Abstract
The effectiveness of rituximab in hepatitis C virus (HCV)–related mixed cryoglobulinemia (MC) has been shown. However, the risk of an increase in viral replication limits its use in cirrhosis, a condition frequently observed in patients with MC. In this prospective study, 19 HCV-positive patients with MC and advanced liver disease, who were excluded from antiviral therapy, were treated with rituximab and followed for 6 months. MC symptoms included purpura, arthralgias, weakness, sensory-motor polyneuropathy, nephropathy, and leg ulcers. Liver cirrhosis was observed in 15 of 19 patients, with ascitic decompensation in 6 cases. A consistent improvement in MC syndrome was evident at the end-of-treatment (EOT) and end-of-follow-up (EOF-U). Variable modifications in both mean viral titers and alanine aminotransferase values were observed at admission, EOT, third month of follow-up, and EOF-U (2.62 × 106, 4.28 × 106, 4.82 × 106, and 2.02 × 106 IU/mL and 63.6, 49.1, 56.6, and 51.4 IU/L, respectively). Improvement in liver protidosynthetic activity and ascites degree was observed at EOT and EOF-U, especially in more advanced cases. This study shows the effectiveness and safety of rituximab in MC syndrome with advanced liver disease. Moreover, the depletion of CD20+ B cells was also followed by cirrhosis syndrome improvement despite the possibility of transient increases of viremia titers.
Introduction
Hepatitis C virus (HCV) chronic infection is a main cause of end-stage liver disease and liver cancer worldwide.1 Much evidence has shown that HCV infection is associated with B-cell lymphoproliferative disorders, such as mixed cryoglobulinemia (MC) and B-cell non-Hodgkin lymphoma. MC is the most investigated of these lymphoproliferative disorders and strictly HCV-related with both epidemiologic and pathogenetic data supporting such an association.2 As an obvious consequence, the possible usefulness of antiviral therapy has been investigated, and its effectiveness proven.3-6 However, such therapy is burdened by a number of side effects and is often not tolerated or contraindicated. In addition, several studies strongly suggest that MC is frequently associated with severe liver disease.7,8 The usefulness and safety of rituximab (RTX), a chimeric monoclonal anti–human CD20 antibody, in MC have been clearly shown in several studies involving patients with contraindications to antiviral therapy. RTX was shown to be highly effective in modifying the dynamics of B cells by deleting expanded clones and markedly improving MC syndrome in most cases.9 Such improvement is generally prolonged for longer than 6 months. However, the possibility that viral replication and aminotransferase values may suffer an immunodepression-related increase limits its use in patients with advanced chronic liver disease (CLD). Consequently, limited data exist about RTX treatment for patients with advanced CLD. In a preliminary study,10 we observed that RTX treatment was followed by improvement of both MC and cirrhotic syndromes in 2 patients. Such results prompted us to perform the present, prospective study involving a consistent number of patients with HCV-positive MC and advanced CLD, to better evaluate its safety and effectiveness.
Methods
Patients
We prospectively studied 19 patients (13 women, 6 men; mean age, 63 years; range, 37-77 years) with HCV-related MC (type II in 17 cases and type III in 2) and advanced CLD who were administered RTX. Patients were consecutively recruited at the Department of Internal Medicine, Center for Systemic Manifestations of Hepatitis Viruses of the University of Florence, Italy. Study inclusion criteria were the presence of HCV-positive MC syndrome with advanced CLD, previously excluded from antiviral therapy because of intolerance or contraindications. Exclusion criteria were all known causes of CLD other than HCV infection, with particular attention focused on the presence of hepatitis B surface antigen and anti-HIV antibodies. All patients provided informed consent in accordance with the principles of the Declaration of Helsinki, and the study was approved by the University of Florence Ethics Committee.
The main demographic, clinical, and virologic characteristics of patients are outlined in Table 1. HCV infection was proven by detecting circulating anti-HCV antibodies (EIA-2 and RIBA-2; Ortho Diagnostic Systems) and/or HCV RNA (AMPLICOR HCV Test, Version 2.0; Roche Diagnostics). All patients had serum anti-HCV antibodies, and all but 1 scored persistently serum HCV RNA–positive. HCV genotype (INNO-LiPA HCV II; Immunogenetics) was 1b in 11 patients, 2a/2c in 6, and 4c/4d in 1 patient. The mean duration of HCV infection was approximately 169 months (range, 75-366 months). The source of infection was unknown in 14 cases and most probably related to intravenous drug abuse in 2 and blood transfusion in 3. No patients were positive for immunoglobulin M antibody to hepatitis B core antigen or hepatitis B virus (HBV) DNA, but 3 patients were positive for total antibodies to hepatitis B core antigen. These patients received lamivudine prophylaxis during the study to avoid the risk of occult HBV reactivation11 and were tested monthly for serum HBV DNA.
Main laboratory and clinical characteristics of the 19 patients with MC and severe CLD
| Pt no. . | Sex . | Age, y . | CLD duration, mo . | MCS duration, mo/cryoglobulin type . | HCV genotype . | Histology [Metavir score] (Child-Pugh Class) . | Arthralgias . | Cutaneous manifestations . | Nephritis . | Neuropathic symptoms . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 44 | 146 | 50/II | 4c/4d | CH [A3 F3] | No | No | No | Yes |
| 2 | F | 74 | 173 | 64/II | 2a/2c | CH [A1 F3] | Yes | P(+) | No | Yes |
| 3 | F | 37 | 114 | 123/III | NA | C (A6) | Yes | P(++); 1 leg ulcer | Yes | Yes |
| 4 | F | 66 | 179 | 71/II | 1b | C (C11) | Yes | P(++) | Yes | Yes |
| 5 | F | 71 | 106 | 106/II | 1b | C (B7) | Yes | P(+) | No | Yes |
| 6 | M | 68 | 251 | 47/II | 1b | CH [A2 F3] | No | P(+++) | Yes | Yes |
| 7 | F | 54 | 188 | 44/II | 2a/2c | C (B7) | Yes | P(++) | No | Yes |
| 8 | M | 60 | 149 | 65/II | 1b | C (B8) | Yes | P(+ +) | Yes | Yes |
| 9 | F | 69 | 75 | 75/II | 2a/2c | C (A5) | Yes | P(+) | No | Yes |
| 10 | F | 75 | 181 | 145/II | 1b | C (A5) | Yes | P(+) | No | Yes |
| 11 | M | 61 | 134 | 134/II | 1b | C (A6) | No | P(++) | No | Yes |
| 12 | M | 77 | 134 | 134/II | 1b | C (A5) | Yes | P(++) | No | Yes |
| 13 | F | 58 | 170 | 86/II | 1b | C (A6) | Yes | No | No | Yes |
| 14 | M | 66 | 173 | 137/II | 1b | C (B9) | No | P(++) | No | No |
| 15 | M | 46 | 132 | 24/II | 1b | C (B9) | Yes | P(+++); 3 leg ulcers | Yes | Yes |
| 16 | F | 68 | 207 | 111/III | 2a/2c | CH [A2 F3] | Yes | P(+++) | No | Yes |
| 17 | F | 66 | 172 | 172/II | 2a/2c | C (A5) | Yes | P(++) | No | Yes |
| 18 | F | 71 | 444 | 180/II | 2a/2c | C (A5) | Yes | P(++); 2 leg ulcers | No | Yes |
| 19 | F | 66 | 366 | 30/II | 1b | C (A6) | Yes | P(+) | No | Yes |
| Pt no. . | Sex . | Age, y . | CLD duration, mo . | MCS duration, mo/cryoglobulin type . | HCV genotype . | Histology [Metavir score] (Child-Pugh Class) . | Arthralgias . | Cutaneous manifestations . | Nephritis . | Neuropathic symptoms . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 44 | 146 | 50/II | 4c/4d | CH [A3 F3] | No | No | No | Yes |
| 2 | F | 74 | 173 | 64/II | 2a/2c | CH [A1 F3] | Yes | P(+) | No | Yes |
| 3 | F | 37 | 114 | 123/III | NA | C (A6) | Yes | P(++); 1 leg ulcer | Yes | Yes |
| 4 | F | 66 | 179 | 71/II | 1b | C (C11) | Yes | P(++) | Yes | Yes |
| 5 | F | 71 | 106 | 106/II | 1b | C (B7) | Yes | P(+) | No | Yes |
| 6 | M | 68 | 251 | 47/II | 1b | CH [A2 F3] | No | P(+++) | Yes | Yes |
| 7 | F | 54 | 188 | 44/II | 2a/2c | C (B7) | Yes | P(++) | No | Yes |
| 8 | M | 60 | 149 | 65/II | 1b | C (B8) | Yes | P(+ +) | Yes | Yes |
| 9 | F | 69 | 75 | 75/II | 2a/2c | C (A5) | Yes | P(+) | No | Yes |
| 10 | F | 75 | 181 | 145/II | 1b | C (A5) | Yes | P(+) | No | Yes |
| 11 | M | 61 | 134 | 134/II | 1b | C (A6) | No | P(++) | No | Yes |
| 12 | M | 77 | 134 | 134/II | 1b | C (A5) | Yes | P(++) | No | Yes |
| 13 | F | 58 | 170 | 86/II | 1b | C (A6) | Yes | No | No | Yes |
| 14 | M | 66 | 173 | 137/II | 1b | C (B9) | No | P(++) | No | No |
| 15 | M | 46 | 132 | 24/II | 1b | C (B9) | Yes | P(+++); 3 leg ulcers | Yes | Yes |
| 16 | F | 68 | 207 | 111/III | 2a/2c | CH [A2 F3] | Yes | P(+++) | No | Yes |
| 17 | F | 66 | 172 | 172/II | 2a/2c | C (A5) | Yes | P(++) | No | Yes |
| 18 | F | 71 | 444 | 180/II | 2a/2c | C (A5) | Yes | P(++); 2 leg ulcers | No | Yes |
| 19 | F | 66 | 366 | 30/II | 1b | C (A6) | Yes | P(+) | No | Yes |
Pt indicates patient; CLD, chronic liver disease; MCS, mixed cryoglobulinemia syndrome; CH, chronic hepatitis; C, cirrhosis; P, purpura; +, limited or fluctuating involvement of the lower limbs; ++, diffuse and persistent involvement of the lower limbs; and +++, diffuse and persistent involvement of the trunk and lower limbs.
MC syndrome was diagnosed according to previously described criteria.12,13 Serum cryoglobulin levels and characterization, levels of complement fractions, rheumatoid factor, and autoantibodies were evaluated as described.13-15 The mean duration of MC was 88 months (range, 24-172 months). The main MC symptoms are presented in Table 1. The most relevant clinical manifestations were purpura in 17 patients and leg ulcers in 3, arthralgia and weakness in 15 patients. Five patients had renal involvement, and all but one experienced peripheral neuropathy.
The diagnosis of liver disease was performed according to standard (histologic and/or clinical and ultrasound) criteria.16,17 Four patients had advanced chronic hepatitis; according to the Metavir score16 1 patient scored A3F3, 2 patients scored A2F3, and 1 patient scored A1F3. A diagnosis of liver cirrhosis, according to clinical and ultrasound data, was made in the remaining 15 patients (Table 1).
Comorbidities
Treatment
RTX was administered as previously described9,10,20-22 and consisted of intravenous injection of 375 mg/m2 body surface area (BSA) once weekly over a 1-month period. Patients who already received low-dose corticosteroids (all but one patient, no. 14) continued this treatment. None of the patients had been previously treated with RTX.
The patients were followed before, at the end of treatment (EOT), at 3 months (3m.F-U), and at 6 months after treatment (EOF-U) with an accurate evaluation of the main clinical, immunologic, and biochemical parameters corresponding to both the MC syndrome, the CLD, and the viral infection.
Evaluation of treatment response
MC syndrome/immunologic data.
The main MC-related parameters were evaluated as previously described.9,23 Overall, a complete clinical response was defined as improvement in all baseline clinical manifestations and a partial response as improvement in at least half of the baseline clinical symptoms. All other patients were classified as clinical nonresponders. In regard to the main MC symptoms, extension of purpura was classified according to 4 main semiquantitative grades as follows: 0 (no purpura), + (limited or fluctuating involvement of the lower limbs), ++ (diffuse and persistent involvement of the lower limbs), +++ (diffuse and persistent involvement of the trunk and the lower limbs). Leg ulcer response was considered as follows: complete (when all the ulcers completely healed), major (reduction of ≥ 75% of the diameter and/or recovery of ≥ 75% of the ulcers), minor (reduction of 25%-74% of the diameter of ≥ 1 ulcers and/or recovery of 25%-74% of the ulcers), no response (< 25% reduction in the diameter of ≥ 1 ulcers and/or recovery of < 25% of the ulcers, or their worsening).
Arthralgia and neuropathic symptoms, including both paresthesias/pain and clinically evident motor deficit, were measured through a patient-scored Visual Analog Scale (VAS) (range, 0-100) and, when possible, by electromyographic analysis. Treatment response was defined as follows: complete (disappearance of symptom/s), major (> 50% VAS reduction), minor (25%-50% VAS reduction), no response (< 25% VAS reduction or worsening); relapse/progression (> 50% VAS increase).
The possibility of a malignant lymphoproliferative disorder was evaluated by total body computed tomography, bone marrow biopsy/aspirate, as well as by biohumoral data.
Renal function was evaluated according to serum creatinine and proteinuria/24 hours. A complete response was defined as the combination of normalization of renal function when abnormal (serum creatinine) and proteinuria of 0.5 g/d or less. A partial response was defined as stable or improved renal function and/or persisting for at least 50% reduction of proteinuria. No response was defined as worsening of renal function not attributable to different causes and/or proteinuria increase or a reduction insufficient for the definition of complete or partial response.
Hepatovirologic evaluation.
The degree of liver function was evaluated with the help of the biohumoral liver function tests and the Child/Pugh score24 ; in addition, the degree of ascites (in the absence of infection or development of hepatorenal syndrome) was graded as follows: grade 1 (mild), only detectable by ultrasound examination; grade 2 (moderate), moderate symmetrical abdominal distension; grade 3 (large), marked abdominal distension.25
The determination and quantification of HCV RNA in serum samples were performed by a sensitive real-time polymerase chain reaction technique that has been extensively described in previous studies.26,27 In some cases, peripheral blood mononuclear cell samples were tested for the presence of isolated HCV infection in this compartment, as previously described.27
Evaluation of liver ultrastructure.
Transjugular biopsies for microscopy were obtained in 2 patients (no. 12 and no. 14; Table 1) with liver cirrhosis, both before treatment and 3 months later; another biopsy was obtained before treatment from a third patient (no. 19). The samples were fixed with 2% formaldehyde and 2.5% glutaraldehyde in 0.1M cacodylate buffer, pH 7.4, sonicated, and embedded in epoxy resin. Sections (1-2 μm thick) were stained with alkaline toluidine blue and examined by light microscopy. Sections approximately 70 nm thick were stained with lead acetate and uranyl acetate and observed in a Jeol JEM 1010 electron microscope at 80 kV.
Analysis of reticuloendothelial system (RES) function by Resovist-enhanced magnetic resonance imaging (MRI) quantitative evaluation of liver parenchyma.
Three patients with liver cirrhosis (nos. 12, 14, 19) underwent MRI with a 1.5-T unit (Gyroscan ACS NT, Philips). Unenhanced examinations were repeated after slow administration of Resovist (SHU-555A; Bayer Schering Pharma), a superparamagnetic iron oxide contrast agent, as previously shown.28 A simple quantitative analysis of percentage of signal intensity changes of cirrhotic liver parenchyma, in delayed phases (10 minutes of acquisition), after contrast agent administration, was performed.29-31 The dose of 0.9 or 1.4 mL of SHU-555A was administered in patients whose body weight was below or greater than 60 kg, respectively (range, 7.0-12.9 μmol iron/kg). To obtain a wider window of enhancement, the entire administration was prolonged to approximately 30 to 40 seconds. Patients were analyzed before treatment and after the third month of follow-up.
Psychophysical evaluation.
The psychophysical state was evaluated by administration of the Short Form-36 General Health Survey questionnaire.32
Statistical analysis
Data are expressed as the mean plus or minus SEM. Comparisons between baseline and EOF-U values were analyzed with the paired Student t test and paired Wilcoxon test. All tests were 2-sided at a .05 significance level. Analyses were performed with the Stata version 9.0 (StataCorp LP) and True Epistat standard version (Epistat Services) statistical packages.
Results
All patients completed RTX therapy without notable side effects. The overall period of observation ranged from 6 to 48 months.
MC syndrome/immunologic data
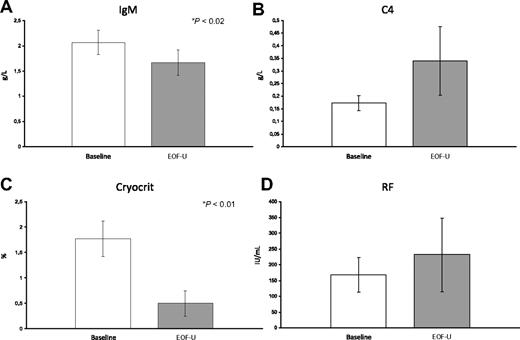
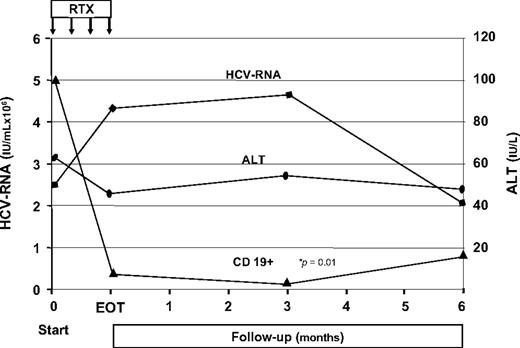
Analysis of the main MC-associated clinical aspects at EOF-U showed a complete response in 12 patients and partial response in 7 patients. The main clinical data gathered during the study are shown in Table 2. At EOF-U, complete disappearance of purpura was observed in 14 of 17 cases; in the remaining 3 patients only a partial response was observed. Patient no. 3 responded early but relapsed at the EOF-U. Pretreatment leg ulcers (1-3) were present in 3 patients. The response was rapid and complete or major. Neuropathic pain was evident in all but one patients (Table 1). A complete or major response was evidenced at EOF-U in 11 patients, a minor response in 3 patients, and no response in 4 patients. Paresthesias were present in all but 1 patient before treatment; a complete or major response was observed in 10 patients, a minor response in 4 patients, and no response in the remaining 4 patients. The electromyographic analysis, available in 5 patients, showed sensitive motor neuropathy with aspects of an axonopathic degenerative process involving the arms and legs in all patients. In 2 patients no consistent modifications were shown after RTX. Arthralgias were present in 15 patients before treatment, and in all a complete or partial response was obtained. Five patients had renal involvement, confirmed by renal biopsy showing a cryoglobulinemic membranoproliferative glomerulonephritis in 2 patients. Three patients showed a partial response, whereas the remaining patients had a complete one. The overall improvement of MC syndrome was also shown by the consistent reduction of the need for corticosteroid therapy that was progressively reduced in most patients and completely interrupted in some cases. The EOF-U analysis of the main MC-associated biochemical parameters showed a consistent reduction of the cryocrit mean values (P < .01) (Figure 1). Complete cryocrit negativization was observed in 9 patients, a cryocrit decrease greater than 50% in 2 patients, or between 25% and 50% in 3 patients, whereas in 5 patients no consistent cryocrit reduction was shown, despite transient negativity at 3m.F-U in 2 cases. Finally, in patient no. 16 we observed an increase of cryocrit values despite a complete clinical response. A consistent reduction of immunoglobulin M (P = .02) as well as an increase of complement C4 component levels were observed (Figure 1). Cytofluorimetric analysis showed a dramatic decrease of CD19+ peripheral blood cells starting early after anti-CD20 infusion (P = .01). This was followed by a partial reconstitution of cells starting at 6 months after EOT (Figure 2). No significant modifications of the T-lymphocyte pool were observed during therapy and follow-up. RTX administration also favorably influenced autoimmune comorbidities (patient nos. 5 and 10) with disappearance of the Coombs test positivity and a progressive improvement of hemoglobin values during the study. In the patient (no. 7) with idiopathic thrombotic thrombocytopenic purpura, platelet values progressively improved (from 140.0 ×109/L to 610.0 ×109/L at EOF-U).
Pattern of response of the main MC symptoms after rituximab therapy
| Patient no. . | Sex . | Skin ulcers . | Purpura . | Neuropathic pain . | Paresthesias . | Nephritis . | Arthralgias . |
|---|---|---|---|---|---|---|---|
| 1 | F | NA | NA | MR (< 6) | MR (< 6) | NA | NA |
| 2 | F | NA | MR (EOT) | MnR | MR (< 1) | NA | CR (< 1) |
| 3 | F | CR (< 1) | MR (< 3)* | NR | NR | PR | PR |
| 4 | F | NA | MR (< 3) | MR (< 6) | MR (< 6) | PR | CR (< 1) |
| 5 | F | NA | MR (< 6) | MR (< 6) | CR (< 1) | NA | CR (< 1) |
| 6 | M | NA | MR (< 3) | CR (< 1) | CR (< 1) | PR | NA |
| 7 | F | NA | MR (< 6) | MnR | CR (< 1) | NA | PR |
| 8 | M | NA | MR (< 3) | MR (< 6) | MnR | CR (< 2) | CR (< 1) |
| 9 | F | NA | MR (< 3) | MR (< 6) | MnR | NA | PR |
| 10 | F | NA | MR (< 3) | NR | NR | NA | PR |
| 11 | M | NA | MnR | MR (< 6) | NR | NA | NA |
| 12 | M | NA | MnR | CR (< 1) | MnR | NA | PR |
| 13 | F | NA | NA | CR (< 1) | CR (< 1) | NA | CR (< 1) |
| 14 | M | NA | MR (< 6) | NA | NA | NA | NA |
| 15 | M | MR (< 1) | MR (< 6) | MR (< 6) | MR (< 6) | CR (< 6) | PR |
| 16 | F | NA | MR (< 6) | MR (< 6) | NR | NA | CR (< 1) |
| 17 | F | NA | MR (< 6) | MnR | MnR | NA | PR |
| 18 | F | CR (< 1) | MnR | NR | NR | NA | CR (< 1) |
| 19 | F | NA | MR (EOT) | NR | CR (< 1) | NA | PR |
| Patient no. . | Sex . | Skin ulcers . | Purpura . | Neuropathic pain . | Paresthesias . | Nephritis . | Arthralgias . |
|---|---|---|---|---|---|---|---|
| 1 | F | NA | NA | MR (< 6) | MR (< 6) | NA | NA |
| 2 | F | NA | MR (EOT) | MnR | MR (< 1) | NA | CR (< 1) |
| 3 | F | CR (< 1) | MR (< 3)* | NR | NR | PR | PR |
| 4 | F | NA | MR (< 3) | MR (< 6) | MR (< 6) | PR | CR (< 1) |
| 5 | F | NA | MR (< 6) | MR (< 6) | CR (< 1) | NA | CR (< 1) |
| 6 | M | NA | MR (< 3) | CR (< 1) | CR (< 1) | PR | NA |
| 7 | F | NA | MR (< 6) | MnR | CR (< 1) | NA | PR |
| 8 | M | NA | MR (< 3) | MR (< 6) | MnR | CR (< 2) | CR (< 1) |
| 9 | F | NA | MR (< 3) | MR (< 6) | MnR | NA | PR |
| 10 | F | NA | MR (< 3) | NR | NR | NA | PR |
| 11 | M | NA | MnR | MR (< 6) | NR | NA | NA |
| 12 | M | NA | MnR | CR (< 1) | MnR | NA | PR |
| 13 | F | NA | NA | CR (< 1) | CR (< 1) | NA | CR (< 1) |
| 14 | M | NA | MR (< 6) | NA | NA | NA | NA |
| 15 | M | MR (< 1) | MR (< 6) | MR (< 6) | MR (< 6) | CR (< 6) | PR |
| 16 | F | NA | MR (< 6) | MR (< 6) | NR | NA | CR (< 1) |
| 17 | F | NA | MR (< 6) | MnR | MnR | NA | PR |
| 18 | F | CR (< 1) | MnR | NR | NR | NA | CR (< 1) |
| 19 | F | NA | MR (EOT) | NR | CR (< 1) | NA | PR |
Values reported are timing of response (in months after EOT).
F indicates female; M, male; NA, not applicable; MR, major response; EOT, end of treatment; MnR, minor response; CR, complete response; NR, no response; and PR, partial response.
This patient had a relapse 6 months after EOT.
Behavior of mean values of the main immunologic data of the 19 patients with MC before RTX therapy and at the end of follow-up (six months after therapy interruption). (A) Immunoglobulin M (IgM) mean values; (B) C4 component of complement mean values; (C) cryocrit mean values; and (D) rheumatoid factor (RF) mean values.
Behavior of mean values of the main immunologic data of the 19 patients with MC before RTX therapy and at the end of follow-up (six months after therapy interruption). (A) Immunoglobulin M (IgM) mean values; (B) C4 component of complement mean values; (C) cryocrit mean values; and (D) rheumatoid factor (RF) mean values.
Pattern of HCV RNA, alanine aminotransferase, and CD19 mean values of the 19 patients with HCV-positive MC during the study. RTX indicates rituximab infusions; EOT, end of treatment; and ALT, alanine aminotransferase.
Pattern of HCV RNA, alanine aminotransferase, and CD19 mean values of the 19 patients with HCV-positive MC during the study. RTX indicates rituximab infusions; EOT, end of treatment; and ALT, alanine aminotransferase.
Hepatovirologic evaluation
Concerning the hepatovirologic data, serum HCV RNA scored persistently positive before the study in all but one patient (no. 3). In HCV RNA–positive patients, mean viremic levels were 2.62 × 106 IU/mL before RTX infusion, reached a mean value of 4.28 × 106 IU/mL at EOT, slightly increased at 3m.F-U (4.82 × 106 IU/mL), and decreased to the pretreatment mean levels at the EOF-U (2.02 × 106 IU/mL; Figure 2). Pretreatment mean alanine aminotransferase value (63.6 IU/L) was not significantly modified by RTX therapy, scoring 49.1 IU/L at EOT, 56.6 IU/L at 3m.F-U, and 51.4 IU/L at EOF-U (Figure 2).
In contrast, serum albumin titers showed important modifications. For all patients, mean albuminemia values were 36.1 g/L, 37.8 g/L, 42.6 g/L, and 39.1 g/L at pretreatment, EOT, 3m.F-U, and EOF-U, respectively (data not shown). This improvement in albuminemia was especially observed in the 6 patients harboring decompensated cirrhosis and pretreatment low values (Table 3). Improved hepatic function in patients with liver cirrhosis was shown by the improved Child-Pugh score observed in most patients with advanced disease (> A score; Table 3). In these patients the score improved from 1 to 4 points in all but one patient who maintained the B7 score (Table 3). An impressive reduction in degree of ascites was observed in the 6 patients with decompensated cirrhosis (Figure 3). In 4 of these 6 patients a complete, persistent disappearance of ascites was observed from the EOT and 3m.F-U. The remaining 2 patients experienced a complete disappearance of ascites at 3m.F-U, with only ultrasound evidence of mild ascitic effusion at EOF-U. This improvement was followed by a progressive reduction of diuretics doses (antialdosterone and furosemide; P = .05), the amount of albumin infusion per week (Figure 3), and the need for paracenteses (patients no. 4 and no. 8, who were previously treated at bimonthly intervals, did not need paracenteses during the study period). Of the 3 patients who received 20 g of albumin weekly during the pretreatment period, only 1 patient needed albumin at the EOF-U (40 g monthly; patient no. 4). Such an impressive effect on the degree of ascites and need for albumin infusions in patients with decompensated cirrhosis did not appear to be justified by a corresponding improvement in proteinuria levels. In the 3 patients with proteinuria, the mean values were 1.072, 0.309, and 0.391 g/24 hours before treatment, at EOT, and at EOF-U, respectively (data not shown).
Modifications of the main hepatovirologic data induced by rituximab therapy in the 6 patients with decompensated cirrhosis
| Treatment phases . | Total protein, g/dL . | Albumin, g/dL . | ALT, IU/L . | HCV RNA, IU/mL ×103 . | Prothrombin activity, % . | Child-Pugh class . |
|---|---|---|---|---|---|---|
| Patient no. 4 | ||||||
| Before RTX infusion | 6.5 | 2.52 | 46 | 350 | 64 | C11 |
| End of treatment | 6.6 | 2.95 | 65 | 835 | 78 | B8 |
| 3rd month PTFU | 6 | 3.1 | 20 | ND | 71 | B7 |
| 6th month PTFU | 6 | 3.1 | 61 | 650 | 80 | B7 |
| Patient no. 5 | ||||||
| Before RTX infusion | 6 | 3.45 | 193 | 451 | 79 | B7 |
| End of treatment | 7.1 | 4.13 | 132 | 450 | 88 | A6 |
| 3rd month PTFU | 6.9 | 3.9 | 180 | 1100 | 82 | A5 |
| 6th month PTFU | 7.1 | 4 | 105 | 1100 | 74 | A6 |
| Patient no. 7 | ||||||
| Before RTX infusion | 7.3 | 3.4 | 153 | 14 100 | 67 | B7 |
| End of treatment | 5.9 | 3.6 | 70 | 4700 | 65 | B7 |
| 3rd month PTFU | 5.7 | 3.6 | 82 | ND | 64 | B7 |
| 6th month PTFU | 5.8 | 3.9 | 46 | ND | 59 | B7 |
| Patient no. 8 | ||||||
| Before RTX infusion | 5.3 | 3.18 | 55 | 575 | 73 | B8 |
| End of treatment | 6.3 | 3.53 | 72 | 950 | 100 | B7 |
| 3rd month PTFU | 6.8 | 3.9 | 31 | ND | 100 | A5 |
| 6th month PTFU | 6.9 | 4.03 | 131 | 750 | 100 | A6 |
| Patient no. 14 | ||||||
| Before RTX infusion | 7.1 | 3.98 | 77 | 691 | 50 | B9 |
| End of treatment | 7.3 | 3.84 | 55 | 597 | 68 | A6 |
| 3rd month PTFU | 6.8 | 3.4 | 68 | 872 | 55 | B7 |
| 6th month PTFU | 6.8 | 3.35 | 62 | 891 | 54 | B7 |
| Patient no. 15 | ||||||
| Before RTX infusion | 5.1 | 2.92 | 20 | 650 | 83.3 | B9 |
| End of treatment | 6.7 | 3.01 | 18 | 800 | 78.2 | B8 |
| 3rd month PTFU | 6.7 | 3.5 | 14 | ND | 63.5 | B8 |
| 6th month PTFU | 6.7 | 3.13 | 15 | 720 | 72.67 | B7 |
| Treatment phases . | Total protein, g/dL . | Albumin, g/dL . | ALT, IU/L . | HCV RNA, IU/mL ×103 . | Prothrombin activity, % . | Child-Pugh class . |
|---|---|---|---|---|---|---|
| Patient no. 4 | ||||||
| Before RTX infusion | 6.5 | 2.52 | 46 | 350 | 64 | C11 |
| End of treatment | 6.6 | 2.95 | 65 | 835 | 78 | B8 |
| 3rd month PTFU | 6 | 3.1 | 20 | ND | 71 | B7 |
| 6th month PTFU | 6 | 3.1 | 61 | 650 | 80 | B7 |
| Patient no. 5 | ||||||
| Before RTX infusion | 6 | 3.45 | 193 | 451 | 79 | B7 |
| End of treatment | 7.1 | 4.13 | 132 | 450 | 88 | A6 |
| 3rd month PTFU | 6.9 | 3.9 | 180 | 1100 | 82 | A5 |
| 6th month PTFU | 7.1 | 4 | 105 | 1100 | 74 | A6 |
| Patient no. 7 | ||||||
| Before RTX infusion | 7.3 | 3.4 | 153 | 14 100 | 67 | B7 |
| End of treatment | 5.9 | 3.6 | 70 | 4700 | 65 | B7 |
| 3rd month PTFU | 5.7 | 3.6 | 82 | ND | 64 | B7 |
| 6th month PTFU | 5.8 | 3.9 | 46 | ND | 59 | B7 |
| Patient no. 8 | ||||||
| Before RTX infusion | 5.3 | 3.18 | 55 | 575 | 73 | B8 |
| End of treatment | 6.3 | 3.53 | 72 | 950 | 100 | B7 |
| 3rd month PTFU | 6.8 | 3.9 | 31 | ND | 100 | A5 |
| 6th month PTFU | 6.9 | 4.03 | 131 | 750 | 100 | A6 |
| Patient no. 14 | ||||||
| Before RTX infusion | 7.1 | 3.98 | 77 | 691 | 50 | B9 |
| End of treatment | 7.3 | 3.84 | 55 | 597 | 68 | A6 |
| 3rd month PTFU | 6.8 | 3.4 | 68 | 872 | 55 | B7 |
| 6th month PTFU | 6.8 | 3.35 | 62 | 891 | 54 | B7 |
| Patient no. 15 | ||||||
| Before RTX infusion | 5.1 | 2.92 | 20 | 650 | 83.3 | B9 |
| End of treatment | 6.7 | 3.01 | 18 | 800 | 78.2 | B8 |
| 3rd month PTFU | 6.7 | 3.5 | 14 | ND | 63.5 | B8 |
| 6th month PTFU | 6.7 | 3.13 | 15 | 720 | 72.67 | B7 |
ALT indicates alanine aminotransferase; ND, not determined; and RTX, rituximab; and PTFU, posttherapy follow-up.
Modifications of pretreatment therapeutic measures and ascites severity in the 6 patients with MC and decompensated cirrhosis. (A) Mean doses of kanrenone before rituximab therapy (baseline) and at 6 months of follow-up after therapy interruption EOF-U. (B) Mean doses of furosemide before treatment and at EOF-U. (C) Mean doses of albumin infusion before treatment and at EOF-U. (D) Modifications of ascites severity degree according to Arroyo et al25 observed before rituximab (baseline), at the end of therapy (EOT), at 3 months of follow-up (3m.F-U), and at EOF-U.
Modifications of pretreatment therapeutic measures and ascites severity in the 6 patients with MC and decompensated cirrhosis. (A) Mean doses of kanrenone before rituximab therapy (baseline) and at 6 months of follow-up after therapy interruption EOF-U. (B) Mean doses of furosemide before treatment and at EOF-U. (C) Mean doses of albumin infusion before treatment and at EOF-U. (D) Modifications of ascites severity degree according to Arroyo et al25 observed before rituximab (baseline), at the end of therapy (EOT), at 3 months of follow-up (3m.F-U), and at EOF-U.
Interestingly, 2 patients (nos. 16 and 18), who were excluded from the antiviral therapy before this study because of cytopenia, could be given viral eradication therapy after RTX treatment. Patient no. 16 (high viral load, > 850 000 IU/mL; HCV genotype 2a/c) had a history of ineffective antiviral treatments and, 3 months after RTX therapy, received standard treatment with pegylated interferon and ribavirin for 6 months. Patient no. 18, who was treatment naive and had similar viral characteristics (high viral load and HCV genotype 2a/c), stopped treatment after only 6 weeks because of the occurrence of severe psychiatric complications. Both patients experienced a sustained virologic response. The analysis of HCV RNA sequences in both peripheral and bone marrow mononuclear cells confirmed the viral eradication. In fact, HCV RNA sequences were not detected in both uncultured and mitogen-stimulated, cultured cells in blood samples obtained at 3 different times (3 and 6 months) after therapy interruption and at the last control visit (12th month after treatment; data not shown).
Evaluation of liver ultrastructure
In patients undergoing transjugular liver biopsy, portal areas hosted a rich infiltrate, mainly made by dendritic cells and lymphocytes with a few macrophages and plasma cells, and many bile ductules. Small foci of dendritic cells and lymphocytes were located among hepatocytes. The sinusoids were scarce. Kupffer and Ito cells had a normal aspect. No consistent modification in such aspects was seen after RTX administration (data not shown).
Analysis of RES function by Resovist-enhanced MRI quantitative evaluation of liver parenchyma
Analysis of MRI data was not dissimilar from mean percentages reported in the literature.31 In the 3 patients who underwent Resovist-enhanced MRI, we did not find any significant change in the mean signal intensity percentage of cirrhotic liver parenchyma before and after therapy either with the T2 (−43% vs −41%) or with T2* (−37% vs −35%) sequence.
Psychophysical evaluation
A consistent improvement in the patient's perception of his/her health was shown by the Short Form-36 General Health Survey questionnaire compiled before treatment and at different times during follow-up (data not shown).
Discussion
MC is both an autoimmune and B-cell lymphoproliferative disorder. This suggested that RTX may represent an interesting alternative to traditional therapeutic approaches for patients with MC, which often have limited efficacy and/or significant side effects. Several previous studies indicate that RTX may be successfully used for treatment of HCV-related MC. However, its efficacy, safety, and cost/benefit in relation to different organ manifestations and duration of clinical response have not been fully investigated.
In the present study, RTX efficacy was confirmed in patients with HCV-related MC and advanced CLD. This study also shows, for the first time, that B-cell depletion induced by RTX can lead to improved concomitant liver cirrhosis syndrome.
Regarding MC syndrome, effects of RTX treatment did not substantially differ from previous reports.9,22,33 RTX was mainly effective in inducing the disappearance or consistent regression of skin manifestations, such as purpura and leg ulcers. Less evident improvement appeared in peripheral neuropathy, especially considering electromyographic changes. Better results were reported in a recent work showing that RTX is effective and safe in the treatment of patients with MC-associated neuropathy.34 The limited number of patients undergoing electromyography before and after therapy in the present study makes it difficult to arrive at a definitive conclusion. In addition, subjective improvement in neuropathic pain and paresthesias, according to the VAS scale, was highlighted in most cases. Although the present study did not include patients with severe renal disease, the use of RTX led to consistent improvement in renal function. For almost all patients with active urinary sediment before treatment, this was significantly reduced or absent after treatment, and, in one-half of patients with proteinuria, a greater than 50% reduction was observed. In addition, in view of the wide range of possible overlapping extrahepatic manifestations frequently observed in patients with chronic HCV infection,6,35 the contemporary resolution of both MC and autoimmune hemolytic anemia or idiopathic thrombotic thrombocytopenic purpura we observed in 3 patients is of great clinical interest.
Concerning the liver disease, previous data showed the absence of direct hepatotoxicity of the treatment. However, in HCV-positive patients, the risk of an immunodepression-related increase in viral replication, with the consequent possibility of worsening liver damage, justified the exclusion of patients with advanced liver disease from previous studies. Such an exclusion would greatly limit the use of RTX for MC. In fact, an association between HCV-positive MC and advanced liver disease was shown in most available studies,7,8,36 although data from some geographical areas may be contrasting, and the limitation of such an association to a specific MC type has been proposed.37,38 In addition, the use of RTX has been especially suggested when antiviral therapy is ineffective or contraindicated, and it is known that liver cirrhosis consistently limits the effectiveness of anti-HCV treatment or represents a contraindication in case of more advanced, decompensated cases. The results of the present study clearly show that this drug may be effective and safe even in this type of patients. In fact, the mean alanine aminotransferase value appeared slightly reduced in our total patients, even though, a moderate increase was observed in some patients. However, such an increase was not followed by a worsening of liver function, but rather by improvement, as shown by increased protein synthesis, mainly evident in patients with cirrhosis complicated by ascites at the time of enrollment. In most of these patients RTX treatment led to a significant decrease in the Child-Pugh class. However, the presence of end-stage liver disease should always prompt a cautious clinical approach and careful clinical monitoring, because of patient variability and the possible presence of comorbidity. In this respect it is noteworthy that none of our patients had evidence of malignant evolution of the lymphoproliferative or liver disorder, or history of spontaneous peritonitis or hepatorenal syndrome.
Improved cirrhotic syndrome after RTX infusion is interesting because of the controversial and complex relationship between hepatic and extrahepatic disease in HCV-related MC. The mechanisms for such a paradoxical positive effect are at present unknown; thus, more specific studies are needed. The initial theory suggesting a role played by the impairment of Kupffer cell function secondary to their massive involvement in the clearance of large circulating immune complexes in MC, with a consequent improvement after cryocrit value reduction,7 may be considered, but, in the present study, we did not obtain supporting data. Liver RES function in some patients was quantitatively investigated by Resovist-enhanced MRI.29 This superparamagnetic T2 contrast agent can label the macrophage system, indicating both access to and clearance of the medium by this cell system. The effect of superparamagnetic iron oxide decreases in the cirrhotic liver because Kupffer cell phagocytic activity is reduced in these patients.30,31 As expected, in patients with cirrhosis the lower SI induced by Resovist was less than in healthy liver, but no evidence of a significant modification after RTX therapy was seen. The lack of consistent modifications of liver ultrastructure, and especially Kupffer cells, after successful therapy, was in agreement with the absence of differences when using Resovist-enhanced MRI. Better knowledge of the main role that B cells play (directly and/or through the modulation of T-cell function) in the pathogenesis of both MC and liver disease would probably help to clarify the observed phenomena and potentially increase the chance of designing personalized protocols.39,40
Another positive result of the present study was the possibility of reducing or interrupting previous treatments, namely the use of steroids, as well as the need for previous weekly albumin infusions and the frequent paracenteses in some patients, resulting in a significant improvement in the patient's quality of life.
Furthermore, because HCV-eradicating treatment should be considered the first-line therapeutic option in HCV-related MC, to be performed as early as possible,5,6,35 but many patients with MC cannot undergo this therapy because of contraindications or intolerance, it is expected that the RTX-driven improvement in patients' overall clinical condition (ie, improved hemochrome values, healing of leg ulcers, reduction of Child-Pugh class) may make it possible, for some patients, to successfully undergo the antiviral therapy.41,42 Accordingly, in the present study, 2 patients who were previously excluded from antiviral therapy, could achieve viral eradication. One of these patients even had history of previous, ineffective antiviral therapy performed in a less severe phase of disease, suggesting that B-cell depletion has a beneficial effect on viral eradication. Further dedicated studies will ascertain this interesting working hypothesis.
In conclusion, our study shows the safety and effectiveness of RTX as treatment of HCV-related MC even in patients with concomitant severe liver disease. The observation of an improvement of cirrhotic syndrome after B-cell depletion strongly suggests the interest for further studies investigating the role, directly or indirectly, played by the B-cell compartment in the HCV-related liver damage.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
We thank Mrs Mary Forrest for precious help in editing the manuscript.
This work was supported by grants from the “Associazione Italiana per la Ricerca sul Cancro” (AIRC; investigator grant 1461), “Istituto Toscano Tumori” (ITT), “Fondazione Istituto di Ricerche Virologiche Oretta Bartolomei Corsi,” and “Ente Cassa di Risparmio di Firenze.” P.C. is supported by a Fondazione Italiana per la Ricerca sul Cancro (FIRC) fellowship.
Authorship
Contribution: A.P. designed and performed research, wrote the paper; L.R., F.V., U.A., M.M., and P.M. performed research; P.C. analyzed data and wrote the paper; S.C. and P.R. performed research, provided vital analytical tools, and wrote the paper; C.G. analyzed data; M.M.-C. and A.B. provided vital analytical tools and performed research; G.L. analyzed data and designed research; and A.L.Z. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Systemic Manifestations of Hepatitis Viruses Study Group appears as a data supplement to the online version of this article (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Anna Linda Zignego, Center for Systemic Manifestations of Hepatitis Viruses (MaSVE), Department of Internal Medicine, University of Florence, Viale GB Morgagni 85, 50134 Firenze, Italy; e-mail: a.zignego@dmi.unifi.it.
References
Author notes
For the MaSVE Study Group.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal