To the editor:
We read with interest the paper by Finberg et al1 that describes the Hjv−/−Tmprss6−/− double mutant mouse and characterizes the hematologic phenotype of Tmprss6+/− mouse. TMPRSS6 encodes a liver-expressed type II transmembrane serine-protease, that, cleaving the membrane Bone Morphogenetic Protein (BMP) coreceptor hemojuvelin (HJV),2 down-regulates the Sons of Mothers Against Decapentaplegic (SMAD)–dependent hepcidin expression. This function is essential for erythropoiesis, since loss of TMPRSS6 activity in both humans3 and mice4,5 causes iron-deficiency anemia.
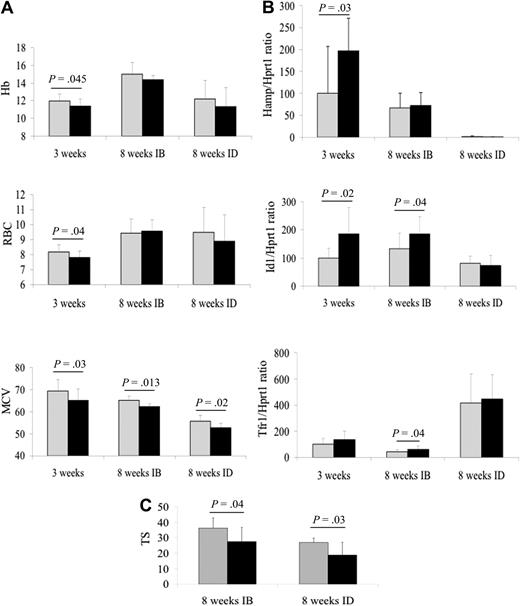
Our recent work on Tmprss6+/− mice complements the results of Finberg et al.1 Tmprss6+/− mice develop normally and are phenotypically indistinguishable from wild-type littermates. At 3 weeks of age they have lower hemoglobin (Hb), red blood cell (RBC) counts, and mean corpuscular volume (MCV; Figure 1A) and higher hepcidin and Id1 levels compared with wild-type (Figure 1B). Adult Tmprss6+/− mice have Hb and RBC similar to wild-type but reduced MCV, as reported.1 Transferrin saturation, which shows a trend toward reduction in 8-week-old animals in the previous study,1 is significantly reduced in our study. Tmprss6+/− mice of this age show mildly elevated Id1 and hepcidin production, inappropriately high for their low levels of transferrin saturation (Figure 1B-C). C-reactive protein mRNA levels are similar in Tmprss6+/− and wild-type mice (data not shown), ruling out a potential contribution of inflammation to hepcidin expression. In agreement with the observation of a more severe anemia in young versus adult patients in a large iron-refractory iron deficiency anemia (IRIDA) pedigree,6 our results show that young Tmprss6+/− mice, which have increased iron demands, are indeed more iron-deficient than adult mice.
Hematologic parameters and hepatic iron-related genes expression in Tmprss6+/− mice. (A) Hb, RBC and MCV as determined using Sysmex KX-21 automated blood cell analyzer (Sysmex). (B) Hepcidin (Hamp), Id1, and transferrin receptor-1 (Tfr1) mRNA levels as quantified by real-time polymerase chain reaction (ABI 7900, Applied Biosystems) from livers dissected and snap-frozen immediately after sacrifice. Hypoxanthine phosphoribosyltransferase 1 (Hprt1) was used to normalize mRNA levels. (C) Serum transferrin saturation (only in 8-week-old animals) calculated as a ratio of serum iron and total iron binding capacity (Randox Laboratories Ltd). Seven to 9 mice for genotype were analyzed. All the animals were maintained in the animal facility of San Raffaele Scientific Institute (Italy) in accordance with the European Union Guidelines. The study was approved by the Institutional Animal Care and Use Committee (IACUC) of our institution. IB indicates iron-balanced (carbonile iron 200 mg/kg, SAFE, Augy, France) diet; ID, iron-deficient diet (SAFE); RBC, red blood cell count (106 cells/μL); Hb, hemoglobin (g/dL); MCV, mean corpuscular volume (fL); and TS, transferrin saturation (%). Gray columns represent wild-type mice; and black columns, Tmprss6+/− mice. Error bars indicate SD. Exact P values are shown over the columns; unpaired, 1-tailed Student t tests were performed for statistical analysis.
Hematologic parameters and hepatic iron-related genes expression in Tmprss6+/− mice. (A) Hb, RBC and MCV as determined using Sysmex KX-21 automated blood cell analyzer (Sysmex). (B) Hepcidin (Hamp), Id1, and transferrin receptor-1 (Tfr1) mRNA levels as quantified by real-time polymerase chain reaction (ABI 7900, Applied Biosystems) from livers dissected and snap-frozen immediately after sacrifice. Hypoxanthine phosphoribosyltransferase 1 (Hprt1) was used to normalize mRNA levels. (C) Serum transferrin saturation (only in 8-week-old animals) calculated as a ratio of serum iron and total iron binding capacity (Randox Laboratories Ltd). Seven to 9 mice for genotype were analyzed. All the animals were maintained in the animal facility of San Raffaele Scientific Institute (Italy) in accordance with the European Union Guidelines. The study was approved by the Institutional Animal Care and Use Committee (IACUC) of our institution. IB indicates iron-balanced (carbonile iron 200 mg/kg, SAFE, Augy, France) diet; ID, iron-deficient diet (SAFE); RBC, red blood cell count (106 cells/μL); Hb, hemoglobin (g/dL); MCV, mean corpuscular volume (fL); and TS, transferrin saturation (%). Gray columns represent wild-type mice; and black columns, Tmprss6+/− mice. Error bars indicate SD. Exact P values are shown over the columns; unpaired, 1-tailed Student t tests were performed for statistical analysis.
Female mice at 18.5 gestational days that have high iron demands, have lower liver iron concentration (LIC) than wild-type, even if hematologic parameters are similar.1 To assess the effect of iron restriction, we fed 4-week-old animals of both genotypes with normal or iron-deficient diet for 4 weeks. The iron-deficient diet induced a more pronounced decrease of transferrin saturation in Tmprss6+/− than in wild-type mice (Figure 1C) and a trend toward a more severe microcytic anemia, although the difference reached statistical significance only for MCV (Figure 1A). However, as in pregnant mice,1 Hamp was strongly down-regulated in both genotypes (Figure 1B), suggesting that iron deficiency overrides the SMAD pathway activation observed in Tmprss6-haploinsufficient mice.
We have not measured LIC that was found decreased in 8-week-old Tmprss6+/− mice.1 However, transferrin receptor 1 (TfR1) mRNA, a LIC indirect measure, is significantly increased only in adult Tmprss6+/− mice fed a standard diet (Figure 1B). Likely high basal TfR1 mRNA levels in young wild-type mice that have high iron requests and in adult wild-type animals on an iron-deficient diet blunt the difference with Tmprss6+/−.
In conclusion, systemic iron homeostasis is mildly compromised in Tmprss6-haploinsufficient mice, which are prone to iron deficiency when iron demands are high for body growth and erythropoiesis expansion or when dietary iron is restricted. In humans, genomewide association studies show that common TMPRSS6 variants influence iron parameters, Hb, and erythrocyte traits.7–10 All these findings suggest that susceptibility to iron deficiency may be modulated by TMPRSS6 mutations even at the heterozygous state.
Authorship
Acknowledgments: We acknowledge Prof Carlos Lopez-Otin (Oviedo University, Spain) for the kind gift of Tmprss6−/− mouse. This work was supported in part by the Telethon Foundation Rome Grant GGP08089 and European Project E-RARE JTCcall 2009 to CC.
Contribution: A.N. and A.P. performed the experiments, analyzed data and wrote the manuscript; L.S. analyzed data; and C.C. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Clara Camaschella, Università Vita-Salute e IRCCS San Raffaele, Via Olgettina, 60, 20132 Milano, Italy; e-mail: clara.camaschella@hsr.it.
References
Author notes
A.N. and A.P. contributed equally.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal