutrophil recruitment in Plg−/− mice is identical to that of wild-type mice,20 suggesting that plasmin does not play a role in neutrophil recruitment in the thioglycollate model. We are currently investigating the possibility that S100A10 may regulate proteases other than plasmin on the surface of the neutrophil. For example, it has been reported that S100A10 regulates the activation of cathepsin B on the surface of certain cancer cells.38
Previous studies have established the presence of S100A10 and its binding partner annexin A2 on the surface of murine macrophages.15 These studies showed that knockdown of annexin A2 resulted in decreased plasmin generation, matrix remodeling, and a dramatic loss in directed migration.13,30,39,40 However, because annexin A2 knockdown results in concomitant loss of S100A10,34 it is difficult to attribute these effects to annexin A2 or S100A10. Because annexin A2 levels were reduced in macrophages isolated from S100A10−/− mice, it was initially unclear whether the reduced macrophage migration in response to thioglycollate-induced peritonitis could be attributable to the loss of S100A10 or annexin A2 or both. Elucidating the role that annexin A2 plays in invasion is complicated by the reports from 3 laboratories that intact annexin A2 does not bind Plg.9,14,29 Because annexin A2-dependent plasmin generation is blocked by the lysine analog ϵ-ACA or by treatment of annexin A2 by CpB,29 it has been proposed that annexin A2 only binds Plg on proteolytic cleavage of the protein and exposure of a new carboxyl-terminal lysine.29 Further proof that Plg binding to annexin A2 requires “activation” by a carboxyl terminal cleavage event was provided by the report that the K307T mutant annexin A2 transfected into HEK 293 cells failed to bind Plg, although a change of a lysine proximal to this site (K328I) bound Plg no differently than the WT.39 The proteinase that has been proposed to cleave annexin A2 has not been identified, but plasmin has been ruled out.39 We addressed this issue of whether annexin A2 processing was required by migrating macrophages in vivo by examining the molecular mass of cell-surface annexin A2 by SDS-PAGE. The macrophages used in this study were isolated from the peritoneal cavity of thioglycollate-stimulated mice. We reasoned that the loss of 29 amino acid residues required for conversion of annexin A2 (Ser1-Asp338) to the processed form (Ser1-Lys307) results in the loss of approximately 3200 Da, which would be detectable on SDS-PAGE. However, as shown in Figure 7D, macrophage cell-surface annexin A2 is similar in molecular mass to unproteolyzed annexin A2. Because the macrophages isolated for these studies were macrophages that had migrated through the ECM into the peritoneal cavity, this result suggests that macrophage cell-surface annexin A2 is not cleaved and therefore is not participating in Plg binding, plasmin generation, or macrophage invasion. Similarly, it has been reported that annexin A2 is not proteolyzed during active plasmin generation by HT1080 cells.38 We have also observed that, in contrast to macrophages, knockdown of S100A10 in fibrosarcoma and colorectal cells did not affect cell-surface annexin A2 levels but inhibited plasmin generation by approximately 75% to 90%.11,26,36 It was also interesting that S100A10 and annexin A2 protein levels were higher for thioglycollate-stimulated peritoneal macrophages compared with resident peritoneal macrophages (supplemental Figure 2), suggesting that macrophages up-regulate S100A10 after activation by inflammatory mediators.
Our observation that annexin A2 levels were lower in macrophages isolated from S100A10−/− mice compared with WT mice was unexpected. Reverse-transcribed polymerase chain reaction analysis suggested that the annexin A2 levels were similar in both macrophages (data not shown). S100A10 has been shown to be ubiquitinated27,41 and rapidly degraded by the proteasome. The binding of S100A10 to annexin A2 protects S100A10 from ubiquitination and proteasomal degradation. In contrast, although annexin A2 is ubiquitinated, ubiquitination does not activate the proteasomal degradation of the protein.42 We have observed that the proteasomal inhibitor MG-132 does not affect the annexin A2 levels (supplemental Figure 1), thereby eliminating proteasomal degradation as a possible mechanism. However, it is known that annexin A2 is mainly cytosolic, whereas the annexin A2-S100A10 complex is associated with the cytoskeleton.43,44 Because the turnover of cytoskeleton-bound annexin A2 (t1/2 = 40-50 hours) is 3 to 4 times slower than for the cytoplasmic annexin A2 (t1/2 = 15 hours),45 it is possible that changes in the subcellular distribution of annexin A2 in the S100A10−/− macrophages could account for the decreased annexin A2 levels.
In conclusion, this study identifies S100A10 as a crucial Plg binding site on murine macrophages. S100A10−/− mice displayed severely compromised leukocyte recruitment in response to inflammatory stress in vivo. In vitro invasion assays showed that S100A10-deficient macrophages had dramatically decreased Plg-dependent invasive capabilities compared with their WT counterparts. Furthermore, S100A10 deficiency resulted in decreased plasmin generation and decreased MMP-9 activation in vitro. Taken together, these data indicate the importance of S100A10 in macrophage cell-surface plasmin regulation and MMP-9 activation and therefore in the invasive and degradative capabilities of macrophages.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Canadian Cancer Society Research Institute and the Canadian Institutes of Health Research. P.A.O. was supported by the Cancer Research Training Program at Dalhousie University. S100A10−/− mice were provided by P.S. and Dr P. Greengard.
Authorship
Contribution: P.A.O. designed and performed research, analyzed data, and wrote the manuscript; A.P.S. performed research and critically evaluated the manuscript; R.S.L. designed research; D.M.W. designed research, analyzed data, and wrote the manuscript; and P.S. critically evaluated the manuscript and supplied the S100A10−/− mice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David M. Waisman, Department of Biochemistry and Molecular Biology, Dalhousie University, Rm 9-B1, Sir Charles Tupper Medical Bldg, 5850 College St, Halifax, Nova Scotia, Canada, B3H 1X5; e-mail: david.waisman@dal.ca.