To the editor:
Hematopoietic stem cells (HSCs) reside in specialized niches that provide signals regulating stem cell function and fate decisions. The canonical Wnt signaling pathway has been implicated in this process, but the role of specific Wnt proteins and possible functional redundancy has remained elusive.1
We recently investigated hematopoiesis in Wnt3a-deficient mice.2 Due to early embryonic lethality,3 this analysis was performed in fetal liver (FL) at embryonic day 12.5. Remarkably, Wnt3a deficiency leads to reduced numbers of long-term HSC and multipotent progenitors, which are severely and irreversibly impaired in long-term reconstitution capacity as observed in serial transplantation assays.2 This severe phenotype suggested that Wnt3a is the most prominent Wnt for FL HSC function. However, it is still unknown to what extent Wnt signaling was affected in these HSC, whether other Wnt genes could take over the role of Wnt3a, and whether its action was autocrine or paracrine.
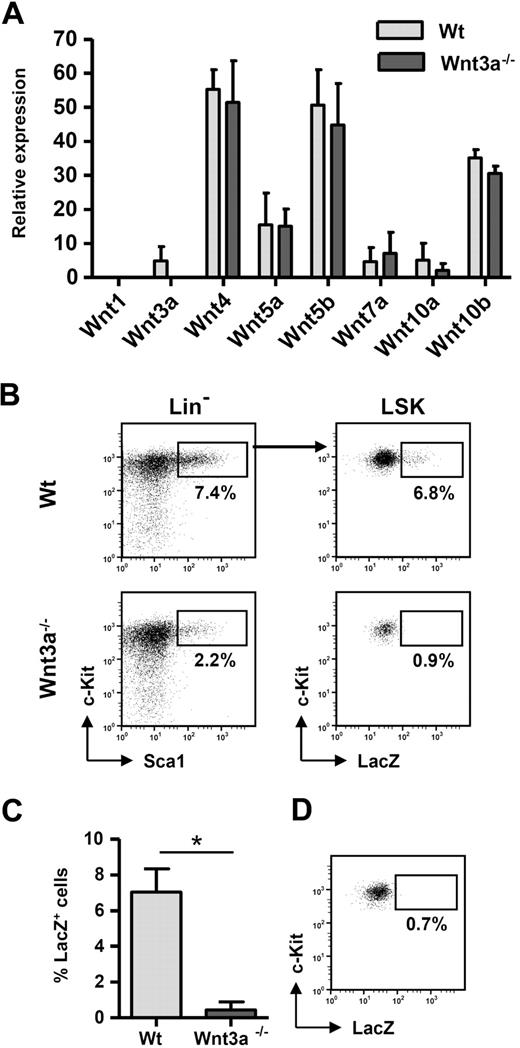
Therefore, we first determined the expression profile in FL of several Wnt genes previously shown to regulate hematopoiesis1 and whether Wnt3a deficiency affects the expression of those Wnt genes. From the panel of Wnt genes analyzed, Wnt4, Wnt5a, Wnt5b, and Wnt10b were expressed at high levels. Wnt3a was expressed at relatively low levels, and, interestingly, Wnt3a deficiency did not significantly influence the expression of the other Wnt genes (Figure 1A), indicating that the lack of self-renewal by the Wnt3a−/− HSCs was not due to an effect on the expression of other Wnt genes.
Wnt3a plays a nonredundant role in the regulation of FL HSC function. (A) Fresh wild-type or Wnt3a−/− FLs were isolated and Ter119+ erythrocytes were depleted before mRNA isolation. Gene expression was analyzed by quantitative polymerase chain reaction for the indicated Wnt genes and expression levels normalized for GAPDH using established primer/probe sets. Results correspond to mean ± SEM of 2 independent experiments. In each experiment a pool of 4 to 8 fetal livers from each genotype was used. Measurements were performed in duplicate for each sample. ND indicates not detectable. Fetal thymic lobes and fetal brain were used as positive controls for all genes analyzed. (B) Canonical Wnt signaling is completely abolished in HSCs from Wnt3a−/− embryos. Wnt3a mice were crossed with Bat-Gal Wnt reporter mice in which a LacZ cassette is under control of Tcf/Lef-binding motifs, resulting in LacZ expression when the pathway is activated. Activation of the pathway was determined by LacZ staining on individual FLs from Bat-GalTg/+ wild-type or Wnt3a−/− littermate embryos. LacZ expression was analyzed by flow cytometry4 on electronically gated LSK cells. (C) Frequency of LacZ+ cells inside LSK population. Results correspond to mean ± SEM of 2 independent experiments with 2 wild-type and 1 Wnt3a−/− littermate embryos each. *P = .03. (D) Littermate wild-type embryos not carrying the reporter transgene were used as negative controls to determine the background staining of LacZ.
Wnt3a plays a nonredundant role in the regulation of FL HSC function. (A) Fresh wild-type or Wnt3a−/− FLs were isolated and Ter119+ erythrocytes were depleted before mRNA isolation. Gene expression was analyzed by quantitative polymerase chain reaction for the indicated Wnt genes and expression levels normalized for GAPDH using established primer/probe sets. Results correspond to mean ± SEM of 2 independent experiments. In each experiment a pool of 4 to 8 fetal livers from each genotype was used. Measurements were performed in duplicate for each sample. ND indicates not detectable. Fetal thymic lobes and fetal brain were used as positive controls for all genes analyzed. (B) Canonical Wnt signaling is completely abolished in HSCs from Wnt3a−/− embryos. Wnt3a mice were crossed with Bat-Gal Wnt reporter mice in which a LacZ cassette is under control of Tcf/Lef-binding motifs, resulting in LacZ expression when the pathway is activated. Activation of the pathway was determined by LacZ staining on individual FLs from Bat-GalTg/+ wild-type or Wnt3a−/− littermate embryos. LacZ expression was analyzed by flow cytometry4 on electronically gated LSK cells. (C) Frequency of LacZ+ cells inside LSK population. Results correspond to mean ± SEM of 2 independent experiments with 2 wild-type and 1 Wnt3a−/− littermate embryos each. *P = .03. (D) Littermate wild-type embryos not carrying the reporter transgene were used as negative controls to determine the background staining of LacZ.
To determine the effect of Wnt3a deficiency on the activation of canonical Wnt signaling, we used an established Wnt reporter mouse (Bat-Gal) in which the LacZ gene (encoding β-galactosidase) is under control of 3 Wnt responsive T-cell factor/lymphoid-enhancer factor (Tcf/Lef)–binding sites.5 Analysis of reporter activity in E12.5 FL LSKs (Lin−c-Kit+Sca1+) showed that approximately 7% of these cells undergo active signaling. Analysis of Wnt3a-deficient embryos carrying the reporter transgene showed a profound reduction in the frequency of LacZ-positive LSKs (Figure 1B-C), which was not significantly higher than background levels of LacZ staining (Figure 1D). Thus, Wnt3a−/− LSKs show a complete abolishment of canonical Wnt signaling in comparison with littermate wild-type embryos.
Activation of the Wnt signaling pathway has been used to expand and enhance HSC function. Interestingly, Wnt3A was shown to preserve HSCs with an immature phenotype in vitro or to induce true stem cell characteristics in hematopoietic progenitors.6,7
Despite expression of other Wnt proteins and although it was expressed at relatively low levels in FL, Wnt3A is the only Wnt protein able to activate canonical Wnt signaling in the HSCs. This may be explained by the requirement of specific ligand/receptor combinations in HSCs or by compartmentalization of Wnts expression in the liver, with specific Wnt proteins being expressed in the stem cell niche. Although Wnt3a may also indirectly regulate HSCs by influencing the niche microenvironment, our data indicate that HSCs are directly affected by Wnt3a deficiency and that Wnt3a acts in a paracrine fashion, since it is not expressed by the HSCs themselves.2 This unanticipated and nonredundant role of one specific Wnt protein in the regulation of HSC self-renewal points specifically to Wnt3A as the Wnt protein of choice to expand the HSCs ex vivo for transplantation- and stem cell–based therapies.
Authorship
Acknowledgments: We thank the members of the Staal laboratory for help with experiments and stimulating discussions. We acknowledge Prof Dr L. Parreira (Lisbon, Portugal) for continuous support and fruitful scientific discussions.
T.C.L. is supported in part by Fundação para a Ciência e a Tecnologia – Portugal. F.J.T.S. is supported in part by the Association of International Cancer Research (AICR) and The Netherlands Organization for Health Research and Development (ZonMW).
Contribution: T.C.L. designed and performed experiments and wrote the manuscript; B.A.E.N. performed experiments; W.E.F. and J.J.M.v.D. wrote the manuscript; and F.J.T.S. designed experiments, wrote the manuscript, and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Dr Frank J. T. Staal, Department of Immunohematology and Blood Transfusion, Room L1-36, Leiden University Medical Center, Leiden, The Netherlands; e-mail: f.j.t.staal@lumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal