Abstract
Compared with adults, pediatric mastocytosis has a relatively favorable prognosis. Interestingly, a difference was also observed in the status of c-kit mutations according to the age of onset. Although most adult patients have a D816V mutation in phosphotransferase domain (PTD), we have described that half of the children carry mutations in extracellular domain (ECD). KIT-ECD versus KIT-PTD mutants were introduced into rodent Ba/F3, EML, Rat2, and human TF1 cells to investigate their biologic effect. Both ECD and PTD mutations induced constitutive receptor autophosphorylation and ligand-independent proliferation of the 3 hematopoietic cells. Unlike ECD mutants, PTD mutants enhanced cluster formation and up-regulated several mast cell-related antigens in Ba/F3 cells. PTD mutants failed to support colony formation and erythropoietin-mediated erythroid differentiation. ECD and PTD mutants also displayed distinct whole-genome transcriptional profiles in EML cells. We observed differences in their signaling properties: they both activated STAT, whereas AKT was only activated by ECD mutants. Consistently, AKT inhibitor suppressed ECD mutant-dependent proliferation, clonogenicity, and erythroid differentiation. Expression of myristoylated AKT restored erythroid differentiation in EML-PTD cells, suggesting the differential role of AKT in those mutants. Overall, our study implied different pathogenesis of pediatric versus adult mastocytosis, which might explain their diverse phenotypes.
Introduction
Mastocytosis is a heterogeneous group of disorders characterized by an abnormal accumulation of tissue mast cells (MCs) in one or more organs, including skin, bone marrow, gastrointestinal tract, liver, spleen, and skeletal systems. Clinical symptoms are the result of MC-derived mediators and, less frequently, destructive infiltration of MCs.1 Mastocytosis may occur also in childhood. In most pediatric cases, the disease is confined to the skin and is diagnosed as urticaria pigmentosa, mastocytoma, or diffuse cutaneous mastocytosis. Spontaneous regression and improvement of skin lesions are often observed in those patients.2 Systemic mastocytosis (SM) is rarely seen in pediatric cases; the disease process may persist through the entire life. In contrast, adult-onset mastocytosis can present persistent and systemic involvement and is sometimes associated with clonal hematologic disorders, such as either myelodysplastic or myeloproliferative syndromes, or acute myeloid leukemia. MC leukemia, a rare malignant subgroup, is mostly found in adults.3 Although it is a sporadic disease, rare family cases have been reported.4,5
In the past decade, increasing evidence has demonstrated that the development of mastocytosis is linked to gain of function (GOF) of the c-kit proto-oncogene.6-8 The c-kit gene encodes the stem cell factor (SCF) receptor, KIT, which belongs to the subclass III of the tyrosine kinase receptor family. This subclass is characterized by an extracellular region consisting of 5 immunoglobulin (Ig)-like domains (extracellular domain [ECD]), a single transmembrane domain (TMD), a regulatory juxtamembrane domain (JMD), and an insert that splits the kinase domain into an adenosine triphosphate-binding region and a phosphotransferase domain (PTD).9 A substitution of aspartic acid by valine residue (D816V) in exon 17 encoding PTD, which results in constitutive KIT activation, has been reported in most adult patients with systemic mastocytosis.7,8 Recent work has shed insight into the causative role of the D816V mutation in differentiation and abnormal clustering of neoplastic cells10 and in induction of mastocytosis.11 The presence of D816V does not appear to correlate with aggressiveness or prognosis of human mastocytosis.12 Other rare mutations involving JMD, TMD, and ECD can be detected in mastocytosis.13-15 Unlike adult cases, the status of c-kit mutations in childhood mastocytosis remains unsolved. D816V mutation was undetectable in 6 pediatric patients reported by Longley et al7 ; 3 of 6 presented a dominant inactivating mutation substituting lysine for glutamic acid at codon 839. Another group found a missense codon 816 mutation in 10 of 12 Japanese childhood-onset patients.16 In our recent study on 50 children with sporadic or familial mastocytosis, 36% harbored the well-known D816V mutation and 44% presented mutations in exons 8 and 9, which encode the fifth Ig-like domain (D5).17 Interestingly, several of these mutations, or their locations, have been described in other tumors, such as acute myeloid leukemia and gastrointestinal tumors (GISTs).18,19 These data suggest an underlying correlation between heterogeneity of c-kit mutations and diverse clinical phenotypes.
Many recent studies have contributed to the delineation of the molecular mechanisms that mediate the biologic effects of c-kit mutations. We and others have reported that GOF c-kit mutations in JMD and PTD can elicit constitutive activation of signaling events, which play an important role in cellular functions, such as survival, proliferation, differentiation, and adhesion.20,21 Some reports also suggested that JMD and PTD mutations could be active through different mechanisms.22 But little is known to date about potential differences in signaling pathways and the biologic consequences of ECD and PTD mutations.
In the present study, although both KIT-ECD and PTD mutants displayed GOF potentiality, they differently induced cellular phenotype, gene expression profile, and functions. We also found that ECD mutants, but not PTD mutants, activated AKT pathway, and the ECD mutant-mediated cellular functions were AKT activity-dependent. Myristoylated AKT restored impaired erythroid differentiation in PTD mutant-expressing cells. In conclusion, our results revealed, for the first time, different activation mechanisms of ECD versus PTD mutants at both cellular and molecular levels, which might help to better understand heterogeneity of mastocytosis.
Methods
Reagents
Mouse monoclonal anti–human KIT antibody (Ab81), polyclonal rabbit anti-ERK2, anti-STAT5b, anti STAT1, anti STAT3, anti STAT5, and anti-Grb2 antibodies were purchased from Santa Cruz Biotechnology. The polyclonal rabbit anti-PY719-KIT, anti-KIT, anti-PS473AKT, anti-PT308AKT, anti-AKT, anti-PS9GSK3b, anti-PY2448mTOR, anti-mTOR, anti-PY701STAT1, anti-PY705STAT3, anti-PY694STAT5, anti-PY183Y185SAPK/JNK, anti-JNK antibodies were obtained from Cell Signaling Technology. Anti–active (pTGpY) p38, anti-p38, and anti–active (pTEpY) MAPK antibodies were purchased from Promega, and anti-p85PI3K antibody from Upstate Biotechnology.
RTK inhibitors imatinib and dasatinib were purchased from Sequoia Research. AKTVIII was purchased from Calbiochem and benzidine dihydrochloride from Sigma-Aldrich.
Stable KIT-expressing cell lines
The murine interleukin-3 (IL-3)–dependent Ba/F3 lymphoid cell line, the immortalized lymphohematopoietic cell line EML, the human erythroleukemia TF1 cell line, and the rat fibroblastic cell line Rat2 were cultured as described before.11,20,23,24 Ba/F3 cells were transfected with different c-kit constructs as described.23 TF1 cells were lentivirally transduced as described in supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Both Rat2 and EML cells were transduced using the Phoenix retroviral expression system (ATCC, LGC-Standards). Phoenix cells were first transfected with pLXSN vectors containing the c-kit constructs or pMSCVpuro-(Myr)EGFP-AKT using Fugene 6 (Roche Applied Science) according to the manufacturer's instructions. At 48 hours later, retroviral supernatants were used to infect either Rat2 or EML cells. At 24 hours after transfection, cells were selected with either 0.4 mg/mL (EML cells) or 1 mg/mL (Ba/F3 and Rat2 cells) of geneticin (Invitrogen SARL) or 1.5 μg/mL puromycin (Calbiochem) in corresponding culture medium for 14 days. Ba/F3 and EML cells transfected with KIT mutants were further grown in growth factor-free medium to obtain autonomous populations. Cells expressing KIT variants were selected using ARIA cell sorter (Becton-Dickinson Biosciences) to obtain homogeneous populations.
Flow cytometry
Expression of ICAM-1 on Ba/F3 cells was tested as described22 with antimouse ICAM-1 antibody (BD Biosciences). Events were collected using a FACScan (BD Biosciences) and analyzed using FlowJo software v.7.2.5 (TreeStar).
Immunoprecipitation and immunoblotting
Cells were starved in RPMI containing 0.5% fetal bovine serum (FBS) at 37°C for 5 hours before stimulation for 5 minutes with or without 250 ng/mL SCF. In some experiments, cells were treated with AKTVIII for 1 hour. Cell lysis and immunoprecipitation were performed as described.23 Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride Immobilon-P membrane (Millipore). Membranes were then incubated with antibodies. Proteins were visualized by incubation with Supersignal West-Pico Chemiluminescent reagent (Pierce Chemical).
Cell proliferation assay
The 104 Ba/F3 cells were cultured in 96-well plates in complete medium with and without SCF or IL-3. In some experiments, the medium was supplemented with inhibitors as indicated. After incubation for 48 hours, 10 μL of WST-1 (Roche Applied Science) was added, and the cells were grown for another 3 hours. Viable cells were quantified by measuring absorbance at 450 nm with a scanning multiwell MultiSkan-MS spectrophotometer (Thermo-LabSystems).
Colony-forming cell assay and benzidine staining
Soft-agar assays were performed with Rat2 cells as described.20 Colony images were captured under inverted microscopy and counted on day 10.
For burst-forming units-erythroid (BFU-E) assay, reconstituted cytokine-free MethoCult (M3231, StemCell Technologies) was plated in 6-well plates in the presence of 250 ng/mL mSCF, 10 U/mL recombinant human erythropoietin (Epo; gift from N. Casadevall, Assistance Publique–Hopitaux de Paris), or no cytokine. Parental and KIT mutants expressing EML cells were seeded at 5 × 102 cells/well per milliliter. Colonies were counted after 7 days.
For benzidine staining, KIT mutants expressing EML cells were cultured in erythroid differentiation medium (Iscove modified Dulbecco medium; Invitrogen SARL, with 10% FBS, 10% BIT; StemCell Technologies) supplemented with 250 ng/mL mSCF and/or 5 U/mL recombinant human Epo for 12 days. Hemoglobin-positive cells were scored after staining with benzidine dihydrochloride.
RNA isolation and reverse-transcriptase PCR
RNA isolation from cell lines and reverse transcription were done as described.23 Resulting cDNAs were mixed with polymerase chain reaction (PCR) solution containing the primers indicated in Table 1. PCR was cycled 35 (for CD25 and β-actin) or 40 times (for MC-carboxypeptidase A [MC-CPA]) at 94°C for 45 seconds, 55°C for 30 seconds, and 72°C for 1 minute. PCR products were resolved by agarose gel electrophoresis. All microarray data have been deposited into the Gene Expression Omnibus (GEO; National Center for Biotechnology Information) public database under accession number GSE21255.
Primers used in PCR
| Gene amplified/primer . | Sequence, 5′ → 3′ . |
|---|---|
| MC-CPA | |
| Sense | GGACTGTGGCATTCATGCACG |
| Antisense | CATCCGTGGCAATCCTTGCAA |
| CD25 | |
| Sense | GACTTCCCACAACCCACAGAAACA |
| Antisense | AGGAAACAGCCGTTAGGTGAATGC |
| β-actin | |
| Sense | GTACCACAGGCATTGTGATGGACT |
| Antisense | CTTTGATGTCACGCACGATTTCCC |
| Gene amplified/primer . | Sequence, 5′ → 3′ . |
|---|---|
| MC-CPA | |
| Sense | GGACTGTGGCATTCATGCACG |
| Antisense | CATCCGTGGCAATCCTTGCAA |
| CD25 | |
| Sense | GACTTCCCACAACCCACAGAAACA |
| Antisense | AGGAAACAGCCGTTAGGTGAATGC |
| β-actin | |
| Sense | GTACCACAGGCATTGTGATGGACT |
| Antisense | CTTTGATGTCACGCACGATTTCCC |
Results
Constitutive tyrosine phosphorylation of KIT mutants in Ba/F3 cells
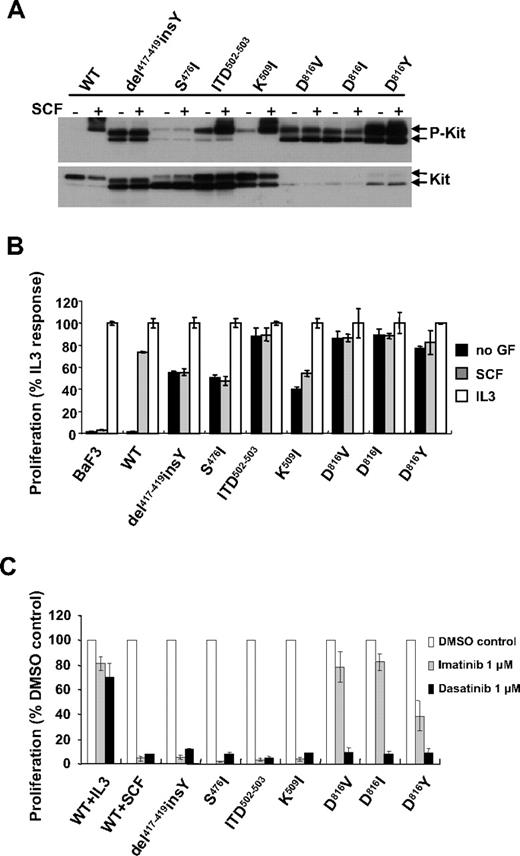
To evaluate the biologic consequences of c-kit mutations identified in pediatric mastocytosis, selected mutations were cloned in the human wild-type (WT) c-kit gene: 4 mutations in the ECD (Del417-419insY, S476I, ITD502-503, and K509I) and 3 in the PTD (D816V or I or Y). Each mutant and WT-KIT were introduced into Ba/F3 cells. The status of tyrosine phosphorylation of KIT mutants was assessed by Western blot. As shown in Figure 1A, constitutive tyrosine phosphorylation was detected in all KIT mutants in the absence of SCF compared with SCF-stimulated phosphorylation of WT-KIT, albeit to a lesser extent for the S476I mutant. SCF stimulation resulted in further phosphorylation of ITD502-503 and K509I mutants, whereas it had minimal effect on phosphorylation of the other mutants. Noticeably, all KIT mutants showed greater or equal expression of immature forms (145 kDa) compared with mature forms (160 kDa), as already described for JMD or PTD mutants.20,22 The mature hyperglycosylated form predominated in WT-KIT.20
Ligand-independent activity of KIT mutants in Ba/F3 cells. (A) Tyrosine phosphorylation of KIT mutants in Ba/F3 cells. Whole-cell lysates obtained from cells unstimulated or stimulated for 5 minutes with 250 ng/mL mSCF were subjected to SDS-PAGE and immunoblotted with anti-PY719KIT antibody. The blot was stripped and reprobed with anti-KIT antibody. Arrows indicate the position of mature (top arrow) and immature (bottom arrow) forms. (B) Cytokine-independent proliferation of Ba/F3 cells carrying KIT mutants. Cells were exposed to 250 ng/mL mSCF or 12.5 ng/mL IL-3 or left unstimulated for 48 hours. Cell proliferation was assessed by measuring mitochondrial conversion of WST-1 into blue formazan dye with a spectrophotometer. Data are shown as percentage of absorbance at 450 nm compared with absorbance of the respective cell line under IL-3 stimulation. (C) Effect of tyrosine kinase inhibitors on ligand-independent proliferation of Ba/F3 cells. Ba/F3 cells expressing WT were introduced in a proliferation assay and incubated for 48 hours with 250 ng/mL mSCF or 12.5 ng/mL mIL-3 and without growth factor for KIT mutants expressing cells and in the presence of 1μM imatinib or dasatinib. Values are presented relative to cell proliferation in the absence of inhibitors. (B-C) Data are mean ± SD of triplicates. Results shown are representative of 3 independent experiments.
Ligand-independent activity of KIT mutants in Ba/F3 cells. (A) Tyrosine phosphorylation of KIT mutants in Ba/F3 cells. Whole-cell lysates obtained from cells unstimulated or stimulated for 5 minutes with 250 ng/mL mSCF were subjected to SDS-PAGE and immunoblotted with anti-PY719KIT antibody. The blot was stripped and reprobed with anti-KIT antibody. Arrows indicate the position of mature (top arrow) and immature (bottom arrow) forms. (B) Cytokine-independent proliferation of Ba/F3 cells carrying KIT mutants. Cells were exposed to 250 ng/mL mSCF or 12.5 ng/mL IL-3 or left unstimulated for 48 hours. Cell proliferation was assessed by measuring mitochondrial conversion of WST-1 into blue formazan dye with a spectrophotometer. Data are shown as percentage of absorbance at 450 nm compared with absorbance of the respective cell line under IL-3 stimulation. (C) Effect of tyrosine kinase inhibitors on ligand-independent proliferation of Ba/F3 cells. Ba/F3 cells expressing WT were introduced in a proliferation assay and incubated for 48 hours with 250 ng/mL mSCF or 12.5 ng/mL mIL-3 and without growth factor for KIT mutants expressing cells and in the presence of 1μM imatinib or dasatinib. Values are presented relative to cell proliferation in the absence of inhibitors. (B-C) Data are mean ± SD of triplicates. Results shown are representative of 3 independent experiments.
C-kit mutations confer cytokine-independent proliferation to Ba/F3 cells
Previous studies have demonstrated that constitutive activation of KIT mutants could promote growth factor-independent cell proliferation.25 We evaluated the growth properties of Ba/F3 cells expressing different KIT mutants. Although Ba/F3-WT-KIT cells proliferated only in the presence of IL-3 or SCF, expression of either ECD or PTD mutants enabled Ba/F3 cells to propagate in a cytokine-independent manner. In line with increased phosphorylation on SCF stimulation, proliferation of Ba/F3-K509I cells was also mildly enhanced in response to SCF (Figure 1B; supplemental Figure 1).
Furthermore, the autonomous growth of the cells carrying KIT mutants was abolished by treatment with a pharmacologic concentration (1μM) of KIT tyrosine kinase inhibitors imatinib or dasatinib. As a control, IL-3-driven proliferation was resistant to the inhibitors (Figure 1C). The same results (ie, constitutive receptor tyrosine phosphorylation, growth factor independence, and inhibitor sensitivity) were reproduced with ECD and PTD mutations ectopically expressed in the granulocyte-macrophage colony-stimulating factor-dependent human TF1 cell line (supplemental Figure 2). These data demonstrate that these c-kit mutations mediate growth factor-independent proliferation of cytokine-dependent cells.
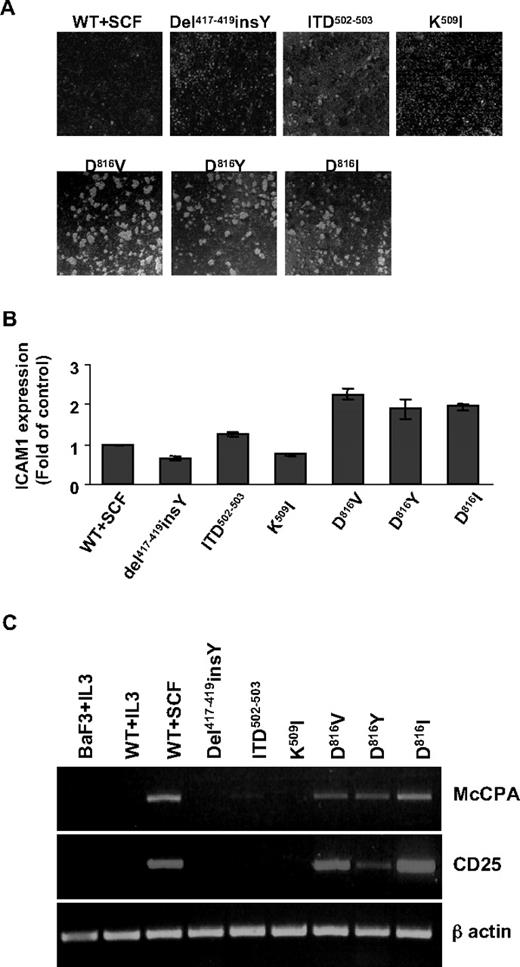
PTD, but not ECD, mutants promote cluster formation and expression of MC differentiation-, adhesion-, and neoplasm-related genes in Ba/F3 cells
KIT D816V has been shown to induce cluster formation and early MC differentiation antigen appearance in Ba/F3 cells.10 We therefore investigated whether other KIT mutants mediate the same effects in the absence of additional growth factors. PTD mutant expression resulted in cluster formation and up-regulation of cell surface expression of ICAM-1 (Figure 2A-B), as reported.10 Conversely, no obvious clusters were found in cells harboring ECD mutants (such as del417-419insY, and K509I), nor was modulation of ICAM-1 expression observed. To note, intermediate effects were observed for ITD502-503 mutant (Figure 2A-B). Reverse-transcribed PCR was performed to analyze expression of MC differentiation and neoplasm-associated molecules. Like WT-KIT followed by SCF stimulation, PTD mutants enhanced expression of the MC-protease MC-CPA and the marker for MC neoplasm CD25, whereas ECD mutants did not. Ba/F3 cells derived from IL-3 culture were negative for both MC-CPA and CD25 transcripts (Figure 2C). The data indicate that PTD and ECD mutants differently regulate genes related with MC differentiation, neoplasm, and adhesion antigens, despite the similar proliferative behavior they exhibit in Ba/F3 cells.
PTD mutants only promote cluster formation, MC adhesion-, differentiation-, and neoplasm-related gene expression in Ba/F3 cells. (A) Cluster formation of Ba/F3 cells harboring various KIT mutants. Cells were seeded at 105/mL in 12-well plates with or without mSCF or mIL-3. Six hours later, cells were photographed under an inverted microscopy (Leica DM IRB microscope with a 10×/0.25 objective lens). Images were captured with a CANON Powershot S50 camera and transferred directly to a PowerPoint file (v.2002 SP3). (B) Surface expression of ICAM-1 on Ba/F3 cells. A total of 1 × 105 Ba/F3 cells were stained with phycoerythrin-conjugated antimouse ICAM-1 antibody or IgG isotype control antibody. Expression level is given as fold of control: (MFIICAM-1-MFIisotype)/(MFIWT+SCF-ICAM-1-MFIWT+SCF-isotype). Data are mean ± SD of 3 independent experiments. (C) Expression of CD25 and MC-CPA mRNA in Ba/F3 cells induced by KIT mutants. RNA was isolated from Ba/F3 cells or KIT expressing Ba/F3 cells cultured as indicated. Expression of MC-CPA, CD25, and β-actin mRNA was analyzed by reverse-transcribed PCR as described in “RNA isolation and reverse-transcriptase PCR.”
PTD mutants only promote cluster formation, MC adhesion-, differentiation-, and neoplasm-related gene expression in Ba/F3 cells. (A) Cluster formation of Ba/F3 cells harboring various KIT mutants. Cells were seeded at 105/mL in 12-well plates with or without mSCF or mIL-3. Six hours later, cells were photographed under an inverted microscopy (Leica DM IRB microscope with a 10×/0.25 objective lens). Images were captured with a CANON Powershot S50 camera and transferred directly to a PowerPoint file (v.2002 SP3). (B) Surface expression of ICAM-1 on Ba/F3 cells. A total of 1 × 105 Ba/F3 cells were stained with phycoerythrin-conjugated antimouse ICAM-1 antibody or IgG isotype control antibody. Expression level is given as fold of control: (MFIICAM-1-MFIisotype)/(MFIWT+SCF-ICAM-1-MFIWT+SCF-isotype). Data are mean ± SD of 3 independent experiments. (C) Expression of CD25 and MC-CPA mRNA in Ba/F3 cells induced by KIT mutants. RNA was isolated from Ba/F3 cells or KIT expressing Ba/F3 cells cultured as indicated. Expression of MC-CPA, CD25, and β-actin mRNA was analyzed by reverse-transcribed PCR as described in “RNA isolation and reverse-transcriptase PCR.”
ECD, but not PTD, mutants mediate transformation of Rat2 cells and Epo-induced erythroid differentiation of EML cells
We next investigated whether ECD and PTD mutants would also mediate transformation of fibroblast-like cell lines. Rat2 cells were transduced with various c-kit constructs and plated in soft-agar with or without SCF. Colony numbers were assessed after 10 days. Rat2-WT-KIT cells formed colonies only in the presence of SCF, whereas Del417-419insY, S476I, and to a greater extent, ITD502-503 and K509I mutants expressing Rat2 cells readily formed colonies without need for additional cytokine. Despite their ability to induce cytokine independence in hematopoietic cells, PTD mutants were almost unable to induce colonies in Rat2 cells even in the presence of SCF (Figure 3A), suggesting differential clonogenic potentials of ECD and PTD mutants in Rat2 cells.
Different biologic effect induced by ECD and PTD mutants in Rat2 and EML cells. (A) Colony formation by Rat2 cells expressing different KIT mutants in soft-agar medium. Cells were plated at a concentration of 3 × 104 per dish under the indicated growth condition. Colonies were photographed (top panel) under inverted microscopy (original magnification ×4) and counted (bottom panel) at day 10. Data are mean ± SD of duplicates. (B) Epo-induced hemoglobinization of EML cells bearing different KIT. EML parental cells, EML-WT cells (with 5 U/mL Epo and mSCF), and KIT mutants expressing EML cells (with 5 U/mL Epo) were grown in erythroid differentiation medium for 12 days. Hemoglobin-positive cells were scored after benzidine staining. Data are percentage of benzidine-positive cells in a field. (C) Epo-induced BFU-E formation by KIT variants expressing EML cells. Different EML cells mixed with 1 mL methylcellulose were seeded in 6-well plates in the presence of 10 U/mL Epo and/or mSCF. Colonies were counted after 7 days. Data are percentage of BFU-E compared with total colonies. Results represent the mean ± SD of duplicates. (A-C) Results are representative of 3 independent experiments.
Different biologic effect induced by ECD and PTD mutants in Rat2 and EML cells. (A) Colony formation by Rat2 cells expressing different KIT mutants in soft-agar medium. Cells were plated at a concentration of 3 × 104 per dish under the indicated growth condition. Colonies were photographed (top panel) under inverted microscopy (original magnification ×4) and counted (bottom panel) at day 10. Data are mean ± SD of duplicates. (B) Epo-induced hemoglobinization of EML cells bearing different KIT. EML parental cells, EML-WT cells (with 5 U/mL Epo and mSCF), and KIT mutants expressing EML cells (with 5 U/mL Epo) were grown in erythroid differentiation medium for 12 days. Hemoglobin-positive cells were scored after benzidine staining. Data are percentage of benzidine-positive cells in a field. (C) Epo-induced BFU-E formation by KIT variants expressing EML cells. Different EML cells mixed with 1 mL methylcellulose were seeded in 6-well plates in the presence of 10 U/mL Epo and/or mSCF. Colonies were counted after 7 days. Data are percentage of BFU-E compared with total colonies. Results represent the mean ± SD of duplicates. (A-C) Results are representative of 3 independent experiments.
To further assess the role of KIT mutants in cellular function(s) other than proliferation, we introduced them into EML cells, a primitive lymphohematopoietic cell line capable of self-renewal in a SCF-dependent manner and differentiating into various hematopoietic lineages on varied cytokine stimulation.26 Both ECD and D816V/I mutants conferred cytokine-independent growth to EML cells (data not shown). The impact of the different KIT mutants on Epo-induced erythroid differentiation was analyzed. Hemoglobin-positive cells were scored by benzidine staining in the presence of Epo for 12 days, when positivity was maximal. EML-WT-KIT cell hemoglobinization was induced to a level comparable with that observed in EML parental cells in the presence of SCF and Epo. Del417-419insY or ITD502-503 mutants enhanced Epo-mediated erythroid differentiation in an SCF-independent manner, with 20% and 8% benzidine positivities, respectively (Figure 3B). Surprisingly, the benzidine-positive population was barely detectable in EML-D816V/I cells (Figure 3B), and colony-forming assays revealed impaired Epo-induced BFU-E formation by these mutants (Figure 3C); addition of SCF failed to restore erythroid differentiation (data not shown). We next analyzed whole-genome transcriptional profiles of EML-ECD and EML-PTD cells (supplemental Figure 3). In EML-D816V cells, underexpression of erythroid cell-related antigens was concomitant with overexpression of Epo-signaling suppressors compared with Del417-419insY or WT-KIT expressing cells. Furthermore, KIT-D816V promoted expression of MC surface markers and MC function-related molecules, including FcϵRI, glycoprotein 49A, MMCP-5, LIF, integrin α E (Itgae), leukotriene B4 receptor (Ltb4r1) (Table 2). These differential expression profiles between EML-ECD and EML-PTD cells are in agreement with their distinctive functional phenotype.
Affymetrix microarray analysis of up- and down-regulated genes in EML-D816V cells compared with EML-Del417-419insY cells
| Gene . | Fold* . |
|---|---|
| MC activation/differentiation-related genes | |
| FceRI | +66.64 |
| Gp49a | +30.35 |
| MMCP-5 | +16.78 |
| MMCP-2 | +3.03 |
| Lgals3 | +2.91 |
| Spp1 | +96.22 |
| Gpr34 | +9.57 |
| Adora3 | +20.81 |
| Rab27b | +15.58 |
| Rab32 | +3.68 |
| C3ar1 | +8.71 |
| Mgst2 | +5.81 |
| MC growth-related genes | |
| Lif | +16.12 |
| Osm | +3.99 |
| Il3rb2 | +3.01 |
| Il4ra | +4.6 |
| MC adhesion and migration-related genes | |
| Tjp1 | +31.46 |
| Itgae | +10.76 |
| Ltb4r1 | +4.03 |
| Suppressors of Epo signaling | |
| Cis | +17.14 |
| Socs2 | +13.99 |
| Socs1 | +4.81 |
| Erythroid cell-related genes | |
| Ermap | −3.28 |
| Gypa | −5.03 |
| Ank | −7.05 |
| Spna1 | −7.51 |
| Gene . | Fold* . |
|---|---|
| MC activation/differentiation-related genes | |
| FceRI | +66.64 |
| Gp49a | +30.35 |
| MMCP-5 | +16.78 |
| MMCP-2 | +3.03 |
| Lgals3 | +2.91 |
| Spp1 | +96.22 |
| Gpr34 | +9.57 |
| Adora3 | +20.81 |
| Rab27b | +15.58 |
| Rab32 | +3.68 |
| C3ar1 | +8.71 |
| Mgst2 | +5.81 |
| MC growth-related genes | |
| Lif | +16.12 |
| Osm | +3.99 |
| Il3rb2 | +3.01 |
| Il4ra | +4.6 |
| MC adhesion and migration-related genes | |
| Tjp1 | +31.46 |
| Itgae | +10.76 |
| Ltb4r1 | +4.03 |
| Suppressors of Epo signaling | |
| Cis | +17.14 |
| Socs2 | +13.99 |
| Socs1 | +4.81 |
| Erythroid cell-related genes | |
| Ermap | −3.28 |
| Gypa | −5.03 |
| Ank | −7.05 |
| Spna1 | −7.51 |
Affymetrix microarrays were performed as described in Supplemental data. Gp49a indicates glycoprotein 49A; Lgals3, galectin-3; Spp1, secreted phosphoprotein 1; Gpr34, G protein–coupled receptor 34; Adora3, adenosine A3 receptor; Mgst2, microsomal glutathione S-transferase 2; Osm, oncostatin M; Tjp1, tight junction protein 1; Itgae, integrin α E; Ltb4r1, leukotriene B4 receptor 1; Ermap, erythroblast membrane-associated protein; Gypa, glycophorin A; Ank, progressive ankylosis; and Spnal, spectrin α 1.
Fold represents median gene expression level in two EML-D816V cells cultured for 48 or 72 hours relative to that in two EML-Del417−419insY cells. + indicates increase; and −, decrease. For all the cited genes, the P value was < .001.
Both ECD and PTD mutants constitutively activate the STAT, but not the MAPK, pathway
To understand the molecular mechanisms by which ECD and PTD mutants mediate different cellular functions, we analyzed the signaling pathways activated by ECD and PTD mutants in Ba/F3 cells. As shown in Figure 4, WT-KIT induced phosphorylation of ERK, JNK, p38, STAT5, STAT3, and STAT1 (at a low level) on transient exposure to SCF. After chronic stimulation with SCF, neither STAT nor MAPK molecules were activated in WT-KIT cells, indicating that these signaling pathways were normally regulated by WT-KIT. KIT mutants expressing Ba/F3 cells showed no or only weak constitutive phosphorylation of 3 MAPK molecules as already reported.20,21,27-29 In contrast, both ECD and PTD mutants led to constitutive activation of all 3 STAT molecules, a robust phosphorylated signal being detected in PTD mutants. Compared with the KIT mutants identified in mastocytosis, a JMD mutant V559D frequently found in GIST apparently displayed an activation profile similar to that of ECD mutants (Figure 4). Altogether, these results indicate that the STAT, rather than the MAPK, pathways may be important for certain functions induced by all KIT mutants.
STAT pathways rather than MAPK pathways are constitutively activated by both ECD and PTD-KIT mutants. Parental or KIT-expressing Ba/F3 cells were serum starved for 5 hours in medium containing 0.5% FBS before stimulation for 5 minutes with or without 250 ng/mL mSCF as indicated. Ba/F3-WT (PS) cells were cultured in the presence of 250 ng/mL mSCF as a control for permanent stimulation (PS) of the KIT receptor. Whole-cell lysates were analyzed by SDS-PAGE, followed by Western blotting with indicated antibodies. (A) After immunoblotting with phospho-specific antibodies toward STAT1, STAT3, and STAT5 (top panels), membrane was stripped and reprobed with anti-STAT antibodies as loading controls. (B) Blots were stained with the indicated activation-specific antibodies. (Top panels) Antiphospho-ERK1/2, antiactive P38, and antiphospho-JNK antibodies. Blots were stripped and reprobed with anti-ERK2 and anti JNK antibodies to illustrate equal loading. These results are representative of 3 independent experiments.
STAT pathways rather than MAPK pathways are constitutively activated by both ECD and PTD-KIT mutants. Parental or KIT-expressing Ba/F3 cells were serum starved for 5 hours in medium containing 0.5% FBS before stimulation for 5 minutes with or without 250 ng/mL mSCF as indicated. Ba/F3-WT (PS) cells were cultured in the presence of 250 ng/mL mSCF as a control for permanent stimulation (PS) of the KIT receptor. Whole-cell lysates were analyzed by SDS-PAGE, followed by Western blotting with indicated antibodies. (A) After immunoblotting with phospho-specific antibodies toward STAT1, STAT3, and STAT5 (top panels), membrane was stripped and reprobed with anti-STAT antibodies as loading controls. (B) Blots were stained with the indicated activation-specific antibodies. (Top panels) Antiphospho-ERK1/2, antiactive P38, and antiphospho-JNK antibodies. Blots were stripped and reprobed with anti-ERK2 and anti JNK antibodies to illustrate equal loading. These results are representative of 3 independent experiments.
ECD and PTD mutants differentially activate AKT and its downstream target
The PI3K-AKT pathway is a major pathway for cell proliferation, survival, and growth.30 An Akt1 mutation newly identified in human cancers showed a transforming potential both in vitro and in vivo.31 PI3K is known to be constitutively activated by D816V mutant and contributes to D816V-induced proliferation.21 To verify whether the p85 subunit of PI3K was also constitutively recruited by ECD mutants, KIT was immunoprecipitated from various KIT-expressing Ba/F3 lysates. Western blot analysis showed that p85 constitutively associated with both ECD and PTD mutants, whereas it associated with WT-KIT only after SCF stimulation (Figure 5A).
Differential activation of AKT pathway by ECD and PTD mutants. (A) Constitutive association of p85 with all KIT mutants. Cells were serum starved for 5 hours and then stimulated with or without mSCF for 5 minutes. KIT proteins were immunoprecipitated, fractionated by SDS-PAGE, and blotted with anti-P85 (top panel) or anti-KIT antibodies (bottom panel). (B) Activation of AKT and its downstream pathway by ECD and PTD mutants. Total lysates from serum-starved Ba/F3 cells were subjected to SDS-PAGE and immunoblotted with antiphospho-AKT (T308), antiphospho-AKT (S473), and antiphospho-GSK3β (S9) antibodies. Membranes were stripped and reprobed with anti-AKT antibody. The experiment was performed 3 times with similar results.
Differential activation of AKT pathway by ECD and PTD mutants. (A) Constitutive association of p85 with all KIT mutants. Cells were serum starved for 5 hours and then stimulated with or without mSCF for 5 minutes. KIT proteins were immunoprecipitated, fractionated by SDS-PAGE, and blotted with anti-P85 (top panel) or anti-KIT antibodies (bottom panel). (B) Activation of AKT and its downstream pathway by ECD and PTD mutants. Total lysates from serum-starved Ba/F3 cells were subjected to SDS-PAGE and immunoblotted with antiphospho-AKT (T308), antiphospho-AKT (S473), and antiphospho-GSK3β (S9) antibodies. Membranes were stripped and reprobed with anti-AKT antibody. The experiment was performed 3 times with similar results.
We next tested whether these KIT mutants affect the AKT signaling pathway. After a 5-minute SCF stimulation, AKT was dramatically phosphorylated at both T308 and S473 residues in Ba/F3-WT-KIT cells. Although the total level of AKT was unaffected, continuous exposure to SCF resulted in suppression of AKT phosphorylation at T308 and reduced at S473 in these cells. AKT phosphorylation profiles of ECD and KIT-V559D mutants were similar to that observed in continuously activated WT-KIT. No or weak phosphorylation of AKT was detected in Ba/F3-PTD cells (Figure 5B), in agreement with previous reports.11,21,29,32 Accordingly, GSK3β, a known downstream target of AKT, was phosphorylated in both Ba/F3-ECD and V559D cells to a higher level than in Ba/F3-PTD cells (Figure 5B), suggesting that GSK3β activation may be linked to phosphorylation of AKT S473 in Ba/F3 cells. This discrepancy in AKT signaling pathways was also observed in TF1 and EML cells transduced with different KIT mutants (supplemental Figures 2,4).
The AKT inhibitor AKTVIII inhibits ECD-induced proliferation, transformation, and erythroid differentiation
To evaluate the role of AKT pathway in cellular functions mediated by KIT mutants, Ba/F3 cells expressing KIT variants were treated with AKTVIII, a highly specific allosteric AKT1/2 inhibitor targeting the PH domain.33,34 Like SCF-stimulated WT-KIT, ECD mutant-driven proliferation was more sensitive to AKTVIII (2.5μM) treatment than PTD mutants and IL-3-induced cell growth (Figure 6A). Biochemical analysis confirmed a specific inhibitory effect of AKTVIII on the AKT pathway (Figure 6B). ECD mutants were strongly sensitive to AKTVIII in EML cell proliferation and Rat2 cell clonogenicity assays (supplemental Figure 5A-B). Low-dose AKTVIII suppressed approximately 50% of Epo-induced BFU-E formation mediated by Del417-419insY mutant and WT-KIT in EML cells, without influencing the total number of CFU-GM. AKTVIII had little inhibitory effect on erythroid differentiation induced by the MyrAKT, whose activation is independent on the PH domain (Figure 6C).
Effect of AKT inhibitor AKTVIII on ligand-independent proliferation, colony formation, and erythroid differentiation mediated by KIT ECD mutants. (A) Effect of AKTVIII on proliferation of KIT variants expressing Ba/F3 cells. Ba/F3 cells expressing WT or KIT mutants were plated in 96-well plates and grown for 48 hours with or without mSCF or mIL-3 and in the presence of 2.5μM AKTVIII. Cell growth was assessed by measuring mitochondrial conversion of WST-1 into blue formazan dye with a spectrophotometer. Values are presented relative to cell proliferation in the absence of AKTVIII. Data are mean ± SD of triplicates. Results shown are representative of 3 independent experiments. (B) Effect of AKTVIII on activation of AKT and its downstream substrates in Ba/F3-Del417-419insY cells. Cells were serum starved for 5 hours and treated with 0 to 2.5μM AKTVIII for 1 hour before lysis. Phosphorylation of AKT, GSK3β, mTOR, STAT3, and KIT was analyzed by Western blotting using the indicated activation-specific antibodies. The blot was stripped and reprobed with different antibodies as loading controls. (C) Inhibition of Epo-induced BFU-E formation mediated by Del417-419insY mutant. EML cells expressing WT-KIT, Del417-419insY mutant, or MyrAKT were plated in Epo and/or SCF containing methylcellulose in the presence or absence of 0.06μM AKTVIII. Results are percentage of BFU-E or CFU-GM colonies in the presence of AKTVIII relative to those in the absence of AKTVIII. Data are mean ± SD of duplicates. Experiments were performed twice.
Effect of AKT inhibitor AKTVIII on ligand-independent proliferation, colony formation, and erythroid differentiation mediated by KIT ECD mutants. (A) Effect of AKTVIII on proliferation of KIT variants expressing Ba/F3 cells. Ba/F3 cells expressing WT or KIT mutants were plated in 96-well plates and grown for 48 hours with or without mSCF or mIL-3 and in the presence of 2.5μM AKTVIII. Cell growth was assessed by measuring mitochondrial conversion of WST-1 into blue formazan dye with a spectrophotometer. Values are presented relative to cell proliferation in the absence of AKTVIII. Data are mean ± SD of triplicates. Results shown are representative of 3 independent experiments. (B) Effect of AKTVIII on activation of AKT and its downstream substrates in Ba/F3-Del417-419insY cells. Cells were serum starved for 5 hours and treated with 0 to 2.5μM AKTVIII for 1 hour before lysis. Phosphorylation of AKT, GSK3β, mTOR, STAT3, and KIT was analyzed by Western blotting using the indicated activation-specific antibodies. The blot was stripped and reprobed with different antibodies as loading controls. (C) Inhibition of Epo-induced BFU-E formation mediated by Del417-419insY mutant. EML cells expressing WT-KIT, Del417-419insY mutant, or MyrAKT were plated in Epo and/or SCF containing methylcellulose in the presence or absence of 0.06μM AKTVIII. Results are percentage of BFU-E or CFU-GM colonies in the presence of AKTVIII relative to those in the absence of AKTVIII. Data are mean ± SD of duplicates. Experiments were performed twice.
Altogether, the data suggest that AKT activation is required for certain cellular functions mediated by ECD mutants but not for those induced by PTD mutants.
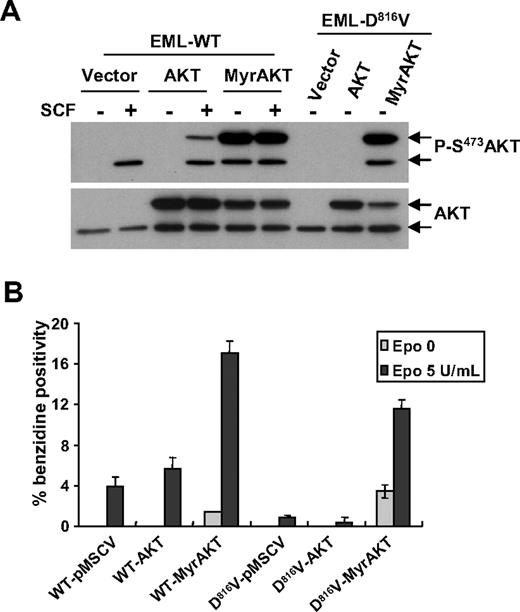
MyrAKT expression restores Epo-dependent erythroid differentiation of EML-D816V cells
To determine whether activated AKT can bypass the blockade of erythroid differentiation by D816V mutant in EML cells, we introduced both WT-AKT and a constitutively active form, MyrAKT, into EML cells expressing WT-KIT or the D816V mutant. Overexpression of MyrAKT, but not of WT-AKT, induced a sustained phosphorylation of the chimeric AKT protein as well as of endogenous AKT in both EML-WT-KIT and EML-D816V cells in the absence of SCF (Figure 7A). MyrAKT expression restored full erythroid differentiation, overrode the need for Epo, and induced a hemoglobin-positive population in EML-D816V cells as revealed by benzidine staining. Benzidine-positive cells were not or barely detectable in either vector- or WT-AKT-transduced EML-D816V cells, with or without Epo (Figure 7B). Noticeably, Epo promoted more erythroid differentiation in both EML-D816V and EML-WT-KIT cells expressing MyrAKT compared with EML-WT-KIT cells transduced with either WT-AKT or control vector (Figure 7B). These results suggest that lack of AKT signaling might be responsible for deficient erythroid differentiation in EML-D816V cells.
Expression of MyrAKT restores erythroid differentiation in EML-D816V cells. (A) Phosphorylation of AKT in EML cells expressed different forms of AKT. EML-WT or EML-D816V cells transduced with vector, EGFP-AKT1, or MyrEGFP-AKT1 were serum starved for 3 hours before stimulation for 5 minutes with or without 250 ng/mL mSCF. Immunoblot was done with antiphospho-AKT (S473) antibody. Membranes were stripped and reprobed with anti-AKT antibody. Arrows represent the positions of hybrid (Myr) EGFP-AKT (top arrow), and endogenous AKT (bottom arrow). (B) Active form AKT promotes erythroid differentiation in EML-D816V cells. EML-WT cells and EML-D816V cells transduced with different forms of AKT were grown in Iscove modified Dulbecco medium 10 containing 10% BIT with Epo and/or mSCF for 12 days. Hemoglobin-positive cells were scored after benzidine staining. Data are shown as percentage of benzidine-positive cells compared with total cells in a field. Data are mean ± SD of 3 fields. A representative of 3 separate experiments is shown.
Expression of MyrAKT restores erythroid differentiation in EML-D816V cells. (A) Phosphorylation of AKT in EML cells expressed different forms of AKT. EML-WT or EML-D816V cells transduced with vector, EGFP-AKT1, or MyrEGFP-AKT1 were serum starved for 3 hours before stimulation for 5 minutes with or without 250 ng/mL mSCF. Immunoblot was done with antiphospho-AKT (S473) antibody. Membranes were stripped and reprobed with anti-AKT antibody. Arrows represent the positions of hybrid (Myr) EGFP-AKT (top arrow), and endogenous AKT (bottom arrow). (B) Active form AKT promotes erythroid differentiation in EML-D816V cells. EML-WT cells and EML-D816V cells transduced with different forms of AKT were grown in Iscove modified Dulbecco medium 10 containing 10% BIT with Epo and/or mSCF for 12 days. Hemoglobin-positive cells were scored after benzidine staining. Data are shown as percentage of benzidine-positive cells compared with total cells in a field. Data are mean ± SD of 3 fields. A representative of 3 separate experiments is shown.
Discussion
Our study reports functional and downstream signaling differences between ECD and PTD mutations present in the c-kit tyrosine kinase receptor of mastocytosis patients. Both types of mutations are GOF mutations as substantiated by (1) tyrosine autophosphorylation of mutated receptors in the absence of ligand stimulation, (2) constitutive activation of downstream KIT-activated signaling pathways, and (3) autonomous growth of cytokine-dependent hematopoietic cell lines, but they induce different functional consequences at the cellular level. The common PTD mutations (substitution on codon 816) found in the majority of adult mastocytosis patients show either absent or reduced transforming capacity of Rat2 cells and induce a MC differentiation program in Ba/F3 and EML cells, whereas ECD mutations found in half of the pediatric mastocytosis patients do not. These functional differences are correlated with the induction of distinct signaling pathways for each type of mutations. Whereas both ECD and ligand-stimulated WT receptor induced AKT kinase phosphorylation and its activation, PTD mutations showed either a limited or a negatively regulated activation of this pathway. As a consequence, PTD mutations are unable to induce an AKT-dependent erythroid differentiation program in EML cells in response to Epo. This is the first report on differences in signaling pathway that could explain distinct functional properties between 2 activating mutations in a tyrosine kinase receptor. These differences could explain the heterogeneity between adult and pediatric mastocytosis as well as prognosis of this latter form of the disease.
Are pediatric-associated mutations gain-of-function mutations?
In a previous work, we found c-kit somatic mutations in pediatric forms of 43 of 50 mastocytosis patients. Besides the well-known somatic D816V substitution and its variants D816I and D816Y, encoded by the PTD in the cytoplasmic kinase region, we identified mutations located in the ECD of the receptor in half of the mutated pediatric patients.17 To analyze the GOF potentiality and the involvement of these mutations in the pathology, we selected 7 mutations of the pediatric variants: 4 ECD mutations (Del417-419insY, S476I, ITD502-503, and K509I) and 3 TKD mutations (D816V, D816Y, and D816I)
All the mutations analyzed show constitutive autophosphorylation of the receptor in Cos cells17 as well as in Ba/F3, TF1, and EML hematopoietic cell lines; they confer clear cytokine-independent growth to Ba/F3 cells (Figure 1B; supplemental Figure 1) and TF1 cells (supplemental Figure 2A). This cell growth is completely dependent on the mutated receptors because tyrosine kinase inhibitors, imatinib and dasatinib, inhibit their growth to selective concentrations (Figure 1C; supplemental Figure 2B). It is interesting to note that the mutants show different patterns of migration in immunoblot. The 3 PTD mutants show a high phosphorylation status compared with their relatively low expression level as seen by flow cytometry (supplemental Figure 6) and immunoblotting (Figure 1A). Similar findings have been reported for this PTD type of mutations in KIT, as in other class III–related receptors. This hyperphosphorylation has been associated with a high degradation status of the PTD-mutated forms.35 In contrast, ECD mutants are well expressed at the surface of Ba/F3 cells, despite the presence of immature forms detected by PAGE. Such expression patterns give rise to very different receptor phosphorylation profiles (Figure 1A) and suggest for ECD mutants an attenuated level of degradation. Only distal D5/ECD mutants are still ligand-responsive as shown by an additional phosphorylation activity in the presence of SCF (Figure 1A). This pattern seems to correlate with better cell surface expression of these mutants (supplemental Figure 6).
Structural importance of D5 domain of KIT in its constitutive activation
A recent crystallographic study revealed that residues involved in ECD mutations lie at an interface between 2 D5 domains in the ligand-induced dimers of KIT, suggesting that oncogenic mutations at these sites act by enhancing homotypic interactions between D5 domains.36 We recently reported that mutations in the c-kit D5 domain are very common in the highly aggressive dog mast cell tumors (MCTs), and pointed out 2 mutation hot spots: the dog c-kit codons 417 to 421 (equivalent to human 417-419) and the dog N508I substitution (equivalent to human residue 505).23 These combined results strongly support the idea that regions encoding 417 to 419 and 501 to 509 residues play key roles in KIT activation. They additionally show that D5-activating mutations are selectively involved in childhood-onset mastocytosis. One possible explanation for the activation mechanism of D5-mediated mutants is an increased affinity in the homotypic interaction coupled with overexpression of the receptor.36 This type of ECD GOF mutations has not been identified previously in the KIT-related receptors FLT3 and PDGFRα.37,38
Differences in functional activity between PTD and ECD mutants
These different mutations were introduced in varied cell lines to analyze and compare their functional activities in different cellular assays. No differences were observed for cell proliferation and/or survival after an irradiation stress between the different mutants versus the activated WT-KIT cells (data not shown). In contrast, Mayerhoffer et al10 recently reported that expression of KITD816V in Ba/F3 cells is able to induce cell-cluster formation accompanied by expression of several MC differentiation- and adhesion-related antigens. We observed such cluster formation with Ba/F3D816V cells, as with other PTD mutants. No or fewer clusters were observed with ECD mutants, similar to SCF-treated Ba/F3-WT-KIT cells. The absence of cluster formation might be correlated with a defect in up-regulation of adhesion molecules, as seen for ICAM-1 in PTD mutants. Cluster formation was suggested to reflect an SM phenotype,10 but ECD mutations were identified in a large cohort of children in which SM is generally not detected. These data are in agreement with a correlation between an SM phenotype and the presence of cluster in vitro. Several proteins were expressed by both types of mutants, as already described for one PTD mutant.10 In contrast, significant differences were seen in MC differentiation antigen expression: MC-CPA and CD25 were induced in SCF-treated WT-KIT as well as PTD mutant cells, whereas IL-3-treated Ba/F3 cells and ECD mutants were unable to induce them. CD25 is an important marker because it is expressed by neoplastic MCs.39 The whole-genome transcriptional profiles performed on EML cells confirmed the transcriptional induction of an MC differentiation program by PTD mutant, in contrast to ECD mutant (supplemental Figure 4); genes reported either in MC function (growth, activation, adhesion, and chemotactism) or as MC surface antigens were up-regulated in PTD-expressing EML cells (Table 2).
Unlike PTD mutants, ECD mutants seem to lack the capacity to induce a complete MC differentiation profile in either Ba/F3 or EML cells. Because decreased differentiation/maturation is in general associated with a gain in proliferation/transformation levels, ECD mutant MCs could represent a more aggressive form of MC neoplasia. This suggestion is supported by the fact that ECD mutants in Rat2 cells are able to form colonies in soft-agar, whereas PTD mutants demonstrate a clear differentiation capacity program associated with limited oncogenic activity. This incapacity of PTD mutants to transform cells was also reported for other PTD mutants of class III receptors FMS40 and FLT3.41 PTD mutants are either not or only rarely observed in dog MCT and human GIST, confirming the lack of agressivity of this class of mutants.23,42
Whereas the absence of oncogenic activity of PTD mutants seems to agree with the biologic behavior of neoplastic clones in SM,10 the high transforming capacity of ECD mutants is intriguing. ECD mutations have been identified in tumoral, aggressive, and metastatic pathologies, such as GIST and dog MCT, but also in pediatric mastocytosis, which is often a cutaneous, localized, and nonaggressive form of the disease that regresses at puberty. This contradiction is confirmed by the observation that germline mutations have been observed with ECD or TMD mutants but not with PTD mutants.4,5,14 Globally, it seems that ECD mutations are not fully transforming in human MCs, contrasting with their high oncogenic potential in Cajal cells that can give rise to GIST. This could be explained by a difference in cell-specific signaling, either through selective expression of positive regulator(s) in Cajal cell lineage or via a negative regulation pathway only in MC lineage.
Difference in signaling could explain functional differences between PTD and ECD mutants
Many similarities have been observed between SCF-activated WT-KIT and mutated receptors in the regulation of most KIT second messengers, such as ERK, JNK, and p38 MAPKinases, PI3kinase, and PDK1 activation as well as several adaptator molecules. Signaling pathways could be the angular stone of the functional differences observed between mutants. In this study, STAT molecules stayed as the only examined substrates that showed different activation profiles between chronic SCF-stimulated WT-KIT and chronic mutation-stimulated receptors (Figure 4A). STAT5 and, to a lesser extent, STAT3 were highly activated by all mutant forms as well as WT-KIT receptors, but this activation was down-regulated in chronic SCF-stimulated WT-KIT cells. STAT5 has been identified as a key regulator of MC development,43 and both STAT3 and STAT5 have been reported to be important in the D816V-dependent functions of neoplastic cells.27,44 In addition, STAT1 has been reported as a negative regulator in some pathway through mitochondrial functions and could explain the lack of aggressiveness of neoplastic MCs.45
The observed differences in the capacity of ECD and PTD mutants to induce Epo-mediated erythroid differentiation in EML cells are of special importance. PTD mutants were unable to induce Epo-mediated erythroid differentiation as assessed by benzidine staining and BFU-E formation (Figure 3B-C) as well as by the overexpression of suppressor of Epo-signaling proteins or by the down-regulation of erythroid-related antigens (Table 2). Erythroid differentiation was restored by ectopic expression of a dominant GOF form of AKT kinase (Figure 7). These data agree with the absolute requirement of AKT activation in Epo-mediated erythropoiesis of early progenitors46,47 ; and most of all, it highlights the role of AKT kinase regulation in the signaling pathway of PTD mutants. We and others have reported that PI3kinase was not altered in mutant KIT21 ; we additionally showed that downstream substrates of PI3kinase are well activated (Figure 5B and data not shown, respectively) but that phosphorylation of AKT S473 and AKT activity are down-regulated in PTD mutants, as confirmed by GSK3β hypophosphorylation (Figure 5B). Under SCF stimulation, WT-KIT as well as D816V mutant were phosphorylated on AKT S473, but phosphorylation was less intense and was rapidly down-regulated with D816V mutant compared with WT-KIT (data not shown). Preliminary results using okadaic acid treatment suggest that negative AKT activation could be mediated through a PP2A phosphatase-dependent pathway. Altogether, these data suggest that PTD mutants could induce a signal different from that of ECD mutants, involving phosphatase(s) acting as negative regulator(s) on the AKT pathway. This difference in signaling, especially downstream of AKT, was also suggested by a higher sensitivity to rapamycin of PTD compared with JMD mutants.48 Although major effectors of this pathway are known, crosstalk and substrate deregulation that occur during neoplasia are still ill defined. A recent proteomic approach led us to identify the FES kinase as a specific effector of PTD mutants,49 confirming the use of specific downstream pathway in mutant receptors, which could represent an attractive alternative approach to targeted therapies. Further studies are required to elucidate the functional consequences of the involvement of such “new” proteins and/or the resulting pathways on oncogenic KIT-mediated signaling so as to better understand heterogeneity of mastocytosis and of KIT-mediated pathologies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank F. Birg for editing the manuscript.
This work was supported by Inserm, la Ligue Nationale Contre le Cancer (Equipe labellisée), and Agence Nationale pour la Recherche, grant Maladies Rares. Y.Y. was the recipient of a PhD fellowship from Association de Recherche sur le Cancer. M.V. was the recipient of a PhD fellowship from Fondation pour la Recherche Medicale. L.B. was supported by an ANR RIB grant. K.H. was supported by the Association Française pour les Initiatives de Recherche sur le Mastocyte et les Mastocytoses.
Authorship
Contribution: Y.Y. and P.D. designed research, analyzed data, and wrote the paper; F.B. analyzed data and wrote the paper; S. Létard, L.B., A.C., K.H., S. Lopez, M.V., and P.F. performed research; and D.B., S.G., and P.d.S. analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Patrice Dubreuil, Inserm U891, Centre de Recherche en Cancérologie de Marseille, 27 Bd Leï Roure Marseille, F-13009, France; e-mail: patrice.dubreuil@inserm.fr.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal