To the editor:
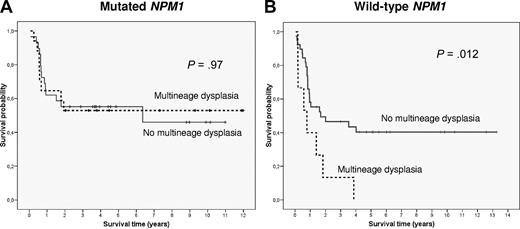
The prognostic significance of multilineage dysplasia (MLD) in acute myeloid leukemia patients with intermediate-risk cytogenetics (IR-AML) presenting as de novo disease is unclear.1-4 Falini et al have recently analyzed the biologic and prognostic significance of MLD in IR-AML and did not find any impact of MLD on survival in patients harboring NPM1 mutations.5 Moreover, in a subgroup of IR-AML patients with wild-type NPM1 from one of the participating institutions (Munich Leukemia Laboratory), no difference in outcome according to the presence of dysplastic features was observed. To clarify the prognostic significance of MLD in this cytogenetic category, we analyzed a cohort of 130 patients (51% female; median age, 53 years, range, 18-74 years) diagnosed consecutively with de novo IR-AML in our institution from 1994 to March 2010 and treated with intensive chemotherapy. Evaluation of dysplasia was performed by 2 independent observers (M.R., J.Ll.A.) according to the WHO criteria.2 Multilineage dysplasia was detected in 32 cases (25%), a frequency similar to that reported by Falini, and was associated with a higher proportion of normal karyotype (93% vs 60%; P < .001), lower leukocyte count at diagnosis (32 × 109/L vs 69 × 109/L; P = .01), and lower bone marrow infiltration (51% vs 72% blast cells, P < .001). Interestingly, the frequency of NPM1 and FLT3 internal tandem duplication (FLT3-ITD) mutations did not differ between patients with and without MLD (59% vs 50%, and 31% vs 38%, respectively). NPM1 mutations were found in 68 patients (52%). MLD was observed in 19 patients (28%) with mutated NPM1 and in 13 (21%) with wild-type NPM1. Outcomes in patients with mutated NPM1 were similar for those with and without MLD; response rate was 95% and 85%, 5-year relapse incidence was 35% ± 26% and 47% ± 16%, and 5-year survival was 56% ± 23% and 46% ± 14%, respectively. In contrast in patients with wild-type NPM1, those patients with MLD showed an inferior response rate to induction chemotherapy (53% vs 85%; P = .02). When the analysis was restricted to younger patients (≤ 60 years) those with MLD showed a lower 5-year survival (0% vs 40% ± 16%, P = .012; Figure 1). The unfavorable prognostic value of MLD on response rate (P = .034; relative risk, 4.8; 95% confidence interval, 1.1-20) and survival (P = .036; hazard ratio = 2.5; 95% confidence interval, 1.1-6) was confirmed in a multivariate analysis.
Survival curves of patients up to 60 years with intermediate-risk cytogenetics AML depending on NPM1 status and presence of multilineage dysplastic features (MLD). (A) In NPM1-mutated AML no significant differences in OS were observed between cases with (discontinuous line) and without (continuous line) MLD (P = .97). (B) On the contrary, MLD identified a subgroup of patients with an adverse outcome among wild-type NPM1 IR-AML (P = .012).
Survival curves of patients up to 60 years with intermediate-risk cytogenetics AML depending on NPM1 status and presence of multilineage dysplastic features (MLD). (A) In NPM1-mutated AML no significant differences in OS were observed between cases with (discontinuous line) and without (continuous line) MLD (P = .97). (B) On the contrary, MLD identified a subgroup of patients with an adverse outcome among wild-type NPM1 IR-AML (P = .012).
These results confirm that, although dysplastic features are a common trait in NPM1-mutated AML, they do not confer a worse prognosis. Falini et al found that gene expression profiling did not identify any distinctive MLD-associated gene signature in the mutated NPM1 cohort.6 The correlation found in the present study between an unfavorable outcome and dysplastic features in wild-type NPM1 IR-AML patients leads us to suggest that a search for novel genetic or epigenetic markers in this AML subgroup might reveal a specific biologic identity.
In conclusion, the prognostic relevance of MLD in IR-AML might be dependent on NPM1 mutational status. Whereas MLD predicts an adverse outcome in patients with wild-type NPM1, it lacks prognostic value in NPM1-mutated AML. Nonetheless, this observation requires further confirmation in a larger series of patients.
Authorship
Acknowledgments: This work has been partially supported by grants FIS03/0423, PI080158, and RD06/0020/0004 from “Instituto de Salud Carlos III” (ISCIII), Spanish Ministry of Health, Spain.
Contribution: M.D.-B. updated the database of patients included in the analysis, performed all statistical analysis, and wrote the manuscript; M.R. performed morphologic review of all cases and reviewed the article; M.P. updated the database, performed molecular analysis, and reviewed the article; M.T. and M.C. performed molecular analysis and reviewed the article; J.Ll.A. performed morphologic review of all cases and reviewed the article; and J.E. designed the study, supervised statistical analysis, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Jordi Esteve, Hospital Clinic of Barcelona, Villarroel 170, Barcelona 08036, Spain; e-mail: jesteve@clinic.ub.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal