Abstract
Hemochromatosis is a common genetic disorder in which iron may progressively accumulate in the liver, heart, and other organs. The primary goal of therapy is iron depletion to normalize body iron stores and to prevent or decrease organ dysfunction. The primary therapy to normalize iron stores is phlebotomy. In this opinion article, we discuss the indications for and monitoring of phlebotomy therapy to achieve iron depletion, maintenance therapy, dietary and pharmacologic maneuvers that could reduce iron absorption, and the role of voluntary blood donation.
Introduction
Hemochromatosis is a common heritable disorder characterized by the progressive accumulation of iron attributable to increased absorption from dietary sources.1 The most common hemochromatosis genotype is homozygosity for HFE C282Y, although the role of HFE protein in the pathogenesis of the iron overload has not been clearly elucidated. Other mediators that affect iron absorption, transport, and mobilization include hepcidin, bone morphogenic protein 6, hemojuvelin, transferrin receptor-1 and -2, and ferroportin.2 The use of phlebotomy therapy to reduce body iron stores followed the clinical description of hemochromatosis by at least 50 years.3 Phlebotomy stimulates erythropoiesis and mobilizes iron from parenchymal cells and other storage sites.
In this opinion article, we focus on the treatment of typical HFE C282Y homozygotes with iron overload. Many of these management concepts also are applicable to the treatment of patients with other types of iron overload, although the severity of iron overload and response to phlebotomy vary depending on the genetic subtype of hemochromatosis or other iron overload disorder. Further, the success of phlebotomy therapy depends on normal erythropoiesis. The diagnosis of iron overload is beyond the scope of this article.4 More patients have hyperferritinemia that is caused by inflammatory conditions and liver diseases than to iron overload, and the risk for adverse events caused by inappropriate phlebotomy therapy in such patients may be great. Phlebotomy treatment of other conditions such as hepatitis C, fatty liver disease, and porphyria cutanea tarda is beyond the scope of this article.
Rationale for iron depletion therapy
It had been previously considered that most patients with hemochromatosis have progressive iron accumulation. Excess iron is toxic to cells, an effect mediated through the production of free radicals and the Fenton reaction,5 although the authors of many in vitro iron toxicity studies used supraphysiologic concentrations of iron. The previous rationale for phlebotomy therapy for all patients with hemochromatosis was that iron depletion would reduce or eliminate the potential for iron-mediated tissue injury.6 Iron toxicity has been difficult to demonstrate in animal models.7 An extrapolation unfounded by clinical studies was that iron depletion would improve the quantity and quality of life of all patients with hemochromatosis and that the benefits of therapy would outweigh any potential risks or adverse effects of phlebotomy therapy.
Do all C282Y homozygotes require phlebotomy therapy?
Since the development of diagnostic genetic testing for hemochromatosis, it has become apparent that approximately 50% of female and 20% of male adult C282Y homozygotes have normal serum ferritin (SF) levels and may never require phlebotomy therapy.8 The guidelines for the initiation of phlebotomy therapy published by expert groups before HFE mutation analysis was widely available recommended starting therapy in C282Y homozygotes with an increased SF (> 200 μg/L in women, > 300 μg/L in men).9 This recommendation stems from the assumption that a person with hemochromatosis and an increased SF level at presentation has already demonstrated accumulation of excess iron that would likely be progressive. Because most patients have been treated with phlebotomy after clinical or biochemical presentation, there has been little opportunity to collect observations on the natural history of untreated hemochromatosis.
The authors of several large population-based studies have performed hemochromatosis genetic testing on participants many years into the study and used stored blood samples to measure SF over time in participants found to have C282Y homozygosity.10-15 These studies demonstrate that not all C282Y homozygotes, including those with an increased SF level, are destined to have progressive iron overload.16,17 The degree of elevation of the SF level at presentation can be used to predict the risk of progression.13 An adult C282Y homozygote with a normal SF level at diagnosis is very unlikely to develop iron overload later.14 These observations from population screening studies may not be relevant to a C282Y homozygote with symptoms referred to initiate phlebotomy therapy. It is widely agreed that persons with hemochromatosis whose SF is 1000 μg/L or greater should undergo phlebotomy to achieve iron depletion.18 The decision to treat C282Y homozygotes with moderate elevations of SF (200-1000 μg/L) should consider patient preferences and clinical judgment (Table 1). The conventional approach is to treat all C282Y homozygotes with SF above the reference range. New information from population studies has demonstrated that SF levels do not increase progressively in all patients, and thus observation with follow-up SF measurements can be considered as an alternative management strategy. The presence of symptoms or increased serum levels of liver enzymes are not a prerequisite for initiating phlebotomy therapy.
Treatments for iron overload caused by hemochromatosis
| Treatment . | Usual route of treatment . | Advantages . | Principal route/form of iron elimination . | Compliance with treatment . | Disadvantages . | Adverse effects . |
|---|---|---|---|---|---|---|
| Phlebotomy | Venipuncture | Much experience; effective on the part of the clinician, widely available, safe, inexpensive; reversal of cirrhosis in some cases; may improve left ventricular diastolic function | Blood as hemoglobin (1 mL of erythrocytes = 1 mg of Fe) | Excellent for iron depletion; good for maintenance | Requires repeated visits to health-care facility; requires normal erythropoiesis; some patients report intolerance | Transient hypovolemia; fatigue; increases iron absorption; iron deficiency if monitoring inadequate or inappropriate |
| Erythrocytapheresis | Venipuncture | Rapid, safe; may be preferred for patients with severe iron overload | Blood as hemoglobin (1 mL of erythrocytes = 1 mg of Fe) | Excellent in selected patients | Limited clinical experience; requires special apparatus and facility, limited availability; expensive | Transient hypovolemia; fatigue; increases iron absorption; citrate reaction; iron deficiency if monitoring inadequate or inappropriate |
| Deferoxamine (DFO) chelation | Subcutaneous infusion | Much clinical experience in iron overload patients without hemochromatosis; widely available; consider its use in patients intolerant of phlebotomy | Urine as chelate; daily iron excretion variable | Fair | Few reports of use in hemochromatosis, mostly to achieve iron depletion; inadequate chelation of cardiac iron in some cases; expensive | Infusion site reactions; hearing, vision, growth, skeletal abnormalities; zinc deficiency; Yersinia infection |
| Deferasirox (DFX) chelation | Oral | Good chelation of hepatic iron; consider its use in patients with inadequate venous access or intolerant of phlebotomy | Stool as chelate; daily iron excretion variable | Fair | Few reports of use in hemochromatosis to achieve iron depletion; no clear benefit for patients with iron-induced cardiomyopathy; expensive | Toxicity often dose dependent; gastrointestinal symptoms; transaminase elevations; elevation of serum creatinine; rash; rare hearing, vision abnormalities; severe (sometimes fatal) liver, kidney, or marrow toxicity |
| Treatment . | Usual route of treatment . | Advantages . | Principal route/form of iron elimination . | Compliance with treatment . | Disadvantages . | Adverse effects . |
|---|---|---|---|---|---|---|
| Phlebotomy | Venipuncture | Much experience; effective on the part of the clinician, widely available, safe, inexpensive; reversal of cirrhosis in some cases; may improve left ventricular diastolic function | Blood as hemoglobin (1 mL of erythrocytes = 1 mg of Fe) | Excellent for iron depletion; good for maintenance | Requires repeated visits to health-care facility; requires normal erythropoiesis; some patients report intolerance | Transient hypovolemia; fatigue; increases iron absorption; iron deficiency if monitoring inadequate or inappropriate |
| Erythrocytapheresis | Venipuncture | Rapid, safe; may be preferred for patients with severe iron overload | Blood as hemoglobin (1 mL of erythrocytes = 1 mg of Fe) | Excellent in selected patients | Limited clinical experience; requires special apparatus and facility, limited availability; expensive | Transient hypovolemia; fatigue; increases iron absorption; citrate reaction; iron deficiency if monitoring inadequate or inappropriate |
| Deferoxamine (DFO) chelation | Subcutaneous infusion | Much clinical experience in iron overload patients without hemochromatosis; widely available; consider its use in patients intolerant of phlebotomy | Urine as chelate; daily iron excretion variable | Fair | Few reports of use in hemochromatosis, mostly to achieve iron depletion; inadequate chelation of cardiac iron in some cases; expensive | Infusion site reactions; hearing, vision, growth, skeletal abnormalities; zinc deficiency; Yersinia infection |
| Deferasirox (DFX) chelation | Oral | Good chelation of hepatic iron; consider its use in patients with inadequate venous access or intolerant of phlebotomy | Stool as chelate; daily iron excretion variable | Fair | Few reports of use in hemochromatosis to achieve iron depletion; no clear benefit for patients with iron-induced cardiomyopathy; expensive | Toxicity often dose dependent; gastrointestinal symptoms; transaminase elevations; elevation of serum creatinine; rash; rare hearing, vision abnormalities; severe (sometimes fatal) liver, kidney, or marrow toxicity |
It is not feasible to estimate net iron loss or gain attributable to diet or medications in individual patients using routine clinical techniques. Some patients with juvenile-onset hemochromatosis, severe iron overload, and iron-induced cardiomyopathy may benefit from combined treatment with phlebotomy and DFO or DFX.
Clinical benefits of phlebotomy therapy
A randomized study of phlebotomy therapy has not been done; therefore, it has been difficult to assess the clinical benefits of therapy. In a large survey of hemochromatosis patients in the United States and Canada, 76% of patients received phlebotomy in a hospital or physician's office and 30% at a blood center. More than three-quarters of patients reported that their phlebotomy therapy was beneficial, whereas 15% expressed a negative opinion about therapy. The primary negative aspects were venous access problems, time (for travel, waiting, and the procedure), and the fact that in many cases the blood is not used for donation. Fifty-nine percent reported that if they could take a pill with a 5% chance of serious adverse effects, they would prefer that treatment to phlebotomy.19,20
Liver fibrosis, cirrhosis, and hepatocellular carcinoma are the most serious complications of iron overload. In population-based studies, these complications occur in approximately 5% of male and less than 1% of female C282Y homozygotes.21,22 The presence of symptoms related to iron overload in C282Y homozygotes found in a population screening study was 28.4% in men and 1.2% in women.21 Several studies in which the authors evaluated liver biopsies before and after phlebotomy therapy demonstrated that hepatic fibrosis can be reversed by phlebotomy therapy.23,24 Hepatic elastography is a noninvasive tool that may be useful to monitor this potential benefit without the need for liver biopsy.25 Liver biopsy has moved from a diagnostic test to a prognostic test in many patients with hemochromatosis. It should be considered in C282Y homozygotes with SF 1000 μg/L or greater and in patients with noniron risk factors for liver disease (eg, alcohol, obesity, viral hepatitis). Liver iron concentration was most useful when the iron concentration/age (hepatic iron index) was used as a diagnostic test for hemochromatosis. This index is rarely useful in the current era of genetic testing.
Severe myocardial siderosis does not develop in most persons with HFE hemochromatosis. Forty-three C282Y homozygotes with iron overload and New York Heart Association functional class I had increased strain rate, a sensitive indicator of left ventricular diastolic function. Strain rate was significantly correlated with biomarkers of oxidative stress but not with serum iron measures. These abnormalities were not observed in control subjects who lacked HFE C282Y and H63D.26 Cardiac dysfunction as the result of myocardial siderosis is typically more severe in persons with hemochromatosis caused by HJV or TFR2 mutations than in persons with HFE hemochromatosis and may manifest as dyspnea, orthopnea, or arrhythmia that often improves after iron depletion.26
Diabetes mellitus, infrequently the result of iron overload alone, is often unchanged by phlebotomy therapy, although some patients report improvement in blood glucose management.27 Skin hyperpigmentation often decreases after phlebotomy. Many patients who report pretreatment fatigue indicate that this symptom improves with phlebotomy. Some patients report being energized by phlebotomy; some are reluctant to discontinue phlebotomy treatment after iron depletion is achieved.
A distinctive form of arthropathy occurs in some patients with hemochromatosis, especially women28 ; many other patients have age-related or other types of arthritis similar to those observed in persons without hemochromatosis.29 Consistent with previous reports,19,28,29 our informal observations suggest that arthralgias increase during phlebotomy therapy in some patients; some report decreased joint pain or swelling after iron depletion is achieved. Arthropathy in many cases appears to be unaffected by phlebotomy management. There are no data from prospective studies that would predict the effect of therapeutic phlebotomy on future arthritis outcomes.
Does phlebotomy treatment improve survival?
Studies from tertiary referral centers have documented that survival is significantly less in hemochromatosis patients with cirrhosis than in those without cirrhosis.30,31 Diabetes mellitus can also adversely influence survival in hemochromatosis patients, but most diabetic patients with hemochromatosis also have cirrhosis. The major goal of early diagnosis and iron depletion therapy is to prevent cirrhosis. Hepatocellular carcinoma occurs predominantly in hemochromatosis patients who have cirrhosis, and therefore prevention of cirrhosis would likely also reduce the risk of hepatocellular carcinoma. Patients with cirrhosis should undergo surveillance for hepatocellular carcinoma with abdominal ultrasonography every 6 months. Because an increase in the prevalence of colon and breast cancer has been described in C282Y homozygotes, patients should be encouraged to undergo colon and breast cancer screening as recommended for the general population.32
Survival benefits of iron depletion, if any, have been difficult to demonstrate because a small minority of persons with hemochromatosis have or will ever develop cirrhosis. Unphlebotomized patients with hemochromatosis have a normal lifespan when the selection criterion is determined by either HFE genotype or biochemical phenotype identified in population screening. There are no data that show definitively that phlebotomy improves survival in such subjects, and such data are unlikely to be obtained because of ethical issues.33 Large population-based studies of cardiac disease have not demonstrated a reduced survival in untreated hemochromatosis patients during follow-up intervals of 17 to 30 years.10,12 Patients discovered to have hemochromatosis in medical care because they have symptoms or biochemical manifestations attributable to iron overload may have greater risk to develop cirrhosis than those discovered in screening programs, but it is unlikely that a randomized clinical trial of phlebotomy therapy in such patients would be acceptable. Thus, observations gleaned largely from population studies in which C282Y homozygotes were identified long after initiation of the study do not imply that a referred symptomatic patient should not be offered phlebotomy therapy. For such patients, there is evidence that liver and cardiac function improve after iron depletion.24,34
Performing phlebotomy therapy
Patients undergo therapeutic phlebotomy in an ambulatory care facility while reclining or sitting; treatments are performed by nurses. One of us uses skilled nurses to oversee phlebotomy therapy; the other performs this management personally. One of us uses a 16-gauge straight needle and collection bag (Blood Pack MR6102; Baxter); the other uses a 19-gauge butterfly needle connected to an evacuated bottle via an extension set (Baxter 1A8503 and 1C8624, respectively). Blood (typically 500 mL) is removed during the course of 15 to 30 minutes. The size of the needle and the venipuncture site can be adjusted by an experienced phlebotomist, as appropriate. A hemoglobin concentration is measured at the time of each treatment. If the value is less than 11 g/dL, the treatment schedule is modified to 500 mL every other week; lower volumes (250-400 mL per session) are optimal for some persons of small body habitus or those whose hemoglobin levels are restored slowly after phlebotomy. Pretreatment hydration and the concomitant administration of a salt-containing sport beverage (eg, Gatorade) is a simple method of maintaining plasma volume during and after phlebotomy treatments.
Monitoring iron measures during phlebotomy therapy
Management of iron overload is determined by measurements of SF because these measures are directly related to the amount of stored iron. Accordingly, we quantify SF every month (or after every 4 phlebotomy sessions) in patients with hyperferritinemia undergoing therapy to induce iron depletion. After SF levels are less than 200 μg/L, we measure SF every 1 to 2 weeks (or after every 1 to 2 phlebotomy sessions).
Transferrin saturation is increased in most patients with hemochromatosis who have hyperferritinemia as the result of iron overload, although day-to-day values fluctuate in the same person. Plasma iron species unassociated with ferritin or transferrin occur in some persons with hemochromatosis, typically when transferrin saturation is increased. These include nontransferrin-bound iron (NTBI) and labile plasma iron (LPI). Both may promote tissue overload and consequent damage.35-39 NTBI and LPI are mainly found when transferrin saturation exceeds 45% and 75%, respectively,39 although NTBI persists in some subjects with low serum iron level and high serum iron-binding capacity.40 Thus, measurement of transferrin saturation during phlebotomy to achieve iron depletion would provide surrogate qualitative assessments of NTBI and LPI. Regardless, no bioclinical correlate of either NTBI or LPI has been reported in HFE C282Y homozygotes, nor is there evidence-based management to reduce NTBI and LPI other than iron depletion.
End points of phlebotomy therapy
There are different ways to follow the progress of phlebotomy therapy. At our centers, patients are treated until the SF is approximately 50 μg/L, a value in the lower reference range that signifies that there is little or no storage iron. Lynch et al41 measured iron absorption from test meals in hemochromatosis patients who were untreated (SF > 1000 μg/L), partially treated with phlebotomy (mean SF 538 μg/L; range, 210-786 μg/L), and “fully” treated by phlebotomy (mean SF 14 μg/L; range, 10-21μg/L).41 Nonheme iron absorption was 9%, 12%, and 42% in these respective groups, whereas heme iron absorption was 41%, 50%, and 39%.
These results demonstrate that nonheme iron absorption is markedly increased by phlebotomy that results in SF of approximately 20 μg/L and could lead to unnecessary induction and maintenance phlebotomy. The absorption of heme iron is relatively high but stable, regardless of the magnitude of iron stores. Accordingly, the approximately 50 μg/L SF criterion enhances absorption of nonheme iron less than would occur at SF levels of approximately 20 μg/L and allows some “room” for iron reaccumulation into the mid- or upper reference range. The decrease in SF levels with phlebotomy is infrequently linear; the rate of iron mobilization may be greater in patients with cirrhosis.42 There are significant positive correlations between the amount of blood removed by phlebotomy to reach iron depletion and either the initial SF level, hepatic iron concentration measured in liver biopsy specimens, or liver iron levels estimated by the use of magnetic resonance imaging configured to measure liver iron.43,44
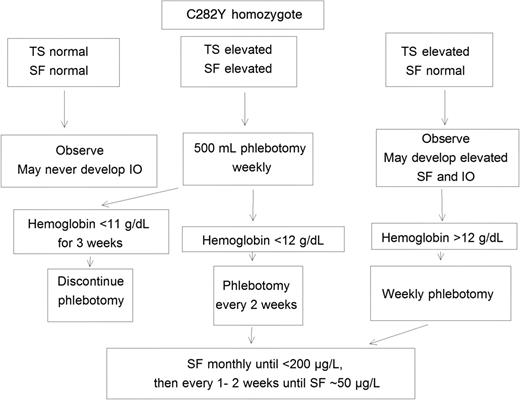
Measuring SF is the preferred means to monitor the progress of therapeutic phlebotomy to induce iron depletion. We discontinue phlebotomy therapy in most patients when their SF level is in the lower reference range (∼ 50 μg/L; Figure 1). Some patient support groups have advocated more intensive phlebotomy therapy to achieve and maintain much lower SF levels, but some patients so treated develop fatigue and other symptoms because they develop iron deficiency. Transferrin saturation, often elevated in hemochromatosis, usually decreases much less rapidly in response to phlebotomy therapy than the SF level. Transferrin saturation may remain increased when body iron stores and SF values are subnormal; phlebotomy that reduces transferrin saturation to low normal values may result in iron deficiency.45
A clinical guide to phlebotomy management of HFE C282Y homozygotes. IO indicates iron overload; SF, serum ferritin; and TS, transferrin saturation.
A clinical guide to phlebotomy management of HFE C282Y homozygotes. IO indicates iron overload; SF, serum ferritin; and TS, transferrin saturation.
Thus, we recommend that SF, not transferrin saturation, be used as the indicator for cessation of phlebotomy therapy. An alternative approach is to defer weekly phlebotomy in subjects without chronic anemia if hemoglobin is less than 12 g/dL and to discontinue weekly phlebotomy to induce iron depletion if hemoglobin is less than 11 g/dL for more than 3 consecutive weeks (Figure 1).9,46,47 Another approach is to discontinue phlebotomy to induce iron depletion when the mean corpuscular volume is 3% or greater below pretreatment baseline.48 Patients are usually more insistent on maintaining low SF levels than physicians. There is little clinical evidence to suggest that a hemochromatosis patient with a SF level in the middle or high reference range experiences subacute iron toxicity or organ dysfunction. The value of maintenance phlebotomy therapy to keep iron stores within the reference range has not been clearly established. We advise patients who had symptoms or signs reasonably attributable to iron overload at diagnosis that were alleviated by iron depletion and those with SF 1000 μg/L or greater at diagnosis to maintain low iron stores with periodic phlebotomy. Reports of achieving and maintaining low iron stores in hemochromatosis patients who also have common liver disorders such as nonalcoholic steatosis or hepatitis C reveal inconsistent results.49,50
Iron accumulation rates vary greatly among C282Y homozygotes; this variability persists after iron depletion. Women are less likely than men to reaccumulate iron rapidly. Some patients with hemochromatosis monitored as long as 11 years after initial iron depletion and not treated with maintenance therapy did not reaccumulate iron.51 We advise that clinicians measure SF 6 months after cessation of therapy to estimate the risk of iron reaccumulation and to personalize maintenance therapy based on these observations.
Intolerance of phlebotomy
Decreasing the phlebotomy interval to every 2 weeks is necessary in some patients, especially women.9 In patients who report hypotension or dizziness, the volume of oral salt drinks can be increased; rarely, an intravenous saline solution must be infused to restore blood volume after phlebotomy. The volume of blood removed can be decreased, but this prolongs the overall duration of therapy to achieve iron depletion. Phlebitis can be treated by warm compresses, nonsteroidal anti-inflammatory drugs, and rotating venipuncture sites. The use of indwelling catheters entails greater risks. Inadequate venous access usually requires a change in the management plan. No phlebotomy therapy may be acceptable for an intolerant patient with SF less than 1000 μg/L with no organ dysfunction. The development of anemia after few phlebotomies may indicate that the increase in SF level was not the result of iron overload. The evidence for iron overload (eg, liver biopsy, magnetic resonance imaging) should be reviewed before further phlebotomy is performed. Anecdotal reports suggest that hypotestosteronemia, erythropoietin deficiency, or the commitment of time required to undergo phlebotomy therapy may account for anemia or fatigue in some cases.52,53
Erythrocytapheresis
Therapeutic erythrocytapheresis (TE), like therapeutic phlebotomy, removes iron predominantly as hemoglobin (Table 1). Like patients treated with phlebotomy, those managed with TE must have no or mild anemia and a rate of effective erythropoiesis that is sufficient to replace erythrocyte losses rapidly. Isovolemic, large-volume erythrocytapheresis removes more blood erythrocytes per session than phlebotomy while sparing plasma proteins, coagulation factors, and platelets. In small case series, erythrocytapheresis reduced iron measures in hemochromatosis patients with severe iron overload, intolerance of phlebotomy, or coinheritance of β-thalassemia.54 Erythropoietin administration may facilitate such treatment in some cases.55
Performing TE requires the apheresis apparatus itself, a skilled technician, careful patient selection, and venipuncture with large-bore needles to accommodate the flow and pressure requirements of apheresis machines. Patients with hemochromatosis treated with erythrocytapheresis to achieve iron depletion should have a preprocedure hemoglobin measurement of 12 g/dL or greater. The volume of erythrocytes removed at each treatment should be adjusted on the basis of sex, height, weight, and pretreatment hematocrit, such that the minimal postprocedure hematocrit is 30%; patients with postprocedure hypovolemia benefit from infusion of normal saline. Additional procedures are performed every 2 weeks in persons whose hemoglobin level has returned to 12 g/dL or more; an interval of 3 weeks between procedures is required in some patients, especially women. SF is monitored in the same manner as previously described for therapeutic phlebotomy.54,56,57
TE achieves iron depletion in persons with hemochromatosis in a shorter treatment period than therapeutic phlebotomy.56 In a pilot study of 6 hemochromatosis patients treated with TE, the total number and the duration of treatments in the TE group was approximately 70% lower than that in historical control subjects managed with therapeutic phlebotomy. Although the costs of a single TE session were greater, the total costs to induce iron depletion were similar to or lower than those with therapeutic phlebotomy.56 TE appears to be safe and rapid, and its use may achieve iron depletion in patients with severe iron overload more rapidly than phlebotomy therapy. Regardless, the availability of and clinical experience with TE for management of iron overload caused by hemochromatosis are limited. The present authors have not treated patients with hemochromatosis by using TE.
Medications
Iron chelation drugs
Deferoxamine (DFO), a parenterally administered iron chelator, induced iron depletion in patients with hemochromatosis who were unable to undergo phlebotomy therapy.58 In short-term studies, treatment with DFO was as effective as phlebotomy of 500 mL weekly in removing iron from the liver.58 Much experience with the poor compliance and acceptability of DFO therapy in patients with nonhemochromatosis iron overload suggests that DFO treatment would not be generally satisfactory for persons with hemochromatosis (Table 1).
Deferasirox (DFX), an oral iron chelator, was administered daily for 24 weeks to hemochromatosis patients with HFE C282Y homozygosity, pretreatment SF 300 to 2000 μg/L, transferrin saturation greater than 45%, and no history of cirrhosis in a phase 1/2 study (Table 1); one of us was an investigator in this clinical trial.59 The primary end point was the incidence and severity of adverse events; secondary end points were change in SF, time to SF normalization (< 100 μg/L), longitudinal course of SF, and pharmacokinetics of DFX. Adverse events, documented in less than 10% of patients, included diarrhea, nausea, abdominal pain, and increased serum ALT or creatinine levels. These events were dose related and resolved with dose reduction or cessation of DFX therapy; 77% of patients who were treated initially completed the study. Time course of the decline in SF was dose dependent. Only the 5- and 10-mg/kg/d doses are being considered for further study in HFE hemochromatosis, largely because of the rate of adverse events at greater doses.59
There are no reports of the treatment of HFE hemochromatosis with the oral iron chelator deferiprone (DFP). The combination of DFO and DFP have been used to treat severe iron overload complicated by cardiomyopathy in a patient with a juvenile hemochromatosis phenotype.60 DFP is not licensed for use in the United States.
Phase 3 trials to compare phlebotomy with oral chelation therapy should be conducted but may need to await a means to detect which C282Y homozygotes will develop progressive, injurious iron overload and therefore potentially benefit from treatment. Clinical trials must be designed to determine whether oral chelation therapy can eliminate hepatic iron deposits without causing additional liver injury and whether hepatic fibrosis and cirrhosis caused by iron overload can be reversed.61 Treatment schedules for maintaining low body iron stores after iron depletion has been achieved must also be established.61
Proton pump inhibitors
Proton pump inhibitors (PPIs) inhibited the absorption of nonheme iron from a test meal and the habitual diet in hemochromatosis patients with HFE C282Y homozygosity diagnosed in medical care.62 Achlorhydria or drugs that decrease gastric acid secretion diminish absorption of inorganic iron by reducing its solubility. In addition, iron uptake into enterocytes mediated by intestinal divalent metal transporter-1 requires proton cotransport; the protons are presumably derived from gastric acid that enters the duodenum.63,64 These observations suggest that chronic use of PPIs by C282Y homozygotes could either diminish the severity of iron overload that would otherwise occur as the result of excess absorption of inorganic iron from dietary sources or reduce requirements for maintenance phlebotomy. Regardless, there are insufficient data to recommend the routine administration of proton-pump inhibitors to patients with hemochromatosis for the purpose of decreasing iron absorption. Histamine receptor-2 antagonists reduced dietary iron absorption in short-term studies of persons without hemochromatosis,65 but continuous use of cimetidine for 3 years did not alter hemoglobin or plasma iron levels significantly.66 The greater influence of PPIs than histamine receptor-2 antagonists on SF levels could be explained by the direct inhibition of gastric acid secretion and longer duration of action characteristic of PPIs.
Dietary management
Evidence-based dietary advice for patients with hemochromatosis could diminish the rate of increase in iron stores and decrease the expressivity of iron overload caused by hemochromatosis in populations. The rate of compliance with recommended changes in diet in Australian HFE C282Y homozygotes detected in workplace screening was high.67 Our dietary management recommendations are displayed in Table 2.
Dietary management recommendations for persons with hemochromatosis
| Recommendation . |
|---|
| Avoid supplemental iron. |
| Consume red meats in moderation. |
| Consume ethanol in moderation.* |
| Limit supplemental vitamin C to 500 mg daily. |
| Use mineral supplements for specific deficiencies only.† |
| Do not consume raw shellfish. |
| Do not consume cooked seafood contaminated with seawater drippings. |
| Avoid contact of seawater with cuts or other open skin lesions. |
| Recommendation . |
|---|
| Avoid supplemental iron. |
| Consume red meats in moderation. |
| Consume ethanol in moderation.* |
| Limit supplemental vitamin C to 500 mg daily. |
| Use mineral supplements for specific deficiencies only.† |
| Do not consume raw shellfish. |
| Do not consume cooked seafood contaminated with seawater drippings. |
| Avoid contact of seawater with cuts or other open skin lesions. |
This recommendation applies to persons without liver abnormalities. Persons with evidence of liver injury such as elevated serum concentrations of hepatic enzymes or hepatomegaly should consume little or no ethanol. Persons with cirrhosis should abstain from consuming ethanol.
Nonferrous metals including cobalt, zinc, manganese, and chromium share absorptive pathways with iron; excess zinc and manganese are retained in the liver.
Iron
Absorption of heme and nonheme iron from unfortified test meals is far greater in hemochromatosis patients diagnosed in medical care than would be predicted from the relationship between iron absorption and SF levels observed in normal volunteers.41,68 Further, there is a significant inverse relationship of absorption of nonheme iron with increasing SF levels. In contrast, heme iron absorption was not significantly related to SF levels.41 In women in the United Kingdom, HFE C282Y homozygotes had SF concentrations 2.4 times greater than women with wild-type HFE genotypes. The association of heme iron intake with SF levels estimated by the use of food frequency questionnaires (from meat, fish, and poultry) was 2.0 times greater (range, 1.2-3.2) in C282Y homozygotes than subjects with other HFE genotypes; estimated nonheme iron intake had little effect on SF levels.69,70
In women in The Netherlands older than 50 years of age, C282Y homozygotes (and C282Y/H63D compound heterozygotes) had significantly greater SF concentrations than women with other HFE genotypes. C282Y homozygotes and C282Y/H63D compound heterozygotes who consumed relatively high amounts of heme iron had the greatest SF concentrations.71 It has been proposed that the high rate of expression of iron overload and associated manifestations in hemochromatosis homozygotes in Australia is caused by the high national rate of meat consumption.72 Red meat consumption was positively associated with greater SF levels in adults in Busselton, Australia, regardless of HFE genotype.73,74 Altogether, some persons with hemochromatosis may be especially susceptible to iron loading from diets in which a high proportion of available iron is present as heme, although they may also absorb increased fractions of nonheme iron.
Fortification of food with inorganic iron may increase the severity of iron overload in persons with hemochromatosis. In 7 treated hemochromatosis patients (SF < 33 μg/L), the absorption of nonheme iron from a test meal was measured by the use of the extrinsic tag technique to simulate the effects of fortification.75 Doubling of the iron dose produced a 43% increase in mean absorbed iron from 1.6 mg to 2.2 mg, a proportional increase similar to that in normal subjects.76 In Sweden, fortification before 1995 accounted for 42% of the mean daily dietary iron content, although Swedish hemochromatosis homozygotes had lower iron burdens, on average, than those in Australia.77 Decreased fortification in Sweden since 1995 is expected to decrease the rate of iron accumulation in persons with hemochromatosis.77 It is likewise predicted that iron fortification of wheat flour at the current US levels would accelerate the initial rate of iron loading in persons with hemochromatosis from 1.7 to 2.1 g; if the same calculations were determined by untreated hemochromatosis patients with SF 1000 μg/L or greater, the predicted difference in absorption between unfortified and fortified diets decreases to approximately 100 mg annually.
These estimates agree with earlier predictions that the greatest impact of iron fortification is at the early stages of the condition and that the accelerated evolution of clinical disease is directly proportional to the amount of fortification iron added.75,78 If dietary iron intake were increased by 20% with fortification, the predicted time required to develop clinical manifestations is likewise shortened by 20%. Another predicted influence of iron fortification is its effect on the frequency of maintenance phlebotomies required to maintain low iron reserves in fully treated patients. With a typical phlebotomy rate of 4 to 6 times annually, a 20% increase in dietary iron by fortification of wheat flour would necessitate 1 to 2 additional bleedings per year.77,79
Some HFE C282Y homozygotes absorb supplemental nonheme iron readily. The hemoglobin levels of C282Y homozygotes with iron-deficiency anemia who took ferrous sulfate daily (325 mg) returned to normal within 2 to 6 weeks.45 A man who took daily ferrous sulfate for beta-thalassemia minor (153 g of Fe over 7 years) was subsequently diagnosed to have hemochromatosis, C282Y homozygosity, and diabetes mellitus; 32 g of iron was removed by phlebotomy to achieve iron depletion.80 Urinary hepcidin levels did not increase in C282Y homozygotes given a single 65 mg of iron dose as ferrous sulfate, regardless of their state of iron depletion by therapeutic phlebotomy.81 Although 27% of persons diagnosed to have hemochromatosis reported that they had previously taken supplements that contained iron,19 the effect of supplemental iron taken before diagnosis of hemochromatosis on the expression of iron overload in large populations of C282Y homozygotes is unknown.
Noniron diet items
Alcohol loading down-regulates hepatic hepcidin expression and increases iron absorption in humans and mice without hemochromatosis.82,83 Mice of alcohol-susceptible strains that were also homozygous for a knockout of Hfe (orthologue of human HFE gene) had reduced iron absorption and serum hepcidin levels in response to alcohol treatment.84 This finding indicates that either maximal suppression of hepcidin levels had already occurred as a result of the Hfe knockout or that Hfe protein was a component of the pathway used by ethanol in suppressing hepcidin production and increasing iron absorption.84 Direct measures of stored iron were similar in 2 groups of persons with hemochromatosis: those who consumed more than 100 g of alcohol daily and nondrinkers or moderate drinkers who consumed less than 100 g of alcohol daily; complications of alcoholism were common in the first group.85 If dietary iron intake differs between persons with hemochromatosis who are or are not heavy drinkers, such differences would appear to have little effect on iron phenotypes. Altogether, it appears that the most likely reason why heavy alcohol consumption accentuates the expression of hemochromatosis is the additive cofactor effect of iron and alcohol, both of which cause oxidative stress, hepatic stellate cell activation, and hepatic fibrogenesis.84
Black tea inhibits the absorption of nonheme iron, presumably the result of the intraluminal binding of iron by tannate. In 18 German patients with hemochromatosis, iron absorption was significantly lower when a test meal was accompanied by drinks of tea instead of water. After iron depletion was achieved by phlebotomy in the same patients, regular tea drinking with meals reduced the frequency of maintenance phlebotomies required during an interval of one year.86 In Australian subjects, reports of tea consumption on a food questionnaire were not significantly associated with SF concentrations, regardless of HFE genotype.73
Reports of greater levels of noncitrus fresh fruit intake were associated with significantly lower SF levels in men in Busselton, Australia, irrespective of HFE genotype. Mean SF levels were lower by 20% in men who consumed 2 or more pieces of fruit per day, on average, than in those whose average consumption was less than one piece of fruit daily. This effect was not observed in women. Consumption of citrus fruits and citrus juices had no significant effects in either sex.73 We advise patients to limit their intakes of supplemental vitamin C to 500 mg daily.9
Some patients with hemochromatosis with or without cirrhosis have developed infection with Vibrio vulnificus, a free-living Gram-negative bacillus that grows as normal marine flora and is distributed world-wide in warm, coastal waters.87 In the United States, most infections have occurred in regions adjacent to the Gulf of Mexico; some infections are fatal. Abundance of iron in blood and tissues and decreased plasma hepcidin in hemochromatosis may increase risk for infection. In persons with hemochromatosis, V vulnificus has been reported to cause primary septicemia as the result of ingestion of uncooked oysters or other raw shellfish (or of cooked food contaminated with seawater drippings) and wound infections caused by contact of superficial wounds with seawater that contains V vulnificus. Thus, physicians who treat patients with hemochromatosis or iron overload should educate their patients about V vulnificus infections. Like expert hemochromatosis management groups, we recommend that patients diagnosed to have hemochromatosis or iron overload not consume raw shellfish, regardless of iron overload status.52 It is also prudent that persons diagnosed to have hemochromatosis or iron overload prevent contact of wounds with seawater, and avoid direct handling of uncooked saltwater finfish or shellfish.87 Thoroughly cooked seafood uncontaminated with seawater drippings does not contain viable V vulnificus, and is thus safe for consumption.
Hemochromatosis patients as voluntary blood donors
Many persons with hemochromatosis donate blood or qualify as blood donors,88-90 and blood removed for therapeutic phlebotomy can be used as a means of augmenting the blood supply for transfusion.91,92 In August 2001, the US Food and Drug Administration (in a publication that included the US Department of Health and Human Services and the Center for Biologics Evaluation and Research) issued a policy of variances for collecting blood from hemochromatosis donors that accomplished at least 4 major aims: (1) the policy allowed the transfusion of blood from patients with hemochromatosis if the blood met all known safety standards; (2) the policy eliminated financial incentives for hemochromatosis patients to falsify responses to blood bank questions about health status and about possible behaviors that increased the risk of transmissible infections; (3) blood banks that accepted the blood for transfusion from any hemochromatosis patient were required to provide phlebotomy therapy without charge to all hemochromatosis patients who came to the blood bank; and (4) the policy authorized a blood bank to perform phlebotomy therapy according to the prescribing physician's written orders about frequency and hematocrit/hemoglobin limits (Title 21, Code of Federal Regulations, section 640.120 [21 CFR 640.120]).
In practice, some US blood banks obtained the variances, are involved in therapy of patients with hemochromatosis, and report informally that such patients supplement their blood supplies. Most patients treated at blood banks must undergo SF and other evaluations at the offices of their physicians. We have also encountered patients who donated blood for transfusion without revealing their diagnosis of hemochromatosis and without obtaining SF measurements from their physicians, presumably due to financial considerations. Some have had symptomatic iron deficiency with anemia; others had uncontrolled iron overload. Many US blood banks have declined to obtain the variances and prefer to obtain blood units solely from volunteer donors.
It has been postulated that the use of hemochromatosis blood could augment the national blood supply. Two ethical issues germane to this postulate are that no person with hemochromatosis has the right to insist that his/her blood be used for transfusion and that hemochromatosis patients are not obligated to donate therapeutic phlebotomy blood for transfusion.93 The results of a study at the National Institutes of Health demonstrated that recruitment of healthy hemochromatosis donors augmented the intramural blood supply significantly.91 In a questionnaire survey of donors from 8 US blood centers, 0.8% of the 52 650 responding donors reported that they had hemochromatosis.94 In 16 blood centers that did not recruit hemochromatosis patients, only 0.4% of 1.67 million units of red blood cells were donated by hemochromatosis patients.95 Both of us refer patients with hemochromatosis to blood centers for therapeutic phlebotomy, as appropriate.
In Canada, blood from hemochromatosis patients whose blood meets other safety criteria has been used for transfusion since 1991.89 In Wales and Sweden, hemochromatosis patients whose blood meets safety standards is used for transfusion. Because these patients had iron overload, they are allowed to donate blood more frequently than other volunteer donors.96,97 In France, members of a working group recommended continuation of the policy not to allow blood donation by hemochromatosis patients who had an increased SF concentration or evidence of iron-associated organ injury or illness.98 In 2000, investigators in New Zealand recommended that a national blood banking policy be developed to allow transfusion of the blood that is donated by healthy hemochromatosis patients if the blood meets all safety standards.99
Summary
For most hemochromatosis patients, standard therapy is the weekly removal of blood to bring the SF level into the low reference range. The major medical benefit of this therapy is the prevention or reversal of liver fibrosis; some patients report decreased dyspnea, pigmentation, fatigue, or arthralgia, or improved control of diabetes mellitus. The natural history of untreated hemochromatosis has demonstrated that some patients do not have progressive iron overload. Iron chelation therapy is rarely ideal for hemochromatosis patients because of cost, potential toxicity, and relative lack of documentation of benefits. Dietary restrictions and medications to reduce iron absorption seem rational but have not been evaluated in prospective randomized clinical trials. Future studies that would enable us to predict the progression of iron overload in an individual C282Y homozygote could lead to personalized therapeutic approaches.
Authorship
Contribution: P.A. and J.B. designed and wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Paul C. Adams, University Hospital, 339 Windermere Rd, London, ON, Canada N6A 5A5; e-mail: padams@uwo.ca.