Abstract
The development of mature blood cells from hematopoietic stem cells requires coordinated activities of transcriptional networks. Transcriptional repressor growth factor independence 1 (Gfi-1) is required for the development of B cells, T cells, neutrophils, and for the maintenance of hematopoietic stem cell function. However, the mechanisms by which Gfi-1 regulates hematopoiesis and how Gfi-1 integrates into transcriptional networks remain unclear. Here, we provide evidence that Id2 is a transcriptional target of Gfi-1, and repression of Id2 by Gfi-1 is required for B-cell and myeloid development. Gfi-1 binds to 3 conserved regions in the Id2 promoter and represses Id2 promoter activity in transient reporter assays. Increased Id2 expression was observed in multipotent progenitors, myeloid progenitors, T-cell progenitors, and B-cell progenitors in Gfi-1−/− mice. Knockdown of Id2 expression or heterozygosity at the Id2 locus partially rescues the B-cell and myeloid development but not the T-cell development in Gfi-1−/− mice. These studies demonstrate a role of Id2 in mediating Gfi-1 functions in B-cell and myeloid development and provide a direct link between Gfi-1 and the B-cell transcriptional network by its ability to repress Id2 expression.
Introduction
The development of mature blood cells from multipotent hematopoietic stem cells (HSCs) is a highly orchestrated process, with transcription factors playing key roles in lineage commitment and differentiation. For example, the transcription factors PU.1 and Ikaros are required for primitive lymphoid progenitor formation, whereas E2A, EBF, and Pax5 are essential for commitment to the B-cell fate.1 These transcription factors are part of a network connected by transcriptional regulation or direct protein interaction and function in collaboration to activate B-cell lineage-specific genes during B-cell development. Similarly, T lymphopoiesis, myelopoiesis, and erythropoiesis are controlled by their transcriptional networks.2-4
Growth factor independence 1 (Gfi-1) is a zinc finger transcriptional repressor originally identified in an insertional mutagenesis screen for T-cell lymphomas acquiring interleukin-2 (IL-2) growth independence.5,6 Studies of Gfi-1–deficient mice revealed that Gfi-1 functions in T and B lymphopoiesis, neutrophil development, and HSC maintenance. Specifically, Gfi-1–deficient mice display reduced thymic cellularity as the result of decreased survival and proliferation7 and impaired B-cell development with compromised IL-7 signaling.8 Gfi-1−/− mice also lack mature neutrophils. Immature neutrophils accumulate in the bone marrow and spleen of Gfi-1−/− mice because of myeloid hyperplasia and maturation arrest.9,10 Mutations in the Gfi-1 gene have been reported in a group of patients with severe congenital neutropenia.11 In addition, Gfi-1 acts to restrict the proliferation of HSCs, thereby preserving their functional integrity.12,13 However, the mechanisms by which Gfi-1 controls hematopoietic cell proliferation and differentiation are largely unknown.
Gene expression profiling identified inhibitor of DNA binding 1 (Id1) and Id2 as prominently affected genes by loss of Gfi-1 in thymocytes.7 Id genes encode a family of 4 helix-loop-helix proteins (Id1, Id2, Id3, and Id4) that play important roles in regulating cell proliferation, differentiation, and apoptosis.14-16 Id proteins act as dominant-negative regulators of other transcription factors. Target proteins of Id include transcription factors from the E protein family, ETS family, Pax family, and retinoblastoma protein.17-21 As negative regulators of E proteins, high levels of Id expression block both B- and T-lymphocyte development.22-27 Overexpression of Id1 promotes the proliferation of myeloid progenitors and leads to myeloid proliferative disease in vivo.28
We demonstrate here that Id2 is a transcriptional target of Gfi-1. Id2 expression was shown to be up-regulated in several hematopoietic lineages as the result of Gfi-1 deficiency. Knock-down of Id2 expression in Gfi-1−/− bone marrow cells (BMCs) partially rescued B-cell development and myeloid development when these BMCs were transplanted into mice. Furthermore, we observed that heterozygosity at the Id2 locus partially rescued the B-cell and myeloid cell phenotypes of Gfi-1−/− mice. These data indicate that Id2 is a direct physiologic target of Gfi-1, and repression of Id2 by Gfi-1 is required for proper B-cell and myeloid development.
Methods
Mice
Gfi-1–deficient mice and Id2-deficient mice have been previously described.10,29 NCI-Frederick is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the Guide for Care and Use of Laboratory Animals.30 All animal experiments were approved by the NCI-Frederick Animal Care and Use Committee.
Plasmids
Moloney Murine leukemia virus–based retroviral vector-encoding GFP (MMP) and MMP-Gfi-1 plasmids have been previously described.10 Id2pro-3507 plasmid was cloned by polymerase chain reaction (PCR) amplification of the 3507-bp murine Id2 promoter and ligation into XhoI BglII sites of pGL-4.10 vector (Promega). Id2pro-1905 plasmid was cloned by XhoI and PflMI restriction digestion of Id2pro-3507, followed by ligation of the purified and blunted 6147-bp fragment. Id2pro-1036 plasmid was cloned by XhoI and SnaBI restriction digestion of Id2pro-3507, followed by ligation of the purified and blunted 5278-bp fragment.
Retroviral transduction of DA-1, EML, and 5FU-BMC
MMP, MMP-Gfi-1, murine stem cell virus (MSCV), or MSCV-Id2 plasmid was transfected into Phoenix packaging cells (a gift from Dr Gary Nolan, Stanford University) with pCL-eco plasmid by FuGENE 6 (Roche Applied Science) to produce infectious ecotropic retrovirus. The viral supernatants were collected 48 hours after transfection and used to infect DA-1 cells, EML cells, or fluorouracil (5FU)–BMC in the presence of 4 μg/mL Polybrene (Sigma-Aldrich). Cells were transduced 3 times during a 36-hour period, and then green fluorescent protein+ (GFP+) cells were isolated by fluorescence-activated cell sorting (FACS).
Real-time reverse transcription PCR
Total RNA was purified by the use of RNeasy kit (QIAGEN) and converted to cDNA with iScript cDNA synthesis kit (Bio-Rad). Real-time quantitative reverse transcription (qRT)–PCR was performed in triplicates on ABI 7500 Fast Real-Time PCR System (Applied Biosystems). All samples were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Luciferase reporter assays
Id2 promoter reporter plasmid (Id2pro-1036 or Id2pro-1905 or Id2pro-3507, 100 ng) and Gfi-1 expression plasmid (pcDNA-Gfi-1 or pcDNA control plasmid, 100 ng) were cotransfected into 293T cells by the use of FuGENE 6. Luciferase activities were examined 24 hours after transfection with the luciferase assay system (Promega) and normalized per microgram of protein extract.
LightShift electrophoretic mobility shift assays
LightShift electrophoretic mobility shift assays (EMSAs) were performed by the use of LighShift EMSA kit following the manufacturer's protocols (Pierce Biotechnology Inc). In brief, nuclear extracts from pcDNA- or pcDNA-Gfi-1–transfected 293T cells were incubated with biotin-labeled oligonucleotides. After electrophoresis, the protein-oligonucleotide complexes were transferred and cross-linked to nylon membrane. Membrane was incubated with Streptavidin-Horseradish Peroxidase Conjugate (GE Healthcare) after blocking, and double-stranded biotin-labeled DNA was detected with chemiluminescent substrate. Oligonucleotide sequences were as follows:
Site 1: ATGTGACACCAGAAATCACGATTTGTGCAT
Site 2: GACTCAGATAGTAAATCACTTCCAGGCTTA
Site 3: CCTTCTTCCCCCAAATCACTCGAAACTTAA
Underlined letters represent the core Gfi-1 binding site.
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) assays were performed with 1 × 106 EML cells following standard procedures. In brief, chromatin was cross-linked with formaldehyde and sheared by sonication. Immunoprecipitation was performed with immunoglobulin G (IgG) or anti–Gfi-1, followed by incubation with protein agarose/salmon sperm DNA (Upstate Biotechnology Inc). DNA isolated from antibody bound fraction was eluted after wash, extracted with phenol/chloroform, and precipitated with ethanol. Real-time PCR quantification of immunoprecipitated DNA was carried out with the SYBR Green PCR Master Mix (Applied Biosystems) and primers designed to amplify regions covering each Gfi-1 binding site in the Id2 promoter.
Purification of hematopoietic cells
Purification of B cells was accomplished by staining BMC from Gfi-1+/+ or Gfi-1−/− mice with phycoerythrin (PE)–conjugated B220, fluorescein isothiocyanate (FITC)–conjugated CD43, allophycocyanin (APC)–conjugated NK1.1, and APC-conjugated DX5 antibodies (BD Pharmingen). B220+CD43−, B220+CD43+, and B220+CD43+NK1.1−DX5-B cells were purified by multicolor-based sorting. Purification of T cells was accomplished by staining thymocytes from Gfi-1+/+ or Gfi-1−/− mice with PE-conjugated CD4 and FITC-conjugated CD8 antibodies (BD Pharmingen). CD4−CD8−, CD4+CD8+, CD4+CD8−, and CD4−CD8+ T cells were purified by multicolor-based sorting. Purification of hematopoietic progenitors was accomplished by staining BMC from Gfi-1+/+ or Gfi-1−/− mice with purified rat antibodies specific for the following lineage markers: CD4, CD8, B220, TER119, Gr-1, and Mac-1 (BD-Pharmingen). Lin+ cells were removed with sheep anti–rat IgG-conjugated magnetic beads (Invitrogen), and the remaining cells were stained with PE-Cy5–conjugated anti–IL-7Rα, FITC-conjugated anti-CD34, PE-conjugated anti–c-Kit, APC-conjugated anti–Sca-1, and PE-Cy7–conjugated anti-FcγRII/III (Pharmingen). LSK cells were sorted as Lin− IL-7Rα− Sca-1+ c-Kit+; common myeloid progenitors (CMPs) were sorted as Lin− IL-7Rα− Sca-1− c-Kit+ CD34+ FcγRII/IIIlo; granulocyte/macrophage progenitors (GMPs) were sorted as Lin− IL-7Rα− Sca-1− c-Kit+ CD34+ FcγRII/IIIhi; and megakaryocyte/erythrocyte progenitors (MEPs) were sorted as Lin− IL-7Rα− Sca-1− c-Kit+ CD34− FcγRII/IIIlo.
Bone marrow transplantation and flow cytometric analysis
BMCs from Gfi-1+/+ or Gfi-1−/− mice were transduced with pRetro-Id2-shRNA retrovirus or pRetro-NS-shRNA control retrovirus 3 times during a 36-hour period, and then GFP+ cells were isolated by FACS. 1 × 106 GFP+ cells with 2 × 105 supporting BMC from C57BL/6 mice were transplanted into C57BL/6 recipient mice exposed to 9.5 Gy (950 rad) from 137Cs source. Hematopoietic reconstitution was determined by analyzing GFP+ BMCs. All lineage-specific antibodies and PerCP-Cy5.5–conjugated streptavidin were from BD Pharmingen. B220 and Gr-1 were PE-conjugated antibodies. CD43 and Mac-1 were biotin-conjugated antibodies.
Colony-forming assays
For colony-forming units in culture assays with splenocytes, we plated 2.5 × 104 cells in 1 mL of methylcellulose medium (StemCell Technologies) supplemented with murine IL-3 (30 ng/mL) and murine granulocyte monocyte colony-stimulating factor (mGM-CSF; 20 ng/mL), in 35-mm Petri dishes. Colony formation was evaluated after 7 to 10 days' incubation at 37°C. All growth factors were from PeproTech Inc.
Results
Id gene expression is regulated by Gfi-1 in hematopoietic cells
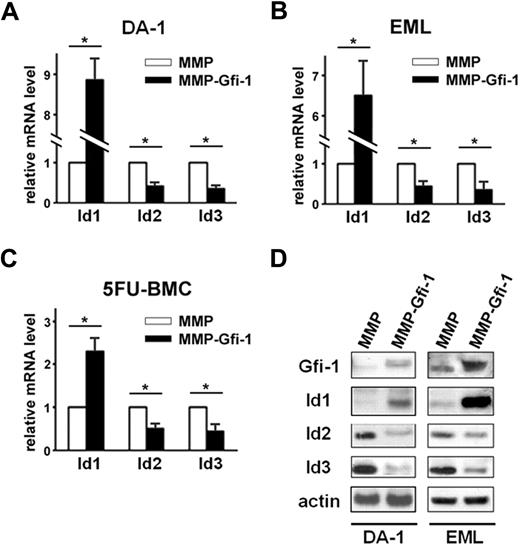
Gfi-1 loss of function in mice leads to multiple hematopoietic phenotypes, including impaired HSC function, impaired myeloid development, and decreased lymphoid development. The authors of previous studies found that Id1 and Id2 expression levels were increased in Gfi-1−/− thymocytes.7 Thus, we asked whether Id genes are regulated by Gfi-1 and whether Id genes could mediate the functions of Gfi-1 in hematopoiesis. To determine whether Gfi-1 regulates Id gene expression, we overexpressed Gfi-1 in hematopoietic progenitor cell lines DA-131 and EML32 and examined Id1, Id2, and Id3 expression levels. DA-1 and EML cells were transduced with MMP-Gfi-1 retrovirus or MMP control retrovirus. RNA and protein were obtained from retrovirus transduced GFP+ cells. Using real-time qRT-PCR, we found that overexpression of Gfi-1 induced Id1 mRNA expression and suppressed Id2 and Id3 mRNA expression in both DA-1 and EML cells (Figure 1A-B).
Enforced expression of Gfi-1 induces Id1 expression and suppresses Id2 and Id3 expression. DA-1 cells (A), EML cells (B), and 5FU-BMC (C) were transduced with MMP-Gfi-1 retrovirus or MMP control retrovirus. RNA was harvested from transduced cells and analyzed by real-time qRT-PCR in triplicates. All samples were normalized to GAPDH. *P < .05 in 2-sided t test. Three independent experiments were performed. (D) DA-1 and EML cells were transduced with MMP-Gfi-1 retrovirus or MMP control retrovirus. Whole-cell lysates were harvested from transduced cells and analyzed by Western blot with anti–Gfi-1, anti-Id1, anti-Id2, anti-Id3, or anti-actin antibodies. Three independent experiments were performed.
Enforced expression of Gfi-1 induces Id1 expression and suppresses Id2 and Id3 expression. DA-1 cells (A), EML cells (B), and 5FU-BMC (C) were transduced with MMP-Gfi-1 retrovirus or MMP control retrovirus. RNA was harvested from transduced cells and analyzed by real-time qRT-PCR in triplicates. All samples were normalized to GAPDH. *P < .05 in 2-sided t test. Three independent experiments were performed. (D) DA-1 and EML cells were transduced with MMP-Gfi-1 retrovirus or MMP control retrovirus. Whole-cell lysates were harvested from transduced cells and analyzed by Western blot with anti–Gfi-1, anti-Id1, anti-Id2, anti-Id3, or anti-actin antibodies. Three independent experiments were performed.
Western blot analysis confirmed that overexpression of Gfi-1 induced Id1 protein expression and suppressed Id2 and Id3 protein expression in both DA-1 and EML cells (Figure 1D). To establish that Gfi-1 regulates Id gene expression in normal hematopoietic cells, we transduced BMCs from 5FU-treated mice with MMP-Gfi-1 retrovirus or MMP control retrovirus and examined Id gene expression levels by real-time qRT-PCR. Similar to the observation in DA-1 cells and EML cells, Gfi-1 induced Id1 mRNA expression and suppressed Id2 and Id3 mRNA expression in 5FU-BMC (Figure 1C). These data indicate that Id gene expression is differentially regulated by Gfi-1 in hematopoietic cells.
Id2 is a direct transcriptional target of Gfi-1
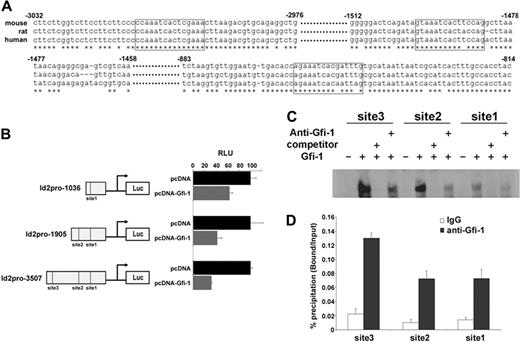
Because Id1, Id2, and Id3 gene expression are regulated by Gfi-1, we asked whether these genes are direct transcriptional targets of Gfi-1. We analyzed 5-kb regions of mouse Id1, Id2, and Id3 promoters for transcription factor binding sites using the MatInspector program (Genomatix). MatInspector identified 4 potential Gfi-1 binding sites in mouse Id1 promoter, 3 in mouse Id2 promoter, and 3 in mouse Id3 promoter (Table 1). Cross-species sequence alignments of Id promoters revealed that Gfi-1 binding sites in the Id2 promoter are phylogenetically conserved (Figure 2A), suggesting functional Gfi-1 binding to these sites. Herein, we focused our studies on Id2. To evaluate whether Gfi-1 regulates Id2 promoter activity, we cloned the mouse Id2 promoter into the pGL4 luciferase reporter plasmid. Specifically, we cloned the following 3 reporter constructs: Id2pro-1036, which contains 1 Gfi-1 binding site; Id2pro-1905, which contains 2 Gfi-1 binding sites; and Id2pro-3507, which contains all 3 Gfi-1 binding sites. We found that Gfi-1 represses 37% of the Id2pro-1036 promoter activity, 58% of the Id2pro-1905, and 68% of the Id2pro-3507 when normalized to pcDNA control. These data suggest that Gfi-1 represses Id2 promoter activity through some or all of these Gfi-1 binding sites.
Potential Gfi-1 binding sites in mouse Id1, Id2, and Id3 promoters
| Promoter . | Position . | Strand . | Sequence . |
|---|---|---|---|
| Id1 | −4751 ∼ −4737 | (−) | caaAATCaactcacc |
| Id1 | −4165 ∼ −4151 | (+) | tttAATCtcagcatt |
| Id1 | −3515 ∼ −3501 | (+) | tttAATCacagcact |
| Id1 | −903 ∼ −887 | (+) | aaaAATCaccagctg |
| Id2 | −3010 ∼ −2996 | (+) | ccaAATCactcgaaa* |
| Id2 | −1498 ∼ −1484 | (+) | gtaAATCacttccag* |
| Id2 | −860 ∼ −846 | (+) | agaAATCacgatttg* |
| Id3 | −2030 ∼ −2016 | (−) | aaaAATCatggcctt |
| Id3 | −280 ∼ −266 | (−) | aaaAATCacttaaaa |
| Id3 | −256 ∼ −242 | (+) | tcaAATCtgtgctgg* |
| Promoter . | Position . | Strand . | Sequence . |
|---|---|---|---|
| Id1 | −4751 ∼ −4737 | (−) | caaAATCaactcacc |
| Id1 | −4165 ∼ −4151 | (+) | tttAATCtcagcatt |
| Id1 | −3515 ∼ −3501 | (+) | tttAATCacagcact |
| Id1 | −903 ∼ −887 | (+) | aaaAATCaccagctg |
| Id2 | −3010 ∼ −2996 | (+) | ccaAATCactcgaaa* |
| Id2 | −1498 ∼ −1484 | (+) | gtaAATCacttccag* |
| Id2 | −860 ∼ −846 | (+) | agaAATCacgatttg* |
| Id3 | −2030 ∼ −2016 | (−) | aaaAATCatggcctt |
| Id3 | −280 ∼ −266 | (−) | aaaAATCacttaaaa |
| Id3 | −256 ∼ −242 | (+) | tcaAATCtgtgctgg* |
Five-kb regions of mouse Id1, Id2, and Id3 promoters were analyzed for potential Gfi-1 binding sites by use of the MatInspector program.
Site is conserved in human and rat.
Id2 is a direct transcriptional target of Gfi-1. (A) Sequence alignment of mouse, rat, and human Id2 promoters. Potential Gfi-1 binding sites are boxed. Numbers indicates nucleotide position in the mouse Id2 promoter. (B) Id2 promoter reporter plasmid (Id2pro-1036 or Id2pro-1905 or Id2pro-3507) and Gfi-1 expression plasmid (pcDNA-Gfi-1 or pcDNA control) were cotransfected into 293T cells. Luciferase activities were examined 24 hours after transfection. To compare Gfi-1 repression on 3 Id2 promoter constructs, Id2 promoter activities in pcDNA-Gfi-1–transfected cells were expressed as the percentage of those in pcDNA-transfected cells. Three independent experiments were performed. (C) LightShift EMSA were performed by the use of nuclear extracts from pcDNA (lanes 1, 5, 9) or pcDNA-Gfi-1 (lanes 2, 3, 4, 6, 7, 8, 10, 11, 12)–transfected 293T cells, and biotin-labeled oligonucleotides from Id2 promoter containing the potential Gfi-1 binding sites, in the presence or absence of a 30-fold excess of nonlabeled competitors or anti–Gfi-1 antibody. Three independent experiments were performed. (D) ChIP assays were performed in EML cells. Immunoprecipitation was performed with IgG or anti–Gfi-1. PCR quantification of immunoprecipitated DNA was performed with the SYBR Green PCR Master Mix and primers designed to amplify regions covering each Gfi-1 binding site in the Id2 promoter. Three independent experiments were performed.
Id2 is a direct transcriptional target of Gfi-1. (A) Sequence alignment of mouse, rat, and human Id2 promoters. Potential Gfi-1 binding sites are boxed. Numbers indicates nucleotide position in the mouse Id2 promoter. (B) Id2 promoter reporter plasmid (Id2pro-1036 or Id2pro-1905 or Id2pro-3507) and Gfi-1 expression plasmid (pcDNA-Gfi-1 or pcDNA control) were cotransfected into 293T cells. Luciferase activities were examined 24 hours after transfection. To compare Gfi-1 repression on 3 Id2 promoter constructs, Id2 promoter activities in pcDNA-Gfi-1–transfected cells were expressed as the percentage of those in pcDNA-transfected cells. Three independent experiments were performed. (C) LightShift EMSA were performed by the use of nuclear extracts from pcDNA (lanes 1, 5, 9) or pcDNA-Gfi-1 (lanes 2, 3, 4, 6, 7, 8, 10, 11, 12)–transfected 293T cells, and biotin-labeled oligonucleotides from Id2 promoter containing the potential Gfi-1 binding sites, in the presence or absence of a 30-fold excess of nonlabeled competitors or anti–Gfi-1 antibody. Three independent experiments were performed. (D) ChIP assays were performed in EML cells. Immunoprecipitation was performed with IgG or anti–Gfi-1. PCR quantification of immunoprecipitated DNA was performed with the SYBR Green PCR Master Mix and primers designed to amplify regions covering each Gfi-1 binding site in the Id2 promoter. Three independent experiments were performed.
To determine whether Gfi-1 binds to these 3 potential Gfi-1 sites in the Id2 promoter, we performed LightShift EMSA. Incubation of biotin-labeled oligonucleotides with nuclear extracts from pcDNA-Gfi-1–transfected 293T cells resulted in the appearance of a protein-DNA complex for all 3 Gfi-1 binding sites in the Id2 promoter, which was not present for pcDNA-transfected 293T nuclear extracts (Figure 2C). This protein-DNA complex was competed by nonbiotin-labled oligonucleotides. The addition of the Gfi-1 antibody reduced the protein-DNA complex, indicating the specificity for Gfi-1 binding.
To determine whether Gfi-1 is present on the Id2 promoter in vivo, we performed ChIP assays in EML cells overexpressing Gfi-1. qPCR analysis with the use of primers specific to the 3 Gfi-1 binding regions in the Id2 promoter generated amplicons that were enriched by anti–Gfi-1 antibody compared with a control IgG (Figure 2D). This finding indicates that Gfi-1 is present on all 3 Gfi-1 binding sites of the Id2 promoter in EML cells. Taken together, luciferase reporter assays, Lightshift EMSA, and ChIP assays demonstrated that Id2 is a direct transcriptional target of Gfi-1.
Id2 gene expression is deregulated in Gfi-1−/− mice
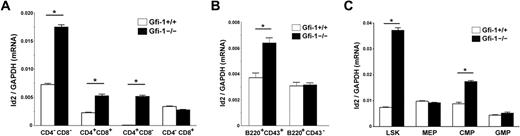
Because Id2 is a direct transcriptional target of Gfi-1, we asked whether loss of Gfi-1 affects Id2 expression in hematopoietic cells. To test this theory, we purified hematopoietic populations from Gfi-1+/+ and Gfi-1−/− mice, including (1) CD4−CD8−, CD4+CD8+, and CD4+CD8− and CD4−CD8+ T lymphocytes; (2) B220+CD43+ and B220+CD43− B lymphocytes; and (3) LSKs, CMPs, GMPs, and MEPs and examined Id2 expression in these populations by real-time qRT-PCR. We found that Id2 mRNA level is up-regulated by loss of Gfi-1 in CD4−CD8−, CD4+CD8+, and CD4+CD8− T cells but not in CD4−CD8+ T cells (Figure 3A). In B-cell populations, the Id2 mRNA level in B220+CD43+ population is significantly increased in Gfi-1−/− mice compared with Gfi-1+/+ mice. However, Id2 mRNA levels are not affected by loss of Gfi-1 in B220+CD43− B cells (Figure 3B). B220+CD43+ population contains natural killer (NK) cells.33
Id2 expression is deregulated in Gfi-1−/− mice. (A) CD4−CD8−, CD4+CD8+, and CD4+CD8− and CD4−CD8+ T lymphocytes were purified from Gfi-1+/+ and Gfi-1−/− thymuses by sorting. RNA was harvested from each population and analyzed for Id2 mRNA expression levels by real-time qRT-PCR. (B) B220+CD43− and B220+CD43+ B lymphocytes were purified from Gfi-1+/+ and Gfi-1−/− bone marrows by sorting. RNA was harvested from each population and analyzed for Id2 mRNA expression levels by real-time qRT-PCR. (C) LSKs, CMPs, GMPs, and MEPs were purified from Gfi-1+/+ and Gfi-1−/− mice by multicolor-based sorting. RNA was harvested from LSK, CMP, GMP, and MEP, and analyzed for Id2 mRNA expression levels by real-time qRT-PCR. All samples were normalized to GAPDH. *P < .05 in 2-sided t test. Three independent experiments were performed.
Id2 expression is deregulated in Gfi-1−/− mice. (A) CD4−CD8−, CD4+CD8+, and CD4+CD8− and CD4−CD8+ T lymphocytes were purified from Gfi-1+/+ and Gfi-1−/− thymuses by sorting. RNA was harvested from each population and analyzed for Id2 mRNA expression levels by real-time qRT-PCR. (B) B220+CD43− and B220+CD43+ B lymphocytes were purified from Gfi-1+/+ and Gfi-1−/− bone marrows by sorting. RNA was harvested from each population and analyzed for Id2 mRNA expression levels by real-time qRT-PCR. (C) LSKs, CMPs, GMPs, and MEPs were purified from Gfi-1+/+ and Gfi-1−/− mice by multicolor-based sorting. RNA was harvested from LSK, CMP, GMP, and MEP, and analyzed for Id2 mRNA expression levels by real-time qRT-PCR. All samples were normalized to GAPDH. *P < .05 in 2-sided t test. Three independent experiments were performed.
Because Gfi-1−/− mice have reduced numbers of B220+CD43+ cells, the proportion of NK cells in this population may be significant. Therefore, we further purified pro-B cells by depleting NK1.1+ and DX5+ cells from the B220+CD43+ population. Id2 mRNA levels in B220+CD43+NK1.1−DX5− pro-B cells was also significantly increased in Gfi-1−/− mice compared with Gfi-1+/+ mice (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Finally, we observed that Id2 mRNA expression is significantly increased in Gfi-1−/− LSKs, which contain HSCs and multipotent progenitors, and Gfi-1−/− CMPs, which contain myeloid progenitors, whereas Id2 mRNA levels in Gfi-1−/− MEPs or GMPs are not changed compared with Gfi-1+/+ controls (Figure 3C). Collectively, these data suggest that loss of Gfi-1 leads to up-regulated Id2 expression in multipotent progenitors, myeloid progenitors, T-cell progenitors, and B-cell progenitors, which could contribute to the hematopoietic defects observed in Gfi-1−/− mice.
High levels of Id2 expression inhibit neutrophil differentiation in vitro and promote myeloid progenitor proliferation in vitro and in vivo
High levels of Id2 expression inhibit T-cell development in transgenic mice, and overexpression of Id2 in hematopoietic stem and progenitor cells blocks B-cell development in vivo.24,27 However, the effects of Id2 overexpression on myeloid development are not clear. Our previous studies suggest Id2 may function in myeloid development.27 To further elucidate Id2 function in myeloid development, we transduced BMC from 5FU-treated mice with retroviral vectors that express Id2 and examined the effects of Id2 overexpression on myeloid development in culture and in transplanted mice. 5FU-BMCs transduced with MSCV control retrovirus gradually lose c-kit expression and differentiate into Mac-1+Gr-1+ neutrophils in culture (Figure 4A-B). In comparison, overexpression of Id2 inhibited neutrophil differentiation and maintained c-kit+ progenitor cells (Figure 4A-B). In addition, we asked whether Id2 affects the proliferation of myeloid progenitors. Cell-cycle analysis showed that 25% BMC-MSCV cells were in the S/G2/M phase, whereas 40% BMC-Id2 cells were in the S/G2/M phase (Figure 4C).
High levels of Id2 expression inhibit neutrophil differentiation in vitro and promote myeloid progenitor proliferation in vitro and in vivo. (A) 5FU-BMCs were transduced with MSCV or MSCV-Id2 retrovirus and cultured in mGM-CSF to induce neutrophil differentiation. Myeloid cells in the culture were analyzed for c-kit, Sca-1, Gr-1, and Mac-1 surface markers. (B) Total cell numbers of Gr-1+Mac-1+ cells and c-kit+ cells from MSCV- or MSCV-Id2–transduced 5FU-BMC culture. *P < .05 in 2-sided t test. (C) Cell-cycle analysis of MSCV- or MSCV-Id2–transduced 5FU-BMCs was analyzed by propidium iodide staining. (D) Myeloid development in recipient bone marrow and spleen was examined 5 months after transplantation by FACS analysis with the use of Gr-1, and Mac-1 antibodies. Total cell number of Gr-1+Mac-1+ cells was shown. *P < .05 in 2-sided t test. Four recipient mice were used in each group. Three independent experiments were performed.
High levels of Id2 expression inhibit neutrophil differentiation in vitro and promote myeloid progenitor proliferation in vitro and in vivo. (A) 5FU-BMCs were transduced with MSCV or MSCV-Id2 retrovirus and cultured in mGM-CSF to induce neutrophil differentiation. Myeloid cells in the culture were analyzed for c-kit, Sca-1, Gr-1, and Mac-1 surface markers. (B) Total cell numbers of Gr-1+Mac-1+ cells and c-kit+ cells from MSCV- or MSCV-Id2–transduced 5FU-BMC culture. *P < .05 in 2-sided t test. (C) Cell-cycle analysis of MSCV- or MSCV-Id2–transduced 5FU-BMCs was analyzed by propidium iodide staining. (D) Myeloid development in recipient bone marrow and spleen was examined 5 months after transplantation by FACS analysis with the use of Gr-1, and Mac-1 antibodies. Total cell number of Gr-1+Mac-1+ cells was shown. *P < .05 in 2-sided t test. Four recipient mice were used in each group. Three independent experiments were performed.
To examine Id2 effect on myeloid development in vivo, we transplanted BMC-Id2 and BMC-MSCV into lethally irradiated mice. In agreement with the effect of Id2 on myeloid proliferation in culture, BMC-Id2 recipient mice developed a myeloid proliferative disease 4 to 6 months after transplantation, with granulocytic hyperplasia in bone marrow and spleen (Figure 4D). Interestingly, although Id2 overexpression inhibited neutrophil differentiation in culture, myeloid maturation in BMC-Id2 recipients was not blocked. This difference may be attributable to the cytokines used in culture (mGM-CSF only) versus the cytokine milieu in recipient mice. Thus, high levels of Id2 promote myeloid progenitor proliferation, suggesting that up-regulated Id2 expression observed in Gfi-1−/− progenitors may contribute to the myeloid defects in Gfi-1−/− mice.
Id2 knockdown in Gfi-1−/− BMC partially rescues hematopoiesis
Because Id2 expression is increased in Gfi-1−/− hematopoietic cells and high levels of Id2 expression disrupt lymphoid and myeloid development, we asked whether impaired hematopoiesis in Gfi-1−/− mice is caused by increased Id2 levels. To address this question, we examined the effects of knocking-down Id2 on myeloid and lymphoid development in Gfi-1−/− BMC. Transduction of BMC with the Id2-specific shRNA (pRetro-Id2-shRNA) results in 70% to 90% Id2 knockdown compared with BMC transduced with the nonspecific shRNA (pRetro-NS-shRNA).27 Gfi-1+/+ and Gfi-1−/− BMCs transduced with pRetro-Id2-shRNA or pRetro-NS-shRNA were transplanted into lethally irradiated wild-type mice with supporting wild-type BMCs. One month after transplantation, we analyzed myeloid and lymphoid development of GFP+ donor cells by FACS.
We could not detect GFP+ T cells in recipients of pRetro-NS-shRNA–transduced Gfi-1−/− BMC or recipients of pRetro-Id2-shRNA–transduced Gfi-1−/− BMCs (data not shown), which confirmed the T-cell developmental defect in Gfi-1−/− mice and suggested that reducing Id2 levels does not rescue T-cell development.
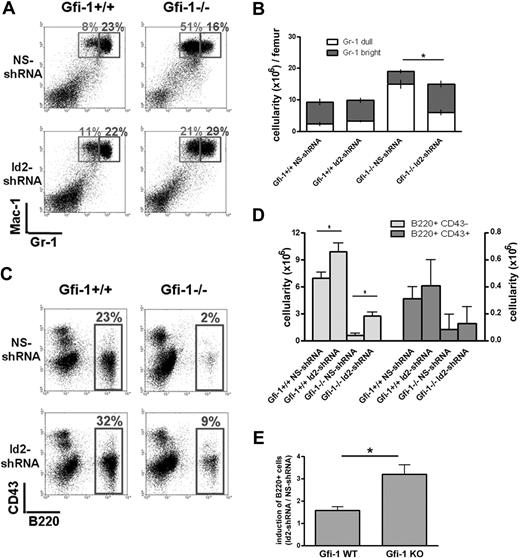
For myeloid reconstitution after transplantation, pRetro-NS-shRNA–transduced Gfi-1+/+ and Gfi-1−/− BMC recipients showed the same myeloid developmental potential that was observed in Gfi-1+/+ and Gfi-1−/− mice, respectively.9 Specifically, pRetro-NS-shRNA–transduced Gfi-1+/+ BMCs produced 31% neutrophils in the bone marrow, with 23% Gr-1bright mature neutrophils and 8% Gr-1dull immature neutrophils. In contrast, pRetro-NS-shRNA–transduced Gfi-1−/− BMC gave rise to 67% neutrophils, with 16% Gr-1bright mature neutrophils and 51% Gr-1dull immature neutrophils (Figure 5A). Thus, pRetro-NS-shRNA–transduced and –transplanted Gfi-1−/− BMCs developed myeloid hyperplasia and maturation arrest phenotypes. Importantly, pRetro-Id2-shRNA–transduced Gfi-1−/− BMCs produced significantly less Gr-1dull immature neutrophils (decreased from 51% to 21%) and more Gr-1bright mature neutrophils (increased from 16% to 29%) compared with pRetro-NS-shRNA–transduced Gfi-1−/− BMC. The total percentage of myeloid cells also decreased from 67% to 50% by Id2 shRNA in Gfi-1−/− BMC. Thus, knocking-down Id2 partially rescued the myeloid differentiation and myeloid hyperplasia phenotypes of the Gfi-1−/− BMCs.
Id2 knockdown in Gfi-1−/− BMC partially rescues hematopoiesis. (A) pRetro-Id2-shRNA– or pRetro-NS-shRNA–transduced Gfi-1+/+ or Gfi-1−/− BMCs (1 × 106 per recipient) with supporting wild-type BMC (2 × 105 per recipient) were transplanted into lethally irradiated C57BL/6 mice. Myeloid reconstitution in recipient bone marrow was examined 4 weeks after transplantation by analyzing the GFP+ donor cells with Gr-1 and Mac-1 surface markers by flow cytometry. (B) Total cell numbers of Gr-1dullMac-1+ and Gr-1brightMac-1+ cells were shown. *P < .05 in 2-sided t test. Four recipient mice were used in each group. Three independent experiments were performed. (C) B-cell reconstitution in recipient bone marrow was examined 4 weeks after transplantation by analyzing the GFP+ donor cells with B220 and CD43 surface markers by flow cytometry. (D-E) Total cell number of B220+CD43+ and B220+CD43− cells, and fold of induction of B220+ cells by Id2 shRNA were shown. *P < .05 in 2-sided t test. Four recipient mice were used in each group. Three independent experiments were performed.
Id2 knockdown in Gfi-1−/− BMC partially rescues hematopoiesis. (A) pRetro-Id2-shRNA– or pRetro-NS-shRNA–transduced Gfi-1+/+ or Gfi-1−/− BMCs (1 × 106 per recipient) with supporting wild-type BMC (2 × 105 per recipient) were transplanted into lethally irradiated C57BL/6 mice. Myeloid reconstitution in recipient bone marrow was examined 4 weeks after transplantation by analyzing the GFP+ donor cells with Gr-1 and Mac-1 surface markers by flow cytometry. (B) Total cell numbers of Gr-1dullMac-1+ and Gr-1brightMac-1+ cells were shown. *P < .05 in 2-sided t test. Four recipient mice were used in each group. Three independent experiments were performed. (C) B-cell reconstitution in recipient bone marrow was examined 4 weeks after transplantation by analyzing the GFP+ donor cells with B220 and CD43 surface markers by flow cytometry. (D-E) Total cell number of B220+CD43+ and B220+CD43− cells, and fold of induction of B220+ cells by Id2 shRNA were shown. *P < .05 in 2-sided t test. Four recipient mice were used in each group. Three independent experiments were performed.
For B-cell reconstitution after transplantation, pRetro-NS-shRNA–transduced Gfi-1+/+ and Gfi-1−/− BMC recipients showed the same B-cell developmental potential that was observed in Gfi-1+/+ and Gfi-1−/− mice, respectively.8 Specifically, pRetro-NS-shRNA–transduced Gfi-1+/+ BMCs produced 23% B220+ cells, whereas pRetro-NS-shRNA–transduced Gfi-1−/− BMCs produced only 2% B220+ cells in the bone marrow (Figure 5C). Consistent with our previous report, knocking-down Id2 enhanced B-cell development in wild-type BMCs.27 Importantly, pRetro-Id2-shRNA–transduced Gfi-1−/− BMC produced more B220+ cells (increased from 2% to 9%) than pRetro-NS-shRNA–transduced Gfi-1−/− BMC. Although knocking-down Id2 expression led to a 50% induction of B220+ cells in Gfi-1+/+ BMC recipients, B220+ cells were induced more than 3-fold by Id2 shRNA in Gfi-1−/− BMC recipients (Figure 5E), indicating that knocking-down Id2 rescued B-cell development of the Gfi-1−/− BMC. However, because Id2shRNA induces B-cell development in wild-type BMC, the rescue of Gfi-1−/− B-cell phenotype is complicated by a concern of additive effect. Therefore, we further analyzed Id2 function in mediating the hematopoietic defects in Gfi-1−/− mice by crossing the Gfi-1 KO mice and Id2 KO mice.
Id2 heterozygosity partially rescues Gfi-1−/− phenotypes
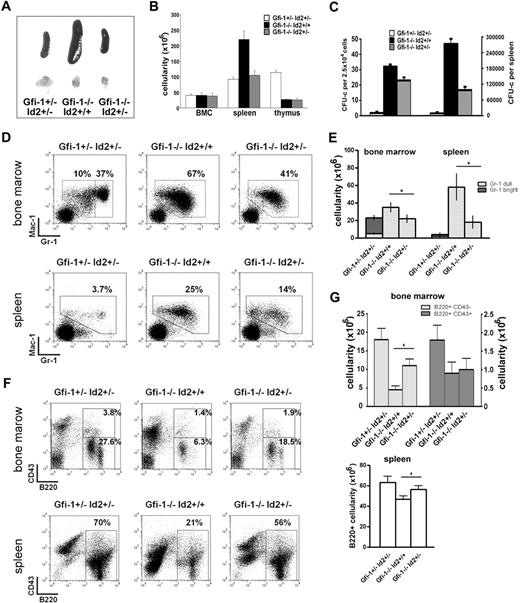
To confirm that Id2 mediates the hematopoietic defects in Gfi-1−/− mice, we crossed Gfi-1+/− and Id2+/− mice to study Gfi-1−/−Id2−/−, Gfi-1−/−Id2+/−, and Gfi-1−/−Id2+/+ mice. In a population of 339 intercross offspring genotyped at 4 weeks of age, no Gfi-1−/−Id2−/− mice were observed. Western blot analysis showed that Id2 protein levels in Id2+/− bone marrow cells are significantly lower than Id2+/+ mice (supplemental Figure 2). Therefore, we compared Gfi-1−/−Id2+/+ mice and Gfi-1−/−Id2+/− mice to determine whether reduced Id2 levels affect Gfi-1−/− phenotypes. We found that Gfi-1−/−Id2+/+ mice had enlarged spleens with increased splenic cellularity. In contrast, the spleen size and cellularity of Gfi-1−/−Id2+/− mice were normal (Figure 6A-B), indicating that Id2 heterozygosity rescues the splenic hyperplasia of Gfi-1−/− mice. Notably, there was no difference in thymus size or number of thymocytes between Gfi-1−/−Id2+/+ mice and Gfi-1−/−Id2+/− mice, indicating that the thymocyte defect observed in Gfi-1−/− mice is not attributable to Id2 deregulation.
Id2 heterozygosity partially rescues Gfi-1−/− phenotype. (A) Picture of spleens and thymuses from Gfi-1+/−Id2+/−, Gfi-1−/−Id2+/+, and Gfi-1−/−Id2+/− mice. Image taken with Canon PowerShot G10 Digital Camera. (B) Cellularity of bone marrow, spleens and thymuses from Gfi-1+/−Id2+/−, Gfi-1−/−Id2+/+, and Gfi-1−/−Id2+/− mice. (C) Splenocytes from Gfi-1+/−Id2+/−, Gfi-1−/−Id2+/+, and Gfi-1−/−Id2+/− mice were plated in methylcellulose medium supplemented with murine IL-3 and mGM-CSF. Colony numbers were counted after 7 to 10 days in culture. (D-E) Bone marrows and spleens of Gfi-1+/−Id2+/−, Gfi-1−/−Id2+/+, and Gfi-1−/−Id2+/− mice were analyzed for Gr-1 and Mac-1 expression by FACS. The percentage and total cell number of myeloid populations were shown. *P < .05 in 2-sided t test. (F-G) Bone marrows and spleens of Gfi-1+/−Id2+/−, Gfi-1−/−Id2+/+, and Gfi-1−/−Id2+/− mice were analyzed for B-cell development by FACS. The percentage and total cell number of B220+CD43+, B220+CD43−, or B220+ cells were shown. *P < .05 in 2-sided t test. Three independent experiments were performed.
Id2 heterozygosity partially rescues Gfi-1−/− phenotype. (A) Picture of spleens and thymuses from Gfi-1+/−Id2+/−, Gfi-1−/−Id2+/+, and Gfi-1−/−Id2+/− mice. Image taken with Canon PowerShot G10 Digital Camera. (B) Cellularity of bone marrow, spleens and thymuses from Gfi-1+/−Id2+/−, Gfi-1−/−Id2+/+, and Gfi-1−/−Id2+/− mice. (C) Splenocytes from Gfi-1+/−Id2+/−, Gfi-1−/−Id2+/+, and Gfi-1−/−Id2+/− mice were plated in methylcellulose medium supplemented with murine IL-3 and mGM-CSF. Colony numbers were counted after 7 to 10 days in culture. (D-E) Bone marrows and spleens of Gfi-1+/−Id2+/−, Gfi-1−/−Id2+/+, and Gfi-1−/−Id2+/− mice were analyzed for Gr-1 and Mac-1 expression by FACS. The percentage and total cell number of myeloid populations were shown. *P < .05 in 2-sided t test. (F-G) Bone marrows and spleens of Gfi-1+/−Id2+/−, Gfi-1−/−Id2+/+, and Gfi-1−/−Id2+/− mice were analyzed for B-cell development by FACS. The percentage and total cell number of B220+CD43+, B220+CD43−, or B220+ cells were shown. *P < .05 in 2-sided t test. Three independent experiments were performed.
We further examined Gfi-1−/−Id2+/+ and Gfi-1−/−Id2+/− myeloid progenitor growth in colony assays. We found that splenocytes from Gfi-1+/−Id2+/− mice produced only 1 to 3 colonies per 2.5 × 104 cells, whereas splenocytes from Gfi-1−/−Id2+/+ mice produced more than 30 colonies per 2.5 × 104 cells (Figure 6C), indicating the presence of myeloid progenitors in Gfi-1−/−Id2+/+ spleens. In comparison, splenocytes from Gfi-1−/−Id2+/− mice produced 22 plus or minus 2 colonies per 2.5 × 104 cells (Figure 6C). The total colony number per spleen in Gfi-1−/−Id2+/− is only 30% of that in Gfi-1−/−Id2+/+ mice (Figure 6C). Thus, the numbers of myeloid progenitors in the Gfi-1−/− spleens were greatly reduced by the loss of 1 Id2 allele.
In addition, we analyzed the spleens of Gfi-1+/−Id2+/−, Gfi-1−/−Id2+/+, and Gfi-1−/−Id2+/− mice by FACS and observed few neutrophils in the Gfi-1+/−Id2+/− spleen (Figure 6D-E). In contrast, an accumulation of immature neutrophils was observed in the Gfi-1−/−Id2+/+ spleens, which accounted for more than 25% of the Gfi-1−/−Id2+/+ splenocytes. Significant reduction of immature neutrophils was seen in Gfi-1−/−Id2+/− spleen, supporting the conclusion that Id2 heterozygosity rescues Gfi-1−/− myeloid hyperplasia. We further examined myeloid development in Gfi-1+/−Id2+/−, Gfi-1−/−Id2+/+, and Gfi-1−/−Id2+/− bone marrow by FACS. We observed normal myeloid development in Gfi-1+/−Id2+/− mice, with approximately 37% Gr-1brigntMac-1+ mature neutrophils and 10% Gr-1dullMac-1+ immature neutrophils in the bone marrow (supplemental Figure 3), whereas, myeloid hyperplasia and arrested myeloid development was observed in Gfi-1−/−Id2+/+ mice, with more than 67% Gr-1dullMac-1+–immature neutrophils and no Gr-1brigntMac-1+–mature neutrophils in the bone marrow (Figure 6D-E). In comparison, only 41% neutrophils were observed in the Gfi-1−/−Id2+/− bone marrow, indicating a reduction in myeloid hyperplasia. However, most neutrophils in the Gfi-1−/−Id2+/− bone marrow were Gr-1dullMac-1+–immature neutrophils, suggesting that Id2 heterozygosity does not induce neutrophil differentiation.
To determine whether Id2 heterozygosity could rescue B-cell development in Gfi-1−/− mice, we analyzed Gfi-1+/−Id2+/−, Gfi-1−/−Id2+/+, and Gfi-1−/−Id2+/− BMC and splenocytes using B-cell markers B220 and CD43. We observed normal B-cell development in Gfi-1+/−Id2+/− mice (supplemental Figure 3) and impaired B-cell development in Gfi-1−/−Id2+/+ mice (Figure 6F-G). Importantly, B220+CD43−, but not B220+CD43+, cells were significantly increased in Gfi-1−/−Id2+/− bone marrow, suggesting that Id2 heterozygosity partially rescues B-cell development after the pro-B stage. However, it is still possible that Id2 may function in pro-B cells or at earlier stages of development with further decreases in Id2 expression. Similarly, Gfi-1−/−Id2+/− mice have more B cells in the spleen compared with Gfi-1−/−Id2+/+ mice. Taken together, our data demonstrated that impaired neutrophil development and impaired B-cell development in Gfi-1−/− mice is, at least partially, because of deregulated Id2 expression.
Discussion
In this study, we show that Id2 is a direct transcriptional target of Gfi-1. In the absence of Gfi-1, high-levels of Id2 expression were observed in hematopoietic progenitors, such as LSK, CMP, pro-B, and CD4−CD8− populations. We found that deregulated Id2 expression mediates the B-cell and myeloid defects in Gfi-1−/− mice but not the T-cell developmental defect. Thus, Gfi-1 is connected to the B-cell transcriptional network via Id2, which is a physiologic regulator of B-cell development through E2A and Pax5.
Gfi-1–deficient mice display impaired B-cell development with compromised IL-7 signaling.8 Previously, the mechanisms by which Gfi-1 regulates B-cell development, and how Gfi-1 integrates into the B-cell transcriptional network, were unclear. B-cell development is regulated by a transcriptional network consisting of, but not limited to, transcription factors PU.1, Ikaros, E2A, EBF, Pax5, and Id2.1 PU.1 and Ikaros are critical for the early stages of lymphoid lineage specification, whereas E2A, EBF, and Pax5 are essential for the commitment of common lymphoid progenitors into B cells. Recent findings also reveal a role of E2A at early stages of lymphoid lineage specification.34-36 Id2 is the physiologically relevant negative regulator of E2A, and Id2 antagonizes the transcriptional activities of PU.1 and Pax5,17,21,27,37,38 indicating that Id2 can function at multiple levels to repress B-cell development. High-levels of Id2 block B-cell development at the pro-B stage, and the absence of Id2 leads to B-cell expansion.27 In addition, feedback controls have been proposed through negative regulation of Id2 by EBF, which enforces B-cell development.39,40 Herein, we discovered that Id2 is a transcriptional target of Gfi-1, and Gfi-1 functions to repress Id2 expression in B-cell progenitors. Functional studies in which the authors used RNA interference or knockout mice revealed that Id2 mediates Gfi-1 function during B-cell development, thus placing Gfi-1 in the B-cell transcriptional network (Figure 7). We noticed in our functional genetic studies that Id2 heterozygosity in Gfi-1−/− mice leads to a 70% to 80% recovery in B-cell development, which strongly supports the hypothesis that Id2 mediates Gfi-1 function in B-cell development. In contrast, Id2 shRNA only leads to a 30% to 40% recovery in B-cell development in mice transplanted with transduced Gfi-1−/− BMC. We have observed that the repopulating ability of Gfi-1−/− cells is impaired compared with Gfi-1+/+ cells, which may contribute to the less efficient rescue in these transplanted recipients.
Id2 is a Gfi-1 target that mediates Gfi-1 function during B-cell and myeloid development. Gfi-1 is a component of the B-cell transcriptional network through Id2 regulation. Repression of Id2 expression by Gfi-1 is required for B-cell development. In Gfi-1–deficient mice, high levels of Id2 expression are observed in B-cell progenitors, which lead to B-cell inhibition. Id2 also mediates Gfi-1 function in myeloid development. In Gfi-1–deficient mice, high levels of Id2 expression are observed in myeloid progenitors, which lead to myeloid hyperplasia and differentiation arrest.
Id2 is a Gfi-1 target that mediates Gfi-1 function during B-cell and myeloid development. Gfi-1 is a component of the B-cell transcriptional network through Id2 regulation. Repression of Id2 expression by Gfi-1 is required for B-cell development. In Gfi-1–deficient mice, high levels of Id2 expression are observed in B-cell progenitors, which lead to B-cell inhibition. Id2 also mediates Gfi-1 function in myeloid development. In Gfi-1–deficient mice, high levels of Id2 expression are observed in myeloid progenitors, which lead to myeloid hyperplasia and differentiation arrest.
The essential function of Gfi-1 in myeloid development is well documented. Several Gfi-1 targets have been identified that contribute to the defects in myeloid development, including PU.1, Csf1, and HoxA9. However, their roles in B-cell development are not known. Gfi-1 physically interacts with PU.1 and antagonizes PU.1 transcriptional activity, which is required for normal myeloid development. Heterozygosity at the PU.1 locus partially rescues the myeloid defects in Gfi-1−/− mice by lowering macrophage-specific gene expression and decreasing the number of mixed lineage myeloid cells.41 Csf1 and HoxA9 are transcriptional targets of Gfi-1. Limiting Csf1 and HoxA9 activities rescues neutrophil development and progenitor proliferation defects in Gfi-1−/− mice, respectively.42,43 In this report, we demonstrated that repression of Id2 by Gfi-1 is also required for myeloid development. Increased Id2 expression was observed in Gfi-1−/− myeloid progenitors. It would be interesting to determine whether Id2 expression was increased in patients with severe congenital neutropenia with Gfi-1 mutation and whether Id2 levels were correlated to the neutrophil defects and myeloid hyperplasia in these patients. Our data show that high levels of Id2 expression inhibits neutrophil expansion, and that reducing Id2 levels partially rescue the myeloid expansion defects in Gfi-1−/− mice, indicating that deregulation of Id2 contribute to the myeloid defects in Gfi-1−/− mice. However, the mechanisms by which Id2 regulates myeloid development are not known. Potential targets of Id2 may include transcription factors of the bHLH protein and Ets protein families, both of which have roles in myeloid proliferation and development.19,44-49
Myeloid hyperplasia and neutrophil maturation arrest in Gfi-1−/− mice are closely related but may be mediated by different mechanisms. We found that reducing Id2 levels by 70% to 90% using the Id2-shRNA not only decreased the number of myeloid cells but also promoted myeloid differentiation of Gfi-1−/− BMC. In contrast, Id2 heterozygosity in Gfi-1−/− BMC rescued the myeloid hyperplasia phenotype, with minimal effects on neutrophil differentiation, suggesting that reducing Id2 levels by 50% in the Gfi-1−/− background is not sufficient to promote myeloid differentiation. It is possible that at different levels, Id2 may target a different subset of proteins in regulating myeloid proliferation versus differentiation. Because we cannot assess to what extent loss of Id2 rescues Gfi-1−/− myeloid development using double knockout Gfi-1 and Id2 mice, our current efforts are focused on developing an Id2 conditional knockout mouse model to address this question.
Although Id1, Id2, and Id3 have similar effects on lymphocyte development when overexpressed, their normal physiologic functions in lymphocyte development are distinct on the basis of studies in knockout mouse models. For example, Id2 was demonstrated to be an intrinsic regulator of B-cell development,27 whereas Id3 regulates thymopoiesis.50 No physiologic role for Id1 has been identified in lymphocyte development; instead, Id1 is required for the proper function of the HSC niche.51 Consistent with its physiologic function, repression of Id2 by Gfi-1 was found to be required for the B-cell development but not T-cell development, even though deregulated Id2 expression was observed in Gfi-1−/− thymocytes. In this regard, it is possible that the thymocyte defect in Gfi-1−/− mice is mediated by other Id genes, such as Id3. This possibility is supported by our finding that the mouse Id3 promoter contains 3 potential Gfi-1 binding sites, 1 of which is conserved across species. Furthermore, the expression of Id3 is repressed by Gfi-1 in hematopoietic cells, suggesting that Id3 is a target of Gfi-1. Currently, we are investigating the role of Id3 in mediating Gfi-1 function in thymocyte development by crossing the Gfi-1+/− mice with the Id3−/− mice.
In summary, we demonstrated that the repression of Id2 by Gfi-1 is required for B-cell and myeloid development. Further studies of Gfi-1 and Id2 interaction in HSC maintenance and specification could lead to better understanding of the hematopoietic transcriptional network.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Hanno Hock for providing Gfi-1 knockout mice and Dr Yoshifumi Yokota for providing Id2 knockout mice. We thank Steve Stull for technical assistance in bone marrow transplantation; Kathleen Noer and Roberta Matthai for technical assistance in FACS; and Drs. Peter Johnson and Sook Lee for instructions on EMSA. We also thank Drs. Linda Wolff and Kristbjorn Orri Gudmundsson for critical review of the manuscript.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. This research was supported, in part, by the Intramural Research Program of National Institutes of Health, National Cancer Institute, Center for Cancer Research.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
National Institutes of Health
Authorship
Contribution: H.L. and M.J. designed and performed research, analyzed data, and wrote the paper; K.D.K. performed research and analyzed data; and J.R.K. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan R. Keller, Bldg 560, Rm 12-03, National Cancer Institute at Frederick, Frederick, MD 21702; e-mail: kellerjo@mail.nih.gov.
References
Author notes
H.L. and M.J. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal