The receptor for advanced glycation end products (RAGE) contributes to the inflammatory response in many acute and chronic diseases. In this context, RAGE has been identified as a ligand for the β2-integrin Mac-1 under static in vitro conditions. Because intercellular adhesion molecule (ICAM)-1 also binds β2-integrins, we studied RAGE−/−, Icam1−/−, and RAGE−/−Icam1−/− mice to define the relative contribution of each ligand for leukocyte adhesion in vivo. We show that trauma-induced leukocyte adhesion in cremaster muscle venules is strongly dependent on RAGE and ICAM-1 acting together in an overlapping fashion. Additional in vivo experiments in chimeric mice lacking endothelium-expressed RAGE and ICAM-1 located the adhesion defect to the endothelial compartment. Using microflow chambers coated with P-selectin, CXCL1, and soluble RAGE (sRAGE) demonstrated that sRAGE supports leukocyte adhesion under flow conditions in a Mac-1– but not LFA-1–dependent fashion. A static adhesion assay revealed that wild-type and RAGE−/− neutrophil adhesion and spreading were similar on immobilized sRAGE or fibrinogen. These observations indicate a crucial role of endothelium-expressed RAGE as Mac-1 ligand and uncover RAGE and ICAM-1 as a new set of functionally linked adhesion molecules, which closely cooperate in mediating leukocyte adhesion during the acute trauma-induced inflammatory response in vivo.
Introduction
Leukocyte recruitment into inflamed tissue follows a well-defined cascade of events, beginning with the capture of free-flowing leukocytes to the vessel wall and subsequent leukocyte rolling along and adhesion to the inflamed endothelial layer.1,2 During rolling, leukocytes get into close contact with the endothelial surface, which allows endothelial bound chemokines to interact with their specific receptors on the leukocyte surface. This triggers the activation of integrins, which leads to firm leukocyte arrest on the endothelium. In addition, integrin-dependent signaling events induce cytoskeletal rearrangements and cell polarization, modifications necessary in helping to prepare the attached leukocyte to spread and crawl in search for its way out of the vasculature into tissue.2,,,–6 Recent evidence has shown that the β2-integrin Mac-1 is crucially involved in transducing Syk-dependent signaling events necessary for sustained leukocyte adhesion.7,–9 The receptor for advanced glycation end products (RAGE) is a pattern recognition receptor that has been identified to be a major player in chronic inflammatory conditions.10,11 This has been mainly attributed to its strong effects on perpetuating nuclear factor-κB (NF-κB) activation and NF-κB-dependent signaling10,11 and its ability to induce its own expression.10,12 Besides its function as a signaling molecule, RAGE also binds to Mac-1, which has been demonstrated under in vitro conditions.13 In addition, a reduction of leukocyte extravasation into the inflamed peritoneal cavity was found in RAGE−/− mice after intraperitoneal application of thioglycollate.13 Subsequently, Orlova et al identified a novel, high-mobility group box 1-dependent mechanism of inflammatory neutrophil recruitment, which requires the presence of neutrophil-expressed RAGE to up-regulate Mac-1.14 In contrast, Zen et al demonstrated in an in vitro assay that fMLP-induced transmigration of human neutrophils through the human intestinal epithelial cell line T84 was dependent on the presence of epithelial RAGE that bound neutrophil-expressed Mac-1.15 These findings implicate an important role of both endothelial and neutrophil expressed RAGE in Mac-1–dependent neutrophil recruitment during inflammation.
Direct in vivo observations of leukocyte recruitment in the absence of RAGE have not been reported. To prove the hypothesis that RAGE is a relevant Mac-1 ligand under in vivo conditions and dissect the contribution of endothelial and neutrophil expressed RAGE in mediating leukocyte recruitment in respect to its role as Mac-1 ligand, we studied RAGE-dependent leukocyte adhesion by intravital microscopy in an acute trauma–induced model of inflammation. Here, we show, for the first time, that endothelial expressed RAGE is a relevant in vivo ligand for Mac-1 and acts in concert with intercellular adhesion molecule (ICAM)-1 in mediating β2-integrin–dependent firm leukocyte adhesion during acute trauma–induced inflammation.
Methods
Animals
RAGE−/− mice, Icam1−/− mice, and LysEGFP mice were generated as described earlier.16,–18 RAGE−/−Icam1−/− mice were generated by cross-breeding RAGE−/− and Icam1−/− mice. All mice were maintained as breeding colonies at the University of Heidelberg, Germany, and the Walter Brendel Center for Experimental Medicine, Ludwig-Maximilians-Universität, Munich, Germany. The generation of bone marrow chimeric mice was conducted as described.19 The animal experiments were approved by the Regierungspräsidium Karlsruhe, Germany (AZ 35-9185.81/G-67/03, AZ 35-9185.81/G-08/08) and the Regierung Oberbayern, Germany (AZ 55.2-1–54-2531-80-07 and -134/08).
Antibodies and cytokines
The following recombinant proteins were used: recombinant murine and human P-selectin, recombinant murine (rm)ICAM-1, recombinant human (rh)ICAM-1, rmTNF-α (all R&D Systems), rmCXCL1 and rhCXCL8 (both PeproTech). Blocking antibodies against murine Mac-1 (Tib128, rat IgG2b) murine LFA-1 (Tib217, rat IgG2a), and murine ICAM-1 (YN1, rat IgG2b) were obtained from ATCC. Blocking antibodies against human Mac-1 (ICRF44, mouse IgG1) and human LFA-1 (HI111, mouse IgG1) were obtained from BD Biosciences PharMingen. Leukotriene B4 (LTB4) was obtained from Sigma-Aldrich.
Preparation of murine sRAGE
A plasmid with the coding sequence of the mouse extracellular domain of RAGE (1030 bp) was cloned into pET-DEST42 (Invitrogen) and transformed into the Escherichia coli strain BL21 before soluble RAGE (sRAGE) expression was induced by isopropyl D-thiogalactopyranoside. The protein was purified using Protino Ni-TED 2000 columns (Macherey-Nagel) and potential endotoxin contamination was removed by affinity chromatography EndoTrap blue 5/1 (Profos AG).
Intravital microscopy
Mice were prepared for intravital microscopy as reported recently.20 Briefly, after intraperitoneal injection of ketamine (125 mg/kg, Parke-Davis) and xylazine (12.5 mg/kg, Phoenix Scientific), intravital microscopy was conducted on an upright microscope (Leica) with a saline immersion objective (SW40/0.75 numerical aperture, Carl Zeiss). Mice were intubated, and the left carotid artery was cannulated for blood sampling and systemic monoclonal antibody (mAb) administration (Mac-1–blocking mAb Tib128 and LFA-1–blocking mAb Tib217, both at 100 μg/mouse).
Cremaster muscle preparation
The surgical preparation of the cremaster muscle and its superfusion with LTB4-containing solution (20nM) were conducted as described previously.20,–22 RmTNF-α (R&D Systems) was injected intrascrotally at 500 ng per mouse 2 to 4 hours before intravital microscopy was started. Microscopic observations were recorded via CCD camera (CF8/1; Kappa) on a Panasonic S-VHS recorder. Time-lapse intravital microscopy experiments were conducted to assess intraluminal leukocyte crawling,5,6 which was defined as cell displacement of more than one cell diameter during the 10-minute observation period and quantified as the number of crawling leukocytes to all attached leukocytes. In a separate set of experiments, cremaster muscle whole mounts were obtained as described before23 and analyzed for intravascular and extravascular leukocytes using a Leica DMRB upright microscope and a 25×/0.75 NA oil immersion objective (both Leica).
Flow chamber assay
Microglass capillaries (VitroCom) were coated with rmP-selectin (2 μg/mL), rmCXCL1 (5 μg/mL), and sRAGE (1 μg/mL) as described24,25 and perfused with freshly isolated bone marrow cells from LysEGFP mice (control) or RAGE−/− mice. In LysEGFP mice, the enhanced GFP (EGFP) is knocked into the murine lysozyme M (lys) locus leading to the expression of EGFP in myelomonocytic cells.18 RAGE−/− mice were generated as EGFP reporter mice by knock-in of the EGFP gene into the murine locus for RAGE.26 Adhesion of GFP positive cells was observed using a BX51 WI microscope with a saline immersion objective 20×/0.95 NA (Olympus Hamburg) for 10 minutes at a wall shear stress of 1 dyne/cm2. In some experiments, cell suspensions were incubated with Mac-1–blocking mAb Tib128 (10 μg/106 cells) or LFA-1 blocking mAb Tib217 (10 μg/106 cells) 20 minutes before the perfusion through the flow chamber. For flow chamber experiments with isolated human neutrophils, coating of flow chambers was performed with rhP-selectin (4 μg/mL), rhCXCL8 (10 μg/mL, both R&D Systems), and sRAGE (4 μg/mL). Mac-1 and LFA-1 on human polymorphonuclear neutrophils (PMNs) were blocked with antihuman Mac-1 and LFA-1 mAbs ICRF44 and HI111, respectively.
Neutrophil adhesion and spreading in vitro
Bone marrow–derived neutrophils from RAGE−/− mice and wild-type (WT) control mice were isolated as described8 and plated onto 10 μg/mL sRAGE or 250 μg/mL fibrinogen-coated 96-well microtiter plates (Greiner). Adhesion was measured in triplicates after staining adherent cells with crystal violet (Sigma-Aldrich) using a microplate reader (Tecan) as described.8 Spreading was assessed on coverslips after fixation of neutrophils with 3.7% formaldehyde using a Zeiss 200M microscope with a Plan-Apochromat 63×/1.4 NA oil immersion objective as described.8
Immunohistochemistry
To investigate the endothelial expression of RAGE and ICAM-1 on unstimulated cremaster muscle venules, or during trauma-induced inflammation, we performed immunohistochemical analysis of whole-mount cremaster muscles as described.27 Briefly, primary antibodies against RAGE (Santa Cruz Biotechnology) or ICAM-1 (YN1; ATCC) were systemically injected, ensuring staining of RAGE and ICAM-1 on the endothelial surface. Excess antibody was washed out with normal saline solution either before killing the mouse (unstimulated) or after a 20-minute superfusion of the cremaster muscle (trauma-stimulated). Surgically prepared cremaster muscle whole mounts were transferred onto adhesive slides (Superfrost)27 and stained for endothelial ICAM-1 and RAGE expression using diaminobenzidine (Vector Laboratories). Analysis of slides was conducted on a Leica DMRB upright microscope and a 25×/0.75 NA oil immersion objective (both Leica). Photographs of the samples were taken using a color CCD camera (KAPPA).
Statistics
Sigma Stat, Version 3.5 (Systat Software), was used for statistical analysis. Comparison between groups and treatments were performed with one-way analysis of variance followed by a multiple pairwise comparison test (Dunn test) or by Wilcoxon rank-sum test, as appropriate. Statistical significance was set at P less than .05.
Results
Leukocyte adhesion in cremaster muscle venules during trauma-induced inflammation
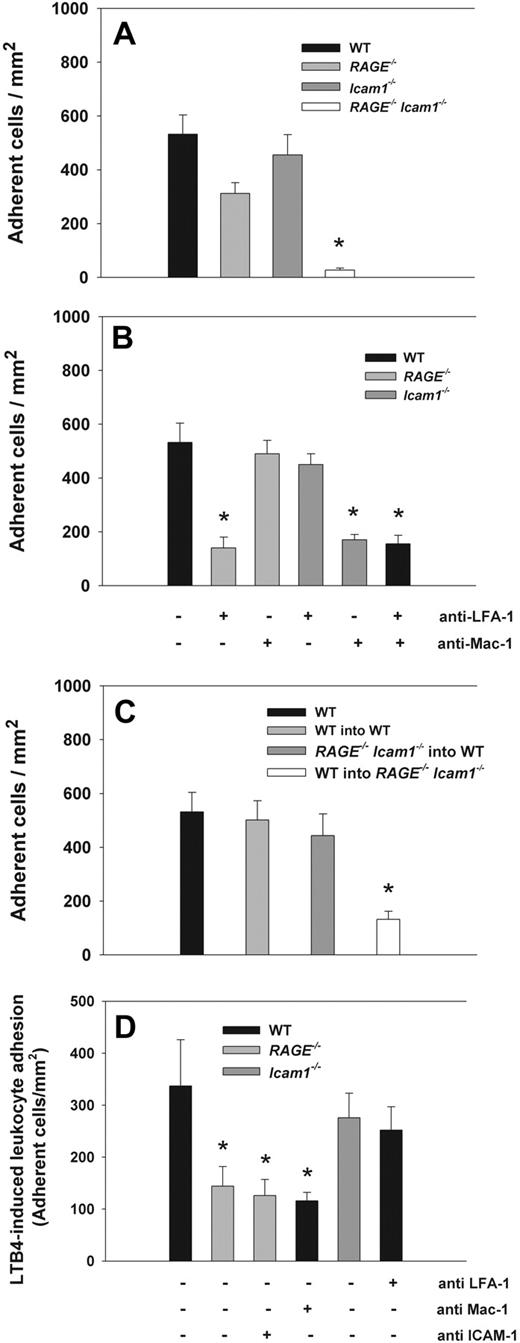
Leukocyte adhesion was observed by intravital microscopy in trauma-stimulated cremaster muscle venules of WT mice, RAGE−/− mice, Icam1−/− mice, and RAGE−/−Icam1−/− mice. Hemodynamic and microvascular parameters were similar between the groups (supplemental Table 1; available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In addition, systemic leukocyte counts were comparable between the groups. In the applied in vivo model, leukocyte adhesion in cremaster muscle venules is induced by the surgical preparation of the cremaster muscle and leukocyte adhesion observed for up to 45 minutes after exteriorization of the cremaster muscle.25 We found that the number of adherent cells in cremaster muscle venules of RAGE−/− mice (Figure 1A) was comparable with the number of adherent cells in WT mice and Icam1−/− mice (Figure 1A). Interestingly, in RAGE−/−Icam1−/− mice, leukocyte adhesion was almost absent (Figure 1A) and significantly different from all other groups (P < .05), suggesting that RAGE and ICAM-1 act in an overlapping manner in mediating firm leukocyte arrest during trauma-induced inflammation. To verify earlier reports that leukocyte adhesion in this model is strongly dependent on β2-integrins,28 we investigated leukocyte adhesion in WT mice pretreated with blocking mAbs against the β2-integrins Mac-1 and LFA-1 (Tib128 and Tib217, respectively). We found a significant reduction in the number of adherent leukocytes (Figure 1B), although this did not reach the low level of adhesion observed in RAGE−/−Icam1−/− mice. Interestingly, leukocyte adhesion was also significantly reduced in RAGE−/− mice pretreated with LFA-1–blocking mAb Tib217 (Figure 1B) and in Icam1−/− mice pretreated with Mac-1–blocking mAb Tib128 (Figure 1B), suggesting that RAGE serves as Mac-1 ligand in this setting whereas ICAM-1 interacts mostly with LFA-1. This was further supported by additional experiments showing that blocking Mac-1 in RAGE−/− mice or LFA-1 in Icam1−/− mice had no effect on leukocyte adhesion compared with WT mice (Figure 1B).
Leukocyte adhesion (mean ± SEM) in trauma-stimulated cremaster muscle venules. Leukocytes adhesion (number of adherent cells/mm2) was investigated in surgically exteriorized cremaster muscle venules of WT control mice (n = 9), RAGE−/− mice (n = 10), Icam1−/− mice (n = 8), and RAGE−/−Icam1−/− mice (n = 3; A). To investigate the contribution of Mac-1 and LFA-1, leukocyte adhesion was also assessed in RAGE−/− mice pretreated with Mac-1–blocking mAb Tib128 or LFA-1–blocking mAb Tib217 (n = 7), Icam1−/− mice pretreated with Mac-1–blocking mAb Tib128 or LFA-1–blocking mAb Tib217 (n = 7), and WT mice pretreated with Mac-1–blocking mAb Tib128 and LFA-1–blocking mAb Tib217 (n = 3). (B) To investigate the role of endothelium-expressed RAGE, leukocyte adhesion was assessed in cremaster muscle venules of bone marrow chimeric mice lacking leukocyte expressed RAGE and ICAM-1 (RAGE−/−Icam1−/− into WT, n = 3) or endothelium-expressed RAGE and ICAM-1 (WT into RAGE−/−Icam1−/−, n = 3; C). WT bars (first bar on the left) are identical in panels A-C. In addition, LTB4-induced leukocyte adhesion was observed in cremaster muscle venules of WT mice (n = 16), RAGE−/− mice (n = 10), and Icam1−/− mice (n = 5) after 3 minutes of superfusion of the cremaster muscle with LTB4 shortly after exteriorization. Values are given as increase in leukocyte adhesion over baseline adhesion values (D). *Significant differences (P < .05) in leukocyte adhesion to WT control mice.
Leukocyte adhesion (mean ± SEM) in trauma-stimulated cremaster muscle venules. Leukocytes adhesion (number of adherent cells/mm2) was investigated in surgically exteriorized cremaster muscle venules of WT control mice (n = 9), RAGE−/− mice (n = 10), Icam1−/− mice (n = 8), and RAGE−/−Icam1−/− mice (n = 3; A). To investigate the contribution of Mac-1 and LFA-1, leukocyte adhesion was also assessed in RAGE−/− mice pretreated with Mac-1–blocking mAb Tib128 or LFA-1–blocking mAb Tib217 (n = 7), Icam1−/− mice pretreated with Mac-1–blocking mAb Tib128 or LFA-1–blocking mAb Tib217 (n = 7), and WT mice pretreated with Mac-1–blocking mAb Tib128 and LFA-1–blocking mAb Tib217 (n = 3). (B) To investigate the role of endothelium-expressed RAGE, leukocyte adhesion was assessed in cremaster muscle venules of bone marrow chimeric mice lacking leukocyte expressed RAGE and ICAM-1 (RAGE−/−Icam1−/− into WT, n = 3) or endothelium-expressed RAGE and ICAM-1 (WT into RAGE−/−Icam1−/−, n = 3; C). WT bars (first bar on the left) are identical in panels A-C. In addition, LTB4-induced leukocyte adhesion was observed in cremaster muscle venules of WT mice (n = 16), RAGE−/− mice (n = 10), and Icam1−/− mice (n = 5) after 3 minutes of superfusion of the cremaster muscle with LTB4 shortly after exteriorization. Values are given as increase in leukocyte adhesion over baseline adhesion values (D). *Significant differences (P < .05) in leukocyte adhesion to WT control mice.
Next, we studied the contribution of endothelium-expressed RAGE and ICAM-1 on leukocyte adhesion in trauma-stimulated cremaster muscle venules. To this end, we generated bone marrow chimeric mice and assessed leukocyte adhesion in trauma-stimulated cremaster muscle venules by intravital microscopy. Hemodynamic and microvascular parameters were comparable between the groups (supplemental Table 1). In lethally irradiated WT mice reconstituted with WT bone marrow, we found no difference in the number of adherent leukocytes in trauma-stimulated cremaster muscle venules compared with WT mice (Figure 1C). Similarly, WT mice reconstituted with RAGE−/−Icam1−/− bone marrow demonstrated comparable numbers of adherent leukocytes to WT mice. However, in lethally irradiated RAGE−/−Icam1−/− mice reconstituted with WT bone marrow, leukocyte adhesion was strongly reduced (Figure 1C), suggesting that the loss of endothelium-expressed (but not leukocyte expressed) RAGE and ICAM-1 mediate firm leukocyte adhesion in this setting. Of note, the reduction in leukocyte adhesion in RAGE−/−Icam1−/− mice reconstituted with WT bone marrow did not completely reach the low adhesion level observed in complete RAGE−/−Icam1−/− mice but was rather comparable with the adhesion defect seen in WT mice pretreated with Mac-1 and LFA-1 blocking mAbs (Figure 1B).
To exclude the possibility that the differences in the expression of Mac-1 and LFA-1 on neutrophils accounted for the observed difference in leukocyte adhesion, Mac-1 and LFA-1 expression was investigated in WT control mice, RAGE−/− mice, Icam1−/− mice, and RAGE−/−Icam1−/− mice by flow cytometry. Supplemental Figure 1 shows that Mac-1 and LFA-1 expression was similar on neutrophils from WT mice, RAGE−/− mice, Icam1−/− mice, and RAGE−/−Icam1−/− mice, excluding a different expression of these β2-integrins to be responsible for the reduction in leukocyte adhesion observed in RAGE−/−Icam1−/− mice.
Leukocyte adhesion in LTB4-stimulated cremaster muscle venules
To confirm the role of RAGE as functional Mac-1 ligand in another in vivo model, we investigated leukocyte adhesion in LTB4-stimulated cremaster muscle venules. Previously, LTB4 has been described to stimulate the up-regulation of Mac-1 and induce Mac-1-dependent leukocyte adhesion.22,29,30 After LTB4 superfusion of the cremaster muscle over 3 minutes shortly after exteriorization, we observed a marked increase in leukocyte adhesion over baseline in WT mice (n = 6 mice, Figure 1D; supplemental Table 1). In RAGE−/− mice (n = 6 mice), the increase in leukocyte adhesion was significantly reduced compared with WT (P < .05) and similar to the increase observed in RAGE−/− mice pretreated with ICAM-1 blocking mAb YN1 (n = 4 mice) or WT mice pretreated with the Mac-1–blocking mAb Tib128 (n = 3 mice, Figure 1D). In contrast, LTB4 superfusion in Icam1−/− mice (n = 5 mice) or WT mice pretreated with LFA-1 blocking mAb Tib217 (n = 7 mice, Figure 1D) led to a comparable increase in the number of adherent leukocytes as in WT mice. These findings suggest that LTB4-induced leukocyte adhesion is mostly dependent on Mac-1 and RAGE but does not require ICAM-1 or LFA-1, which confirms an important role of RAGE in mediating Mac-1-dependent leukocyte adhesion during acute inflammation in vivo.
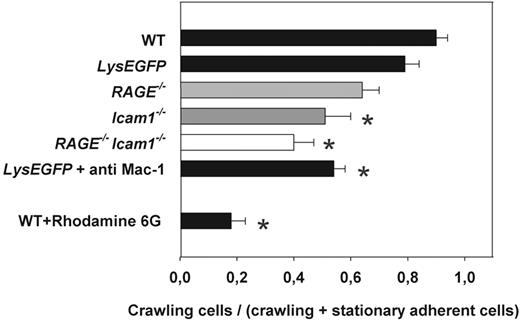
Leukocyte crawling in trauma-induced inflammation in vivo
Intraluminal leukocyte crawling has recently been described as an additional Mac-1-dependent postarrest step during the recruitment of leukocytes into inflamed tissue.5,6 To investigate leukocyte crawling in vivo, we performed time-lapse intravital microscopy experiments in exteriorized cremaster muscle venules of WT control mice. Leukocyte crawling was quantified as the ratio between the number of crawling leukocytes to firmly adherent and crawling leukocytes. Similar to earlier reports,5,6 we found that almost all leukocytes from WT mice crawled along the inflamed endothelium after a short time of firm arrest (Figure 2). Because RAGE−/− mice have been generated as GFP knock-in mice and to exclude any contribution of GFP on leukocyte crawling, we performed additional control experiments in LysEGFP mice, where all myeloid cells are GFP-labeled.18 Similar to WT mice, most leukocytes in LysEGFP mice started crawling on the inflamed endothelium shortly after firm arrest (Figure 2; supplemental Video 1), demonstrating that the presence of GFP did not significantly interfere with leukocyte adhesion and crawling. In RAGE−/− mice, leukocyte crawling was not significantly reduced compared with WT mice or LysEGFP mice (Figure 2; supplemental Video 2), although a trend toward reduced crawling was noted. In Icam1−/− mice, leukocyte crawling was significantly reduced compared with WT mice or LysEGFP mice (P < .05, Figure 2), confirming a role of ICAM-1 in mediating leukocyte crawling during inflammation in vivo.5,6 In RAGE−/−Icam-1−/− mice, the low number of attached leukocytes hampered the assessment of leukocyte crawling. However, analysis of the few adherent leukocytes in RAGE−/−Icam1−/− mice revealed that crawling was observed only for a minority of leukocytes (P < .05 compared with WT or LysEGFP mice, Figure 2), suggesting that both ICAM-1 and (to a minor degree) RAGE contribute to leukocyte crawling during trauma-induced inflammation in vivo (supplemental Video 3). To verify that crawling is indeed Mac-1 dependent, we also investigated leukocyte crawling in LysEGFP mice pretreated with Mac-1–blocking antibody Tib128. This led to a significant reduction in crawling (P < .05 vs WT or LysEGFP mice, Figure 2). Of note, in vivo staining of leukocytes in WT mice using rhodamine 6G significantly blocked leukocyte crawling in this model (P < .05 vs WT or LysEGFP, Figure 2). This may exclude this dye from further use to stain blood cells in leukocyte recruitment studies in vivo.
Intraluminal crawling of leukocytes in trauma-stimulated cremaster muscle venules. Intraluminal crawling was observed in WT mice (n = 3), LysEGFP mice (n = 6), RAGE−/− mice (n = 3), Icam1−/− mice (n = 3), and RAGE−/−Icam1−/− mice (n = 3). Intraluminal crawling was quantified as the number of crawling leukocytes to all leukocytes attached (firmly adherent and crawling) to the endothelial wall. Experiments were recorded by time-lapse intravital microscopy at a frame rate of 20 frames/minute over 10 minutes (supplemental Videos 1-3). *Significant differences versus WT mice (P < .05). Data are mean ± SEM.
Intraluminal crawling of leukocytes in trauma-stimulated cremaster muscle venules. Intraluminal crawling was observed in WT mice (n = 3), LysEGFP mice (n = 6), RAGE−/− mice (n = 3), Icam1−/− mice (n = 3), and RAGE−/−Icam1−/− mice (n = 3). Intraluminal crawling was quantified as the number of crawling leukocytes to all leukocytes attached (firmly adherent and crawling) to the endothelial wall. Experiments were recorded by time-lapse intravital microscopy at a frame rate of 20 frames/minute over 10 minutes (supplemental Videos 1-3). *Significant differences versus WT mice (P < .05). Data are mean ± SEM.
Leukocyte extravasation in surgically prepared cremaster muscle whole mounts
To further investigate the role of RAGE and ICAM-1 on leukocyte recruitment in surgically exteriorized and 20-minute superfused cremaster muscles, Giemsa-staining of whole-mount cremaster muscles was performed and the number of intravascular and perivascular leukocytes assessed as described.23 In RAGE−/− mice, the number of adherent leukocytes (Figure 3A) was comparable with WT mice and Icam1−/− mice. In contrast, in RAGE−/−Icam1−/− mice, the number of intravascular leukocytes was dramatically reduced (P < .05 vs WT) and only slightly higher than the number of intravascular leukocytes in unstimulated cremaster whole-mount preparations of WT mice obtained from postmortem exteriorized preparations (Figure 3A). These results confirm our in vivo findings (Figure 1) and suggest that RAGE and ICAM-1 function in an overlapping fashion in mediating firm leukocyte adhesion in trauma-induced inflammation.
Leukocyte adhesion and adhesion molecule expression in cremaster muscle whole mounts. Intravascular and perivascular numbers of adherent leukocytes (mean ± SEM) were investigated in postcapillary venules of cremaster muscle whole mounts from WT mice (40 vessels in 4 mice), RAGE−/− mice (27 vessels in 3 mice), Icam1−/− mice (35 vessels in 3 mice), and RAGE−/−Icam1−/− mice (40 vessels in 3 mice; A-B). Cremaster muscles were obtained after exteriorization and 20-minute superfusion (trauma-induced inflammation, upper 4 bars of A-B) or directly postmortem (WT unstimulated, last bar at the bottom of A-B). *Significant differences (P < .05) to WT control mice (first bar on top in A-B). In addition, immunostaining was conducted to assess endothelial expression of ICAM-1 (C: n = 8; D: n = 5; E: n = 4; F: n = 4) and RAGE (G: n = 4; H: n = 8; I: n = 3; K: n = 3; L: n = 3; M: n = 3) in postcapillary venules of cremaster muscles obtained directly postmortem (Unstimulated, left side) or after exteriorization and 20-minute superfusion (Trauma, right side). The respective images are representative for the various groups. Application of primary antibody was performed intravenously before harvesting the cremaster muscle to stain RAGE and ICAM-1 on the endothelial surface. Biotinylated secondary antibody, peroxidase-conjugated streptavidin, and diaminobenzidine were used to detect endothelial expression of ICAM-1 and RAGE as brown signal. Counterstaining was performed by Mayer hemalaun. Reference bar for panels C to M is shown in panel M and represents 25 μm.
Leukocyte adhesion and adhesion molecule expression in cremaster muscle whole mounts. Intravascular and perivascular numbers of adherent leukocytes (mean ± SEM) were investigated in postcapillary venules of cremaster muscle whole mounts from WT mice (40 vessels in 4 mice), RAGE−/− mice (27 vessels in 3 mice), Icam1−/− mice (35 vessels in 3 mice), and RAGE−/−Icam1−/− mice (40 vessels in 3 mice; A-B). Cremaster muscles were obtained after exteriorization and 20-minute superfusion (trauma-induced inflammation, upper 4 bars of A-B) or directly postmortem (WT unstimulated, last bar at the bottom of A-B). *Significant differences (P < .05) to WT control mice (first bar on top in A-B). In addition, immunostaining was conducted to assess endothelial expression of ICAM-1 (C: n = 8; D: n = 5; E: n = 4; F: n = 4) and RAGE (G: n = 4; H: n = 8; I: n = 3; K: n = 3; L: n = 3; M: n = 3) in postcapillary venules of cremaster muscles obtained directly postmortem (Unstimulated, left side) or after exteriorization and 20-minute superfusion (Trauma, right side). The respective images are representative for the various groups. Application of primary antibody was performed intravenously before harvesting the cremaster muscle to stain RAGE and ICAM-1 on the endothelial surface. Biotinylated secondary antibody, peroxidase-conjugated streptavidin, and diaminobenzidine were used to detect endothelial expression of ICAM-1 and RAGE as brown signal. Counterstaining was performed by Mayer hemalaun. Reference bar for panels C to M is shown in panel M and represents 25 μm.
When analyzing the number of perivascular leukocytes, we found a similar number of extravasated leukocytes in whole mounts of exteriorized and superfused cremaster muscles of WT and Icam1−/− mice (Figure 3B). In RAGE−/− mice, the number of extravasated leukocytes was significantly reduced compared with WT mice (P < .05, Figure 3B). In RAGE−/−Icam1−/− mice, the number of perivascular leukocytes was further reduced and significantly lower than in WT mice, RAGE−/− mice, and Icam1−/− mice, but comparable with the number of perivascular leukocytes seen in unstimulated WT cremaster muscle whole-mount preparations, which were obtained after the mouse had been killed (Figure 3B). These findings do not only emphasize the importance of RAGE and ICAM-1 for intravascular adhesion but also indicate that RAGE plays a significant role for efficient leukocyte transmigration.
Adhesion molecule expression in unstimulated and surgically prepared cremaster muscle venules
Immunohistochemistry was used to assess the expression of ICAM-1 and RAGE on the endothelial surface of postcapillary venules in cremaster muscles obtained from WT mice, RAGE−/− mice, and Icam1−/− mice either directly postmortem (unstimulated) or after exteriorization and 20-minute superfusion of the cremaster muscle (trauma-induced inflammation). As illustrated in Figure 3C-F, we found endothelial surface expression of ICAM-1 in unstimulated and trauma-stimulated cremaster muscle venules of WT mice and RAGE−/− mice. In contrast, we found no RAGE expression on the endothelium of unstimulated cremaster muscle venules of WT mice (Figure 3G) and Icam1−/− mice (Figure 3I). However, in exteriorized and superfused cremaster muscle venules of WT mice and Icam1−/− mice, RAGE expression could be clearly detected (Figure 3H and Figure 3K, respectively), suggesting that RAGE can be rapidly transferred from intracellular storage pools to the cell surface of endothelial cells. To demonstrate that anti-RAGE antibody is specific for RAGE, we also performed immunostaining of unstimulated and stimulated cremaster muscle venules of RAGE−/− mice, which did not show any obvious staining (Figure 3L-M).
Leukocyte adhesion in TNF-α–stimulated cremaster muscle venules
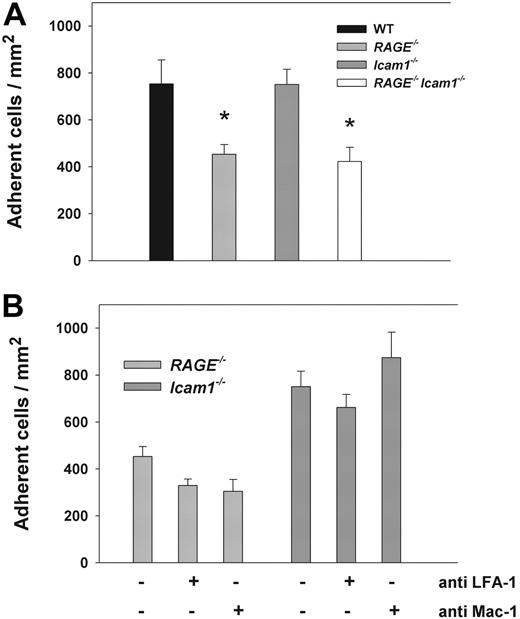
To test whether leukocyte adhesion in RAGE−/−Icam1−/− mice is also affected after stimulation with a proinflammatory cytokine, leukocyte adhesion was assessed 2 to 4 hours after TNF-α injection in cremaster muscle venules of WT mice, RAGE−/− mice, Icam1−/− mice, and RAGE−/−Icam1−/− mice. Hemodynamic and microvascular parameters were similar between the groups (supplemental Table 2). In line with earlier reports, WT mice exhibited a similar number of adherent leukocytes compared with Icam1−/− mice (Figure 4A).31 In RAGE−/− mice, leukocyte adhesion was significantly reduced (Figure 4) compared with WT mice but comparable with the reduction seen in RAGE−/−Icam1−/− mice (Figure 4A). This suggests that the close cooperation of RAGE and ICAM-1 in mediating leukocyte adhesion does not apply after stimulation with TNF-α. In addition, it implies that the RAGE-dependent reduction in leukocyte adhesion after TNF-α stimulation is rather related to the loss of RAGE-dependent signaling and its marked proinflammatory effects.10,32
Leukocyte adhesion (mean ± SEM) in TNF-α–stimulated cremaster muscle venules. Leukocytes adhesion (number of adherent cells/mm2) was observed in (2-4 hours) TNF-α–stimulated cremaster muscle venules of WT control mice (n = 5), RAGE−/− mice (n = 6), Icam1−/− mice (n = 4), and RAGE−/−Icam1−/− mice (n = 3; A). *Significant differences (P < .05) in leukocyte adhesion to WT control mice. In addition, leukocyte adhesion in RAGE−/− mice and Icam1−/− mice (bars identical to panel A) was compared with adhesion in RAGE−/− mice (n = 6) and Icam1−/− mice (n = 6) pretreated with blocking mAbs against either LFA-1 or Mac-1 (B).
Leukocyte adhesion (mean ± SEM) in TNF-α–stimulated cremaster muscle venules. Leukocytes adhesion (number of adherent cells/mm2) was observed in (2-4 hours) TNF-α–stimulated cremaster muscle venules of WT control mice (n = 5), RAGE−/− mice (n = 6), Icam1−/− mice (n = 4), and RAGE−/−Icam1−/− mice (n = 3; A). *Significant differences (P < .05) in leukocyte adhesion to WT control mice. In addition, leukocyte adhesion in RAGE−/− mice and Icam1−/− mice (bars identical to panel A) was compared with adhesion in RAGE−/− mice (n = 6) and Icam1−/− mice (n = 6) pretreated with blocking mAbs against either LFA-1 or Mac-1 (B).
To further address the role of RAGE as Mac-1 ligand in TNF-α-stimulated cremaster muscle tissue, we investigated leukocyte adhesion in TNF-α-treated cremaster muscle venules of RAGE−/− and Icam1−/− mice using blocking mAbs against Mac-1 and LFA-1. In RAGE−/− mice, pretreatment with LFA-1 blocking mAb Tib217 or Mac-1–blocking mAb Tib128 did not lead to a significant change in the number of adherent cells compared with TNF-α-stimulated RAGE−/− mice without antibody treatment (Figure 4B), indicating that alternative, yet undefined, adhesion mechanisms may compensate for the loss of LFA-1 or Mac-1 in this setting. In addition, these results further support a role of RAGE-dependent signaling in mediating leukocyte adhesion after stimulation with TNF-α. In Icam1−/− mice, pretreatment with neither LFA-1 blocking mAb Tib217 nor Mac-1–blocking mAb Tib128 led to a significant change in leukocyte adhesion compared with Icam1−/− mice without antibody treatment (Figure 4B), suggesting that ICAM-1 is dispensable for leukocyte adhesion in TNF-α–stimulated cremaster muscle venules.
Leukocyte adhesion in the microflow chamber assay
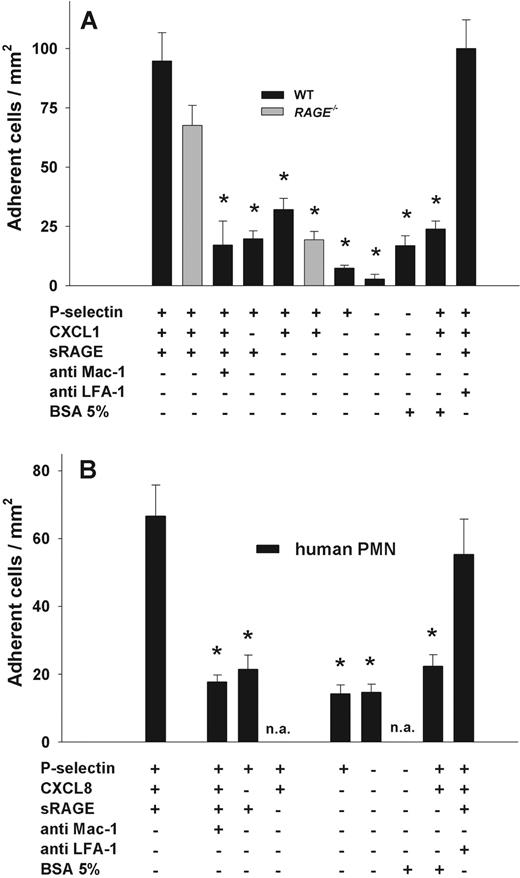
To assess the importance of RAGE/Mac-1 interactions in mediating firm neutrophil adhesion under dynamic in vitro conditions, we used a recently described microflow chamber system.24 Flow chambers were coated with recombinant murine (rm) P-selectin, rmCXCL1, and recombinant sRAGE. To minimize neutrophil activation during cell isolation procedures, we performed experiments with freshly prepared bone marrow cells from LysEGFP mice, where all myeloid cells are GFP-labeled.18 Using fluorescence microscopy and a cell suspension containing 0.25 × 106 GFP+ bone marrow cells per milliliter, we observed adhesion of GFP+ cells over a time period of 10 minutes at constant wall shear stress (1 dyne/cm2). We found that sRAGE was able to mediate firm leukocyte adhesion when coimmobilized with P-selectin and CXCL1 (Figure 5). After a 10-minute perfusion, the number of adherent GFP+ cells was 95 plus or minus 12 cells/mm2 in flow chambers coated with sRAGE, P-selectin, and CXCL1 (Figure 5). Next, we performed flow chamber experiments using RAGE−/− bone marrow cells. RAGE−/− mice were generated as EGFP reporter mice by knock-in of the EGFP gene into the murine locus for RAGE.26 Flow cytometric analysis of bone marrow cells from RAGE−/− mice and LysEGFP mice revealed that 70% and 90% of the Gr-1+ bone marrow cell population was GFP+ in RAGE−/− and LysEGFP, respectively (data not shown). We found that the number of adherent RAGE−/− GFP+ cells was comparable with WT cells, implying that neutrophil-expressed RAGE does not significantly contribute to neutrophil adhesion in the flow chamber. To demonstrate that sRAGE-mediated firm leukocyte adhesion was dependent on Mac-1, we pretreated LysEGFP bone marrow cells with Mac-1–blocking mAb Tib128, which led to a significant reduction in firm leukocyte adhesion in flow chambers surface-coated with P-selectin, CXCL1, and sRAGE (P < .05, Figure 5A) compared with adhesion observed without mAb Tib128 or isotype control mAb (data not shown). To rule out nonspecific leukocyte adhesion in the flow chamber, we also performed experiments using flow chambers coated with P-selectin and sRAGE, P-selectin, and CXCL1 with or without 5% bovine serum albumin, P-selectin alone, 5% bovine serum albumin alone, and without any immobilized protein. Leukocyte adhesion in these flow chambers was significantly reduced compared with adhesion of WT leukocytes in flow chambers coated with P-selectin, CXCL1, and sRAGE (Figure 5A, P < .05). Next, we pretreated leukocytes with the LFA-1–blocking mAb Tib217. This did not lead to a significant change in leukocyte adhesion (Figure 5A) in flow chambers coated with sRAGE, CXCL1, and P-selectin, excluding an important role of LFA-1 in mediating firm arrest under these conditions.
Leukocyte adhesion in the flow chamber. Leukocyte adhesion (mean ± SEM of cells/mm2) under shear (1 dyne/cm2) was observed in the microflow chamber assay using myelomonocytic cells isolated from LysEGFP mice (A) or human PMNs (B) at a final concentration of 0.25 × 106 cells. rmP-selectin (2 μg/mL), rmCXCL1 (KC, 5 μg/mL), and sRAGE (1 μg/mL) were immobilized in flow chambers for experiments with murine neutrophils (A). rhP-selectin (4 μg/mL), rhCXCL8 (IL-8, 10 μg/mL), and sRAGE (4 μg/mL) were used in experiments with human neutrophils (B). Blockade of Mac-1 was achieved by preincubating the cell suspensions for 20 minutes with Mac-1–blocking mAbs Tib128 (antimouse) or ICRF44 (antihuman; 10 μg/106 cells), respectively. LFA-1 was blocked by mAb Tib217 (antimouse) or HI111 (antihuman; 10 μg/106 cells), respectively. Data are mean ± SEM from at least 4 flow chamber experiments per group. *Significant differences in adhesion to control PMNs perfused flow chambers coated with P-selectin, rmCXCL1 (A) or rhCXCL8 (B), and sRAGE (P < .05). n.a. indicates not assessed.
Leukocyte adhesion in the flow chamber. Leukocyte adhesion (mean ± SEM of cells/mm2) under shear (1 dyne/cm2) was observed in the microflow chamber assay using myelomonocytic cells isolated from LysEGFP mice (A) or human PMNs (B) at a final concentration of 0.25 × 106 cells. rmP-selectin (2 μg/mL), rmCXCL1 (KC, 5 μg/mL), and sRAGE (1 μg/mL) were immobilized in flow chambers for experiments with murine neutrophils (A). rhP-selectin (4 μg/mL), rhCXCL8 (IL-8, 10 μg/mL), and sRAGE (4 μg/mL) were used in experiments with human neutrophils (B). Blockade of Mac-1 was achieved by preincubating the cell suspensions for 20 minutes with Mac-1–blocking mAbs Tib128 (antimouse) or ICRF44 (antihuman; 10 μg/106 cells), respectively. LFA-1 was blocked by mAb Tib217 (antimouse) or HI111 (antihuman; 10 μg/106 cells), respectively. Data are mean ± SEM from at least 4 flow chamber experiments per group. *Significant differences in adhesion to control PMNs perfused flow chambers coated with P-selectin, rmCXCL1 (A) or rhCXCL8 (B), and sRAGE (P < .05). n.a. indicates not assessed.
To study the ability of human neutrophils to bind to sRAGE under flow conditions, we performed flow chamber experiments using isolated human neutrophils. In flow chambers coated with rhP-selectin, rhCXCL8 (rhIL8), and sRAGE, we found 67 plus or minus 9 adherent human PMNs/mm2 (Figure 5B). Adhesion was mostly Mac-1-dependent as pretreatment of PMNs with Mac-1–blocking mAb ICRF44 significantly reduced adhesion (P < .05, Figure 5B) to levels found in negative control chambers (Figure 5B). These results suggest that, similar to mouse neutrophils, human neutrophils bind to RAGE in a Mac-1-dependent fashion. In contrast, LFA-1 did not contribute to adhesion as pretreatment of human PMNs with LFA-1–blocking mAb HI111 had no effect on the number of adherent cells (Figure 5B).
Adhesion and spreading of isolated PMNs in vitro
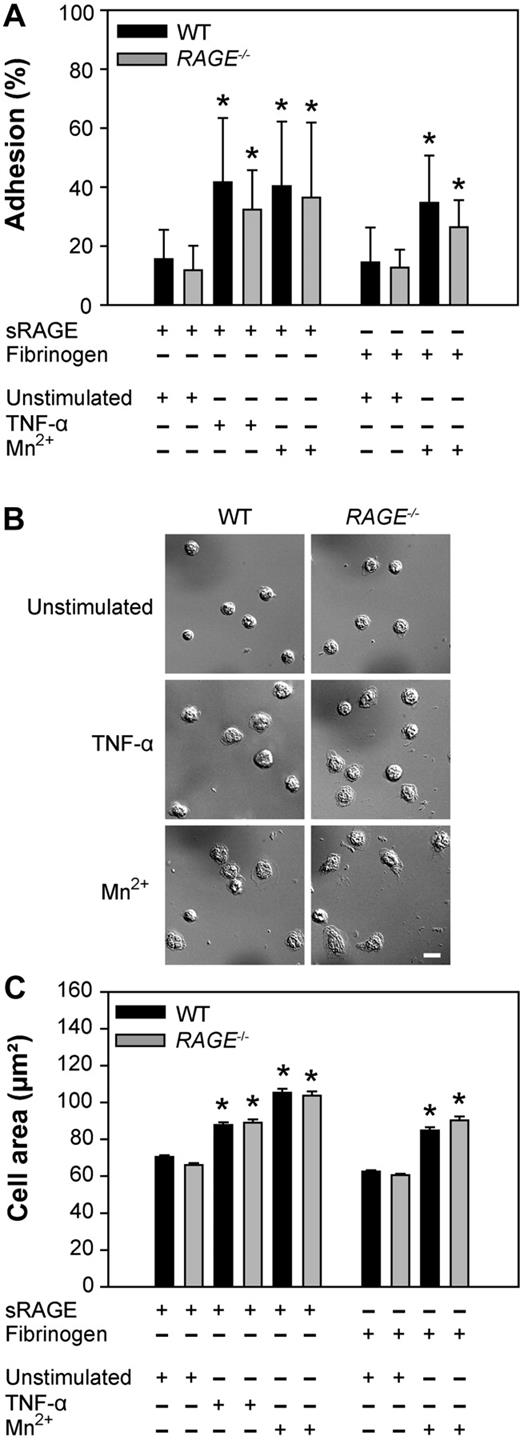
To demonstrate that adhesion and spreading of neutrophils can be directly induced via the immobilized sRAGE and is independent of neutrophil-expressed RAGE, we immobilized sRAGE on plastic dishes and added WT or RAGE−/− neutrophils. When investigating adhesion of unstimulated neutrophils to sRAGE-coated dishes, we found a similar number of WT and RAGE−/− neutrophils bound to the dish (Figure 6A). After 30 minutes of stimulation of neutrophils with TNF-α, the number of adherent neutrophils from WT mice and RAGE−/− mice increased in a similar fashion and was significantly higher than for unstimulated cells (Figure 6A, P < .05 for both groups). Similarly, stimulation of WT neutrophils and RAGE−/− neutrophils with Mn2+ led to a significant increase in neutrophil adhesion in both groups (P < .05 vs unstimulated cells; Figure 6A). To demonstrate that adhesion of stimulated neutrophils also occurs with other immobilized Mac-1 ligands, we performed experiments using fibrinogen-coated dishes and Mn2+-stimulated WT and RAGE−/− neutrophils. Adhesion of Mn2+-stimulated neutrophils to fibrinogen-coated dishes was similar between the 2 groups but significantly different from unstimulated cells (P < .05 vs unstimulated cells; Figure 6A).
Leukocyte adhesion and spreading on sRAGE in vitro. Adhesion and spreading of isolated WT (black bar) and RAGE−/− (gray bar) neutrophils on immobilized sRAGE or fibrinogen on stimulation with TNF-α (100 ng/mL), Mn2+ (1mM), or mock treatment (unstimulated), respectively, at 37°C for 30 minutes. Adherent WT and RAGE−/− neutrophils in percentage of total cells added (n = 7, mean ± SD,; A), microscopic images of spreading on sRAGE (B), and cell area in square micrometers of adherent WT and RAGE−/− neutrophils on immobilized sRAGE and fibrinogen without or with stimulation (n = 500, mean ± SEM; C). *Significant differences (P < .05) in neutrophil adhesion and spreading versus unstimulated WT. Differences between WT and RAGE−/− cells were not significant. Bar in panel B represents 10 μm.
Leukocyte adhesion and spreading on sRAGE in vitro. Adhesion and spreading of isolated WT (black bar) and RAGE−/− (gray bar) neutrophils on immobilized sRAGE or fibrinogen on stimulation with TNF-α (100 ng/mL), Mn2+ (1mM), or mock treatment (unstimulated), respectively, at 37°C for 30 minutes. Adherent WT and RAGE−/− neutrophils in percentage of total cells added (n = 7, mean ± SD,; A), microscopic images of spreading on sRAGE (B), and cell area in square micrometers of adherent WT and RAGE−/− neutrophils on immobilized sRAGE and fibrinogen without or with stimulation (n = 500, mean ± SEM; C). *Significant differences (P < .05) in neutrophil adhesion and spreading versus unstimulated WT. Differences between WT and RAGE−/− cells were not significant. Bar in panel B represents 10 μm.
Next, we analyzed Mac-1-dependent spreading of RAGE−/− and WT neutrophils in vitro (Figure 6B-C). Both WT and RAGE−/− neutrophils underwent substantial spreading on immobilized sRAGE after addition of TNF-α, leading to a significant increase in cell surface area (Figure 6C). This was also true for the induction of spreading by Mn2+ on immobilized sRAGE and fibrinogen (Figure 6C). Thus, RAGE expressed on neutrophils seems not to be required for mediating firm adhesion and spreading under static conditions. Instead, immobilized sRAGE can serve as ligand for Mac-1 and is able to induce β2-integrin-dependent outside-in-signaling in vitro.
Discussion
This study shows that leukocyte adhesion in acutely inflamed venules almost completely disappears in the absence of endothelial expressed RAGE and ICAM-1. In contrast, deficiency in either RAGE or ICAM-1 did not lead to a significant reduction in leukocyte adhesion, thus providing strong evidence that both ICAM-1 and RAGE cooperate in an overlapping fashion in mediating firm leukocyte adhesion during acute trauma-induced inflammation in vivo. Previous reports postulated overlapping functions of leukocyte-expressed β2-integrins LFA-1 and Mac-1 in mediating leukocyte adhesion in vivo,28,33,–35 although a distinct and sequential role of LFA-1 and Mac-1 has been described as well.36 Our experiments reveal both a redundant and distinct contribution of Mac-1 and LFA-1 in mediating leukocyte adhesion during acute trauma-induced inflammation. Most importantly, we provide strong evidence for RAGE as a relevant in vivo adhesion ligand for Mac-1, whereas ICAM-1 mostly operates as adhesion ligand for LFA-1,35 although binding to Mac-1 has been reported.5,6
RAGE belongs to the immunoglobulin superfamily and was found to bind to the I-domain of Mac-1 in a static in vitro assay (50% inhibitory concentration, 150-200nM).13 We have expanded those in vitro findings and investigated the binding of immobilized RAGE to murine and human neutrophils under flow showing that RAGE-mediated adhesion of human and murine neutrophils was dependent on Mac-1, but independent of LFA-1. This indicates that RAGE might also have a role as Mac-1 ligand during inflammation in humans. Two previous studies have provided evidence for a role of RAGE in mediating Mac-1-dependent leukocyte recruitment in mice and humans.14,15 Whereas Orlova et al14 identified a role of neutrophil-expressed RAGE in mediating leukocyte recruitment, Zen et al15 showed that epithelial-expressed RAGE supports leukocyte recruitment. However, both studies differ significantly from our experimental approach in as much as these studies investigated leukocyte recruitment at later time points during inflammation where additional mechanisms triggered by RAGE-mediated signaling events, including activation of NF-κB, may play an important role.10,32 Indeed, we only observed the cooperative effect of RAGE and ICAM-1 in mediating firm leukocyte arrest during the first 30 to 40 minutes of trauma-induced inflammation, whereas at later time points and stimulation with the proinflammatory cytokine TNF-α cooperation between RAGE and ICAM-1 was not observed anymore. Instead, we found a RAGE-dependent decrease in leukocyte adhesion, which was independent of the presence of ICAM-1. This implies that the reduction in leukocyte adhesion in TNF-α-stimulated RAGE−/− mice is the result of absent RAGE-dependent signaling with a concomitant attenuation of the inflammatory response. Furthermore, a recent report demonstrated that soluble RAGE triggered the production of interleukin-6, TNF-α, and macrophage inflammatory protein 2 via interacting with Mac-1.37 This may additionally contribute to the reduction in leukocyte adhesion seen after TNF-α stimulation and in the absence of RAGE.
Recently, several groups reported on leukocyte crawling as a distinct postarrest step during leukocyte recruitment in vivo, affecting almost all adherent leukocytes on inflamed venular endothelium.5,6,38 Leukocyte crawling consists of a slow movement of attached leukocytes along the luminal vessel surface at a velocity of approximately 3 to 10 μm/minute and has been proposed as a mechanisms to find an optimal location for extravasation into tissue.5,6 The crawling process is mostly dependent on Mac-1 but also influenced by ICAM-1.6 This is noteworthy considering the fact that, for firm leukocyte arrest, ICAM-1 is preferentially interacting with LFA-1, but not Mac-1. However, the involvement of ICAM-1 in leukocyte crawling in vivo may be an indication that interactions between ICAM-1 and Mac-1 occur and are relevant for leukocyte crawling. Concerning RAGE, we did not find an important role of RAGE in leukocyte crawling, although a trend toward reduced crawling was noted, particularly when both RAGE and ICAM-1 were absent. A probable explanation for the predominant role of ICAM-1 over RAGE in leukocyte crawling may be that ICAM-1 and RAGE use different binding sites of Mac-1, which could lead to different effector functions.13 In addition, ICAM-1 binding to Mac-1 is also dependent on phosphorylation of the αM-chain,39 a property that has not been described for other Mac-1 ligands so far. Additional studies are warranted to dissect the exact molecular mechanisms leading to Mac-1–dependent leukocyte adhesion and crawling in respect to its ligands.
Finally, our whole-mount histology data indicate a role of RAGE in leukocyte transmigration during acute trauma-induced inflammation in vivo. Using human brain endothelial cells and the myeloid cell lines HL-60 and THP-1, Giri et al investigated β-amyloid-induced transmigration of HL-60 and THP-1 cells across the endothelial monolayer in a static in vitro transmigration assay.40,41 They found that β-amyloid, a known RAGE ligand, caused a significant induction of HL-60 and THP-1 cell transmigration, suggesting that endothelial RAGE triggers leukocyte transmigration across inflamed endothelium.40,41
Taken together, our results identify endothelial RAGE as a functionally relevant Mac-1 ligand under in vivo conditions and demonstrate, for the first time, that endothelial ICAM-1 and RAGE act together and in an overlapping manner to mediate leukocyte adhesion during acute trauma-induced inflammation in vivo. Subsequent studies in clinically relevant acute models of inflammation, including ischemia/reperfusion injury, may be of great interest and could help to uncover a role of RAGE and ICAM-1 under these conditions, which might stimulate the development of new therapeutic approaches.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Melitta Weissinger, Axel Ehrhardt, Inna Babushkina, Marie Stahl, and Susanne Bierschenk for their excellent technical assistance, and Britta Engelhardt, Bern, Switzerland, and Thomas Graf, Barcelona, Spain, for providing Icam1−/− mice and LysEGFP mice, respectively.
This work was supported in part by LMU Innovativ BioImaging and Deutsche Forschungsgemeinschaft (grant SFB405, P.P.N.; grant Wa1048/2-3, B.W.).
Authorship
Contribution: D.F. and M.P. designed research, carried out research, analyzed data, and edited the manuscript; A.K., I.H., I.S., V.Z., K.B., B.L.-S., M.M., and J.S. performed research and analyzed data; P.P.N., I.K.L., A.B., E.R., J.P., and C.K. contributed analytical tools; A.B. edited the manuscript; B.W. designed research and edited the manuscript; and M.S. designed research, carried out research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus Sperandio, Walter Brendel Center of Experimental Medicine, Ludwig-Maximilians-University, Marchioninistr 15, 81377 Munich, Germany; e-mail: markus.sperandio@med.uni-muenchen.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal