The proposed immunomodulatory effects of dasatinib in expanding the cytotoxic T lymphocytes against BCR-ABL–positive cells may supplement the kinase inhibitory effects of the drug against the leukemia clones.
The proposed immunomodulatory effects of dasatinib in expanding the cytotoxic T lymphocytes against BCR-ABL–positive cells may supplement the kinase inhibitory effects of the drug against the leukemia clones.
Among the 20 patients treated with dasatinib, half had lymphocyte expansions with a large granular lymphocyte (LGL) morphology; none of the patients treated with imatinib, with lymphocyte clones at diagnosis, had increased absolute lymphocyte counts during therapy. By analyzing the T-cell receptor (TCR) gene rearrangements, the authors were able to demonstrate that the lymphocytes were clonal in all patients treated with dasatinib who developed LGL lymphocytosis. These clones belonged to distinct lymphocyte subclasses in the γδ+ T-cell and natural killer (NK)–cell populations and did not express BCR-ABL, suggesting that they did not belong to the malignant Philadelphia-positive (Ph+) clone.
The significance of clonal T-cell expansions in hematologic disorders is well established and is best exemplified by their description in aplastic anemia and myelodysplastic syndromes and their disappearance with response to immunosuppressive therapy in these disorders.2 Their role in tumor surveillance and response to therapy has been scrutinized with the hope of harnessing their potential as immune therapy of cancer. Previous studies have suggested their presence in several hematologic cancers. Here, the investigators expand on previous reports of NK or NK/T-cell lineage LGL lymphocytosis in patients with Ph+ leukemias, further establish their existence before the initiation of therapy, and demonstrate differential effects of dasatinib and imatinib with regard to their expansion during therapy.3,4
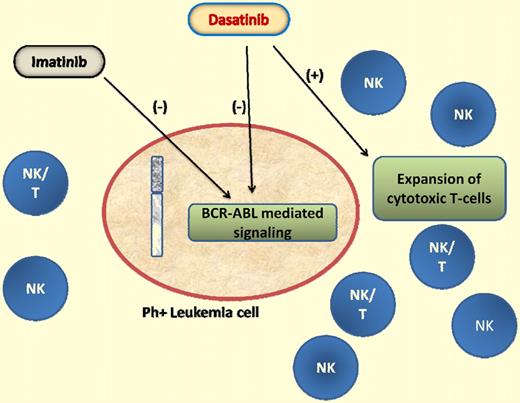
In a previous report, the same investigators demonstrated expansion of NK/T cells in 22 patients with chronic myelogenous leukemia (CML) and Ph+ acute lymphoblastic leukemia and proposed that the expansion was associated with a better response to therapy and higher incidence of toxicity, perhaps mediated through an autoimmune mechanism.4 In the current report, they demonstrate the existence of clonal cytotoxic T-cell populations before the initiation of therapy with tyrosine kinase inhibitors and show that these populations persist during therapy with both imatinib and dasatinib but are expanded only in patients who received dasatinib. This, they report, is manifested by an expansion of a population of LGLs (known to be associated with immune responses to viral and tumor antigens) which are clonal γδ+ T and NK cells further enhancing the hypothesis of a possible immunomodulatory effect for dasatinib that may, at least in part, be responsible for its therapeutic potential in Ph+ leukemias (see figure).
The mechanisms by which dasatinib may mediate this effect are not clear and it is postulated that they may be related to its off-target kinase inhibitory activities mediating the direct activation of LGLs. Alternatively, a potent suppression of the antigen, BCR-ABL, may encourage a more effective immune response previously suppressed by an overwhelming antigen load. It has been reported previously that NK cells from patients with CML are defective in function, and NK-cell numbers decrease as the disease progresses.5
Several in vitro studies have demonstrated that imatinib and dasatinib (as well as nilotinib) have inhibitory effects on T-cell proliferation and activation.6,7 This is mediated through the inhibition of Src kinases, such as Fyn and Lck, involved in the regulation and activation of T cells.6,–8 Imatinib and dasatinib have overlapping as well as distinct target kinase profiles with dasatinib being a more potent inhibitor of Src kinases. Such paradoxical reports of inhibition of TCR-mediated T-cell activation and expansion of NK and NK/T LGLs potentially effective against BCR-ABL–positive clones need to be reconciled to have a clearer understanding of the potential immunomodulatory effects of dasatinib.
If confirmed in larger studies, implications for the treatment of patients with Ph+ leukemias are significant. Clearly, the potential in the postallogeneic stem cell transplantation setting will need to be investigated. Similarly, the role of the drug in association with BCR-ABL–directed vaccines will need to be defined. Therefore, further studies to confirm the presence of cytotoxic T cells in patients with CML at the time of diagnosis, and to illustrate the potential mechanisms governing such clonal selection/expansion by dasatinib, are encouraged. It would also be important to examine the fate of these cells with effective long-term eradication of BCR-ABL.
Conflict-of-interest disclosure: The author has received research funding and honoraria from Bristol-Myers-Squibb. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal