Abstract
Neutrophils play a key role in host defense by releasing reactive oxygen species (ROS). However, excessive ROS production by neutrophil nicotinamide adenine dinucleotide phosphate (NADPH) oxidase can damage bystander tissues, thereby contributing to inflammatory diseases. Tumor necrosis factor-α (TNF-α), a major mediator of inflammation, does not activate NADPH oxidase but induces a state of hyperresponsiveness to subsequent stimuli, an action known as priming. The molecular mechanisms by which TNF-α primes the NADPH oxidase are unknown. Here we show that Pin1, a unique cis-trans prolyl isomerase, is a previously unrecognized regulator of TNF-α–induced NADPH oxidase hyperactivation. We first showed that Pin1 is expressed in neutrophil cytosol and that its activity is markedly enhanced by TNF-α. Inhibition of Pin1 activity with juglone or with a specific peptide inhibitor abrogated TNF-α–induced priming of neutrophil ROS production induced by N-formyl-methionyl-leucyl-phenylalanine peptide (fMLF). TNF-α enhanced fMLF-induced Pin1 and p47phox translocation to the membranes and juglone inhibited this process. Pin1 binds to p47phox via phosphorylated Ser345, thereby inducing conformational changes that facilitate p47phox phosphorylation on other sites by protein kinase C. These findings indicate that Pin1 is critical for TNF-α–induced priming of NADPH oxidase and for excessive ROS production. Pin1 inhibition could potentially represent a novel anti-inflammatory strategy.
Introduction
Neutrophils play an important role in host defense against invading pathogens and in inflammation. In response to stimulating agents, such as the bacterial peptide N-formyl-methionyl-leucyl-phenylalanine (fMLF), neutrophils release large amounts of superoxide anions and other reactive oxygen species (ROS) in a phenomenon called the respiratory burst. ROS produced by the neutrophil nicotinamide adenine dinucleotide phosphate (NADPH) oxidase play a key role in host defenses,1-3 but excessive ROS production can damage healthy bystander tissues, thereby contributing to inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel diseases, and acute respiratory distress syndrome.4,5
Neutrophil ROS production is mediated by the phagocyte NADPH oxidase, also called NOX2. NADPH oxidase is a multicomponent enzyme system that catalyzes NADPH-dependent reduction of oxygen to superoxide anion.6,7 In resting cells, the NADPH oxidase is inactive and its components are distributed between the cytosol and membranes. When cells are activated, the cytosolic components (p47phox, p67phox, p40phox, and Rac2) migrate to the membranes, where they associate with the membrane-bound components (p22phox and gp91phox/NOX2, which form the flavocytochrome b558) to assemble the catalytically active oxidase.7,8 During NADPH oxidase activation, p47phox, p67phox, p40phox, p22phox, and gp91phox/NOX2 become phosphorylated.9-13 p47phox phosphorylation on several serines plays a pivotal role in oxidase activation in intact cells.14,15
Neutrophil ROS production is enhanced or primed by a variety of mediators, including proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α). In vitro, TNF-α induces a very weak oxidative response by neutrophils but strongly enhances ROS release on exposure to a secondary stimulus, such as the bacterial peptide fMLF.16-18 This “priming” of neutrophil ROS production plays a detrimental role in a variety of human inflammatory diseases, in which ROS hyperproduction by primed neutrophils is thought to cause direct tissue insult.18-20 The molecular mechanisms by which TNF-α primes the NADPH oxidase are poorly understood. We have previously shown that phosphorylation of the NADPH oxidase cytosolic subunit p47phox by p38MAPKinase on Ser345 is a key event in TNF-α–induced priming of ROS production by neutrophils, as TNF-α–induced priming is abrogated by Ser345 mutagenesis and by a competitive inhibitory peptide containing the Ser345 sequence.21 Precisely how this phosphorylation potentiates NADPH oxidase activation and ROS production is unknown, and the factor(s) linking p47phox Ser345 phosphorylation to the NADPH oxidase hyperactivation remain(s) to be identified. As phospho-Ser345 is located in a proline-rich region (—PX-phosphoSP—) that can exist in the cis or trans conformation, we suspected a role of Pin1, a unique prolyl isomerase that specifically recognizes phosphorylated serine or threonine residues located immediately N-terminal to a proline, and then isomerizes the peptide bond.22,23 Phosphorylated Ser or Thr adjacent to proline cannot be isomerized by other peptidyl-prolyl isomerase, such as cyclophilin A and FK506 binding protein.
Pin1-dependent isomerization can modulate enzyme activity and protein phosphorylation/dephosphorylation and induce protein degradation.24,25 Pin1 plays important roles in several diseases, including cancer26 and Alzheimer disease.27 Pin1 has been implicated in the control of cytokine expression by activated eosinophils and T lymphocytes.28,29 Very recently, Pin1 overexpression was described in lymphocytes, chondrocytes, and fibroblasts from arthritic mice.30 However, Pin1 expression in neutrophils and its possible role in regulating NADPH oxidase activation and ROS production at inflammatory sites have not been studied. The aim of this study was to investigate the role of Pin1 in the process leading to TNF-α–induced priming of the phagocyte NADPH oxidase and to ROS overproduction by neutrophils. We show that TNF-α induces both Pin1 activation and its colocalization with p47phox in neutrophils. Pin1 inhibitors abrogated TNF-α–induced priming of neutrophil ROS production. In TNF-α–primed neutrophils, Pin1 binds to p47phox via phosphorylated Ser345, thereby inducing conformational changes that facilitate p47phox phosphorylation on other sites by protein kinase C (PKC), resulting in NADPH oxidase hyperactivation. Thus, Pin1-catalyzed prolyl isomerization is a novel mechanism regulating TNF-α–induced neutrophil NADPH oxidase priming.
Methods
Reagents and antibodies
Recombinant human TNF-α was from PeproTech. fMLF, phorbol 12-myristate 13-acetate (PMA), protease, and phosphatase inhibitors were from Sigma-Aldrich. Endotoxin-free buffers and salt solutions were from Invitrogen. The rabbit polyclonal antibodies against phospho-Ser345-p47phox and p47phox have been described elsewhere.21 Anti-Pin1 was from Santa Cruz Biotechnology. To raise antibodies against phospho-Ser315, phospho-Ser320, and phospho-Ser328, rabbits were injected with the ovalbumin-crosslinked phosphopeptide sequences of these serines by PolyPeptide Laboratories. The Pin1-peptide inhibitor sequence (Ac-Phe-D-Thr(PO3H2)-Pip-Nal-Gln-NH2)31 was synthesized by PolyPeptide Laboratories.
Ethics statement
Neutrophils were isolated from venous blood of healthy volunteers with their written informed consent in accordance with the Declaration of Helsinki. All experiments were approved by the Inserm Institutional Review Board and ethics committee. Data collection and analyses were performed anonymously.
Neutrophil preparation
Circulating neutrophils were isolated by Polymorphprep gradient centrifugation.21,32 Briefly, blood was diluted 2-fold in sterile phosphate-buffered saline and cells were isolated by one-step Polymorphoprep gradient centrifugation at 500g for 30 minutes at 22°C. The neutrophil band was collected, and the cells were washed in phosphate-buffered saline and counted.
Neutrophil fractionation
Measurement of ROS production by luminol-amplified chemiluminescence
Neutrophils (5 × 105) were suspended in 0.5 mL of Hanks balanced salt solution containing 10μM luminol at 37°C with or without 250nM juglone or Pin1-peptide inhibitor (PPIn; 50μM)31 for 30 minutes. TNF-α (20 ng/mL) was added for 20 minutes; then the cells were stimulated with 10−7M fMLF. Chemiluminescence was recorded with a luminometer (Berthold-Biolumat LB937).
Bacterial expression of p47phox and Pin1
GST-Pin1 and GST-p47phox were expressed in Escherichia coli (BL21) grown at 37°C and then induced for 3 hours with 0.1mM or 0.2mM isopropylthiogalactoside at 37°C. Cells were harvested by centrifugation (4000g, 20 minutes, 4°C), and the pellet was resuspended in lysis buffer (50mM Tris-HCl, pH 7.5, 50mM NaCl, 5mM MgCl2, 1mM dithiothreitol [DTT], 1% [vol/vol] Triton X-100, and protease inhibitors). Cells were lysed by 6 sonications, each lasting 30 seconds. After centrifugation (3200g, 20 minutes, 4°C), GST-recombinant proteins were purified with glutathione-Sepharose 4B beads (Pharmacia) and recombinant proteins (p47phox and Pin1) were prepared by GST cleavage.
Pin1 activity assay
Pin1 activity was measured using a previously described technique, with some modifications.22,34 Briefly, neutrophils were lysed by sonication, twice for 10 seconds at 4°C, in lysis buffer containing 50mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 100mM NaCl, 0.25% 3-cholamidopropyl)dimethylammonio-1-propanesulfonate, 5mM NaF, 1mM β-glycerophophate, and 1mM ethyleneglycoltetraacetic acid. The assay mixture consisted of 93 μL of N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer (50mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.8, 100mM NaCl, 2mM DTT, 0.04 mg/mL bovine serum albumin), 5 μL of cell lysate (105 cells; or 0.25 nmol of recombinant Pin1) and 2 μL (20 mg/mL) of trypsin solution (Sigma-Aldrich). The reaction was started by adding 50 μL (720μM) of peptide Trp-Phe-Tyr-Ser(PO3H2)-Pro-Arg-pNA (NeoMPS), and p-nitroaniline absorbance was followed at 390 nm for 4 minutes.
GST pull-down assay
A total of 1 μg each of GST-Pin1 and p47phox (phosphorylated or not with p38MAPK) was incubated with glutathione-Sepharose beads in interaction buffer (phosphate-buffered saline; 1% 3-cholamidopropyl)dimethylammonio-1-propanesulfonate; 0.1mM DTT; 5mM NaF, and 1mM β-glycerophosphate) for 2 hours. After washing, the complex was released by cleavage with thrombin protease overnight at 4°C and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot, using specific antibodies.
In vitro p47phox phosphorylation
p47phox was phosphorylated with p38MAPK and PKC in the presence or absence of Pin1. The reaction mixture contained 2.5 μg of p47phox, 100 mU of mitogen-activated protein kinase (MAPK) in 40mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 10mM MgCl2, 1mM DTT, 5 μg/mL diacylglycerol, 150 μg/mL phosphatidylserine, 3mM CaCl2, and 50μM adenosine triphosphate (ATP, containing 1 μCi [γ-32P]-ATP) in a total volume of 50 μL. After 25 minutes of incubation, 1 μg of Pin1 was added for 5 minutes and 2.5 ng of PKC for 2 minutes. The reaction was terminated by adding 1μM staurosporine, and proteins were denaturated by boiling in Laemmli sample buffer35 for 3 minutes.
Dot-blot
Unphosphorylated and phosphorylated peptides corresponding to the Ser345 sequence of p47phox were coupled to ovalbumin (Neosysteme) and dissolved in 50mM Tris-HCl buffer (pH 7.5) at 18 μg/mL. A total of 1 μL of this solution was spotted on a nitrocellulose membrane. After drying, the membrane was blocked for 1 hour at room temperature in Tris-buffered saline containing 3% bovine serum albumin and then incubated with Pin1 (2 μg/mL) for 1 hour in 50mM Tris-HCl, 150mM NaCl, and 1% Triton X-100. After washing, Pin1 was detected with an anti-Pin1 monoclonal antibody followed by a secondary horseradish peroxidase-conjugated antimouse antibody, using an enhanced chemiluminescence method.
Conformational changes of p47phox
p47phox was phosphorylated with p38MAPK for 1 hour, and 25 μg of phosphorylated or nonphosphorylated p47phox was incubated with 2 μg of Pin1, in the absence or presence of juglone, in 100mM Tris buffer for 10 minutes. Digestion was started by adding 0.2 μg of trypsin at 30°C for 1 hour and stopped by adding Laemmli sample buffer.35 Proteins were analyzed with standard SDS-PAGE and Western blot methods.
Statistical analysis
All results are expressed as mean plus or minus SEM. Significant differences (P < .05) were identified with Student t test and one-way analysis of variance followed by a post-hoc test when multiple variables were analyzed. Significance was assumed at P less than .05.
Results
Pin1 is localized in human neutrophil cytosol and TNF-α markedly enhances its activity
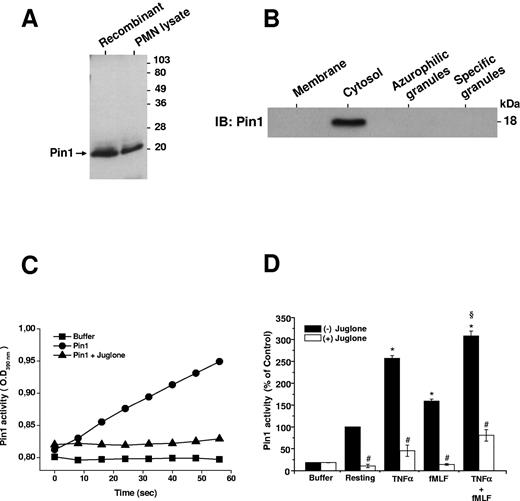
Pin1 is expressed in several immune cells, but its presence in neutrophils has not yet been described. As shown in Figure 1A, a specific antibody against Pin1 protein revealed recombinant Pin1 expressed in E. coli as well as endogenous Pin1 present in human neutrophil lysates. Subcellular fractionation showed that, in resting cells, Pin1 is only present in the neutrophil cytosol (Figure 1B). To investigate the role of Pin1 in TNF-α–primed neutrophils, we studied the effect of TNF-α and fMLF on Pin1 activity in the absence and presence of juglone,36 a selective Pin1 inhibitor. We first checked that juglone 250nM was able to inhibit recombinant Pin1 activity (Figure 1C). We then showed (Figure 1D) that resting human neutrophils possess constitutive basal Pin1 activity, which was markedly enhanced by TNF-α (P < .01 compared with resting cells). fMLF also stimulated Pin1 activity (P < .01). TNF-α induced significantly more Pin1 activity than fMLF (P < .01), although the 2 agents induced more Pin1 activity. Furthermore, incubation of neutrophils for 30 minutes with 250nM juglone inhibited both constitutive and stimulated endogenous Pin1 activity (P < .001; Figure 1D).
Pin1 is expressed in human neutrophils and is activated by TNF-α and fMLF. (A) Recombinant Pin1 (0.5 μg) and a resting human neutrophil (PMN) lysate (2 × 106 cells) were analyzed by SDS-PAGE and Western blot with an anti-Pin1 antibody. (B) Resting human neutrophils were lysed by nitrogen cavitation, and fractions were isolated on a Percoll gradient. Proteins were analyzed by SDS-PAGE and Western blot with an anti-Pin1 antibody. IB indicates immunoblot. (C) Recombinant Pin1 (0.25 nmol) was used to measure activity by recording the absorbance (at 390 nm) of free p-nitroaniline (pNA) cleaved by trypsin from Trp-Phe-Tyr-Ser(PO3H2)-Pro-Arg-pNA in the absence and presence of juglone (250nM). (D) Neutrophils were incubated in the absence and presence of juglone (250nM for 30 minutes) and then treated with TNF-α (20 ng/mL), fMLF (10−7M), or TNF-α, followed by fMLF (TNF-α + fMLF), before lysis. Pin1 activity was determined by measuring the absorbance of free pNA cleaved from Trp-Phe-Tyr-Ser(PO3H2)-Pro-Arg-pNA. Data are mean plus or minus SEM of 6 experiments. *P < .01 compared with resting neutrophils. §P < .01 TNF-α + fMLF compared with fMLF. #P < .001 with versus without juglone.
Pin1 is expressed in human neutrophils and is activated by TNF-α and fMLF. (A) Recombinant Pin1 (0.5 μg) and a resting human neutrophil (PMN) lysate (2 × 106 cells) were analyzed by SDS-PAGE and Western blot with an anti-Pin1 antibody. (B) Resting human neutrophils were lysed by nitrogen cavitation, and fractions were isolated on a Percoll gradient. Proteins were analyzed by SDS-PAGE and Western blot with an anti-Pin1 antibody. IB indicates immunoblot. (C) Recombinant Pin1 (0.25 nmol) was used to measure activity by recording the absorbance (at 390 nm) of free p-nitroaniline (pNA) cleaved by trypsin from Trp-Phe-Tyr-Ser(PO3H2)-Pro-Arg-pNA in the absence and presence of juglone (250nM). (D) Neutrophils were incubated in the absence and presence of juglone (250nM for 30 minutes) and then treated with TNF-α (20 ng/mL), fMLF (10−7M), or TNF-α, followed by fMLF (TNF-α + fMLF), before lysis. Pin1 activity was determined by measuring the absorbance of free pNA cleaved from Trp-Phe-Tyr-Ser(PO3H2)-Pro-Arg-pNA. Data are mean plus or minus SEM of 6 experiments. *P < .01 compared with resting neutrophils. §P < .01 TNF-α + fMLF compared with fMLF. #P < .001 with versus without juglone.
Pin1 inhibitors inhibit TNF-α–induced priming of ROS production by human neutrophils
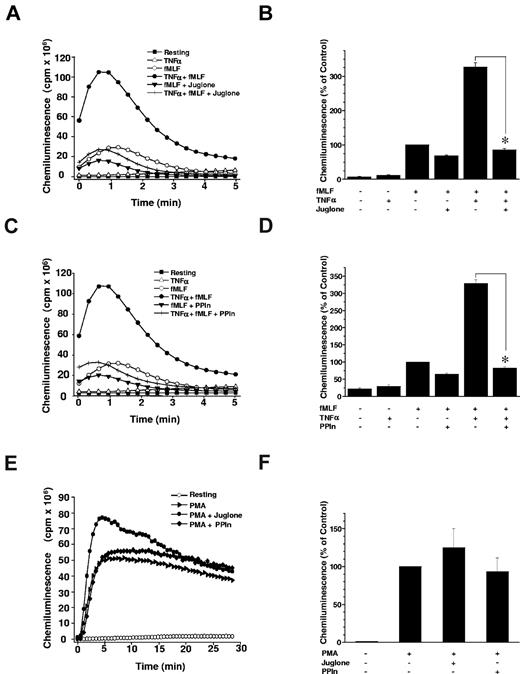
To examine the role of Pin1 in TNF-α–induced priming of ROS production, we first examined the effect of juglone on TNF-α–induced neutrophil priming of ROS production, in the aforementioned conditions that inhibited Pin1 activity. Neutrophils were incubated with juglone (250nM) for 30 minutes, and then treated with TNF-α (20 ng/mL) and stimulated with fMLF (10−7M) before measuring ROS production with a luminol-amplified chemiluminescence method. Resting and TNF-α–treated cells exhibited weak activity, close to baseline (Figure 2A). As expected, TNF-α primed fMLF-induced ROS production by neutrophils in control experiments performed without juglone. However, pretreatment of neutrophils with juglone completely abrogated the priming effect of TNF-α (Figure 2A-B). fMLF-induced activation was also inhibited, although somewhat less strongly, possibly because of neutrophil priming during the isolation procedure itself. Juglone did not affect neutrophil viability at the concentrations tested (data not shown). To rule out a nonspecific effect of juglone, we used a more specific Pin1 inhibitor consisting of a competitive peptide containing a Pin1-binding sequence able to inhibit Pin1 both in vitro and in intact cells.31 This PPIn also abrogated TNF-α–induced priming of ROS production in response to fMLF (Figure 2C-D), whereas its effect on fMLF stimulation alone was not significant. Importantly, neither juglone nor PPIn inhibited neutrophil ROS production triggered by PMA, a PKC direct activator (Figure 2E-F), suggesting that these agents do not inhibit NADPH oxidase activity or the PKC-dependent activation pathway, and that they do not scavenge ROS.
Pin1 is required for TNF-α–induced priming of ROS production. (A) Neutrophils were incubated in Hanks buffer containing juglone (250nM) for 30 minutes; then TNF-α (20 ng/mL) was added for 20 minutes before stimulation with fMLF (10−7M). ROS production was measured with a luminol-amplified chemiluminescence technique. (B) Total chemiluminescence in each experimental condition is expressed as mean plus or minus SEM of 6 experiments. (C) PPIn (50 μM) was tested in the same conditions as juglone. (D) Data are mean plus or minus SEM of 6 experiments. (E) Neutrophils were incubated with juglone or PPIn and then stimulated with PMA (100 ng/mL) before measuring ROS production with a luminol-amplified chemiluminescence technique (one experiment representative of 3). (F) Total chemiluminescence in each experimental condition is expressed as mean plus or minus SEM of 3 experiments. *P < .01 compared with inhibitor-free conditions.
Pin1 is required for TNF-α–induced priming of ROS production. (A) Neutrophils were incubated in Hanks buffer containing juglone (250nM) for 30 minutes; then TNF-α (20 ng/mL) was added for 20 minutes before stimulation with fMLF (10−7M). ROS production was measured with a luminol-amplified chemiluminescence technique. (B) Total chemiluminescence in each experimental condition is expressed as mean plus or minus SEM of 6 experiments. (C) PPIn (50 μM) was tested in the same conditions as juglone. (D) Data are mean plus or minus SEM of 6 experiments. (E) Neutrophils were incubated with juglone or PPIn and then stimulated with PMA (100 ng/mL) before measuring ROS production with a luminol-amplified chemiluminescence technique (one experiment representative of 3). (F) Total chemiluminescence in each experimental condition is expressed as mean plus or minus SEM of 3 experiments. *P < .01 compared with inhibitor-free conditions.
TNF-α enhances fMLF-induced Pin1 and p47phox translocation to the membrane fraction and juglone inhibits this process
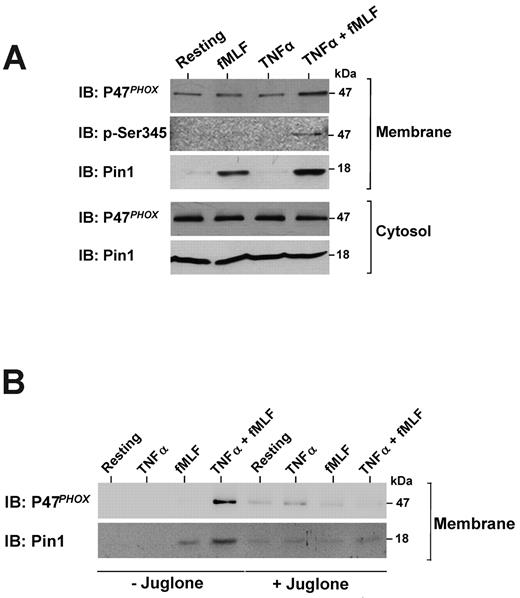
As the NADPH oxidase cytosolic subunit p47phox could be phosphorylated on Ser345,21 a site located in a Pin1 consensus binding sequence, we examined p47phox and Pin1 localization in human neutrophils in different conditions. The subcellular fractionation experiments show that, in resting neutrophils, Pin1 and the NADPH oxidase component p47phox were mainly localized in the cytosol (Figure 3A, Resting). Pin1 and p47phox remained localized in the cytosol in TNF-α–treated cells (Figure 3A). Contrary to TNF-α, fMLF (at 10−7M) induced Pin1 clear translocation and a weak p47phox translocation to the membrane fraction. However, pretreatment with TNF-α enhanced fMLF-induced Pin1, p47phox, and phospho-Ser345-p47phox translocation to the membrane fraction. Interestingly, juglone inhibited p47phox and Pin1 translocation in TNF-α + fMLF-stimulated neutrophils (Figure 3B). These results show that TNF-α enhanced fMLF-induced Pin1 and p47phox translocation to the membranes and that Pin1 activity is required for this process.
TNF-α enhances fMLF-induced Pin1 and p47phox translocation to the membranes in human neutrophils and the effect of juglone. (A) Neutrophils were treated with TNF-α (20 ng/mL), fMLF (10−7M), or TNF-α followed by fMLF (TNF-α + fMLF) and then lysed by nitrogen cavitation. Membranes and cytosols were separated by ultracentrifugation on a sucrose gradient. Proteins were analyzed by SDS-PAGE and Western blotting with anti-Pin1, anti-p47phox, and anti-pSer345 antibodies. (B) Neutrophils were treated with TNF-α, fMLF, or TNF-α + fMLF in the absence or presence of 250nM juglone and then lysed. Membranes were separated by ultracentrifugation on a sucrose gradient, and proteins were analyzed by SDS-PAGE and immunoblotting (IB). (representative of 7 experiments).
TNF-α enhances fMLF-induced Pin1 and p47phox translocation to the membranes in human neutrophils and the effect of juglone. (A) Neutrophils were treated with TNF-α (20 ng/mL), fMLF (10−7M), or TNF-α followed by fMLF (TNF-α + fMLF) and then lysed by nitrogen cavitation. Membranes and cytosols were separated by ultracentrifugation on a sucrose gradient. Proteins were analyzed by SDS-PAGE and Western blotting with anti-Pin1, anti-p47phox, and anti-pSer345 antibodies. (B) Neutrophils were treated with TNF-α, fMLF, or TNF-α + fMLF in the absence or presence of 250nM juglone and then lysed. Membranes were separated by ultracentrifugation on a sucrose gradient, and proteins were analyzed by SDS-PAGE and immunoblotting (IB). (representative of 7 experiments).
TNF-α triggers Pin1 binding to p47phox
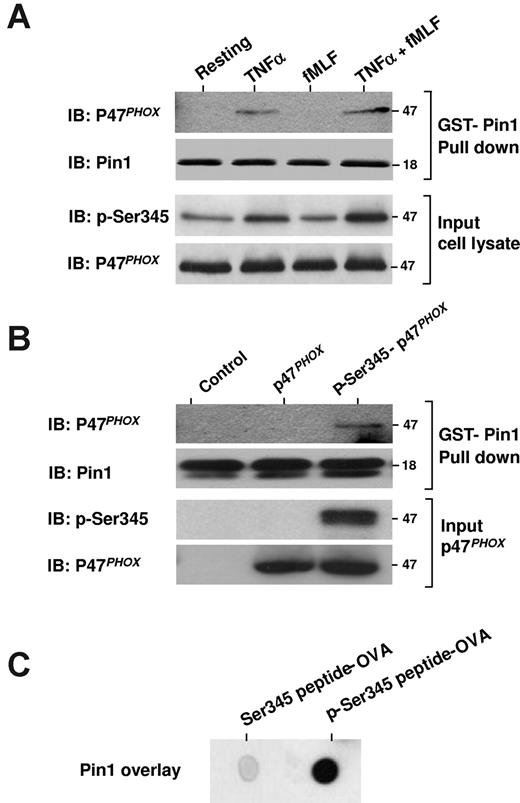
These latter findings suggested that TNF-α, which induces phosphorylation of p47phox on Ser34521 (Figure 4A, p-Ser345), might thereby trigger Pin1 binding to p47phox. To test this possibility directly, we used a GST pull-down assay with GST-Pin1 and cytosol from resting, TNF-α–, fMLF-, and TNF-α + fMLF-treated neutrophils. We found that p47phox from TNF-α– and TNF-α + fMLF-treated neutrophils bound to GST-Pin1 (Figure 4A), whereas p47phox from resting and fMLF-treated neutrophils did not. Exogenous recombinant GST-Pin1 might bind to free phosphorylated p47phox in primed neutrophils or might compete with endogenous Pin1 bound to phosphorylated p47phox.
Pin1 interacts with p47phox via phosphorylated Ser345. (A) Neutrophils were treated with TNF-α alone, fMLF alone, or TNF-α followed by fMLF (TNF-α + fMLF), and then lysed and incubated with GST-Pin1 in the presence of glutathione beads. The beads were then washed, Pin1 was released by thrombin cleavage, and proteins were analyzed by SDS-PAGE and Western blot (IB). (B) Recombinant p47phox was phosphorylated with p38MAPK and then repurified and incubated with GST-Pin1 and glutathione-agarose beads. The beads were washed 3 times, and proteins were analyzed by SDS-PAGE and immunoblotting (IB). (C) p47phox peptides containing phosphorylated or nonphosphorylated Ser345 were coupled to ovalbumin, spotted on nitrocellulose membranes, and overlaid with recombinant Pin1. Pin1 was detected with a specific antibody. Experiments are representative of 3.
Pin1 interacts with p47phox via phosphorylated Ser345. (A) Neutrophils were treated with TNF-α alone, fMLF alone, or TNF-α followed by fMLF (TNF-α + fMLF), and then lysed and incubated with GST-Pin1 in the presence of glutathione beads. The beads were then washed, Pin1 was released by thrombin cleavage, and proteins were analyzed by SDS-PAGE and Western blot (IB). (B) Recombinant p47phox was phosphorylated with p38MAPK and then repurified and incubated with GST-Pin1 and glutathione-agarose beads. The beads were washed 3 times, and proteins were analyzed by SDS-PAGE and immunoblotting (IB). (C) p47phox peptides containing phosphorylated or nonphosphorylated Ser345 were coupled to ovalbumin, spotted on nitrocellulose membranes, and overlaid with recombinant Pin1. Pin1 was detected with a specific antibody. Experiments are representative of 3.
To confirm a direct interaction between Pin1 and p47phox phosphorylated on Ser345, the GST-pull down assay was repeated with GST-Pin1 and recombinant human p47phox phosphorylated on Ser345 by active p38MAPK in vitro. Pin1 effectively bound to phosphorylated p47phox but not to the nonphosphorylated protein (Figure 4B). Furthermore, when a peptide sequence containing the phosphorylated Ser345 or a nonphosphorylated Ser345 was overlaid with Pin1, Pin1 bound to the phosphorylated Ser345 peptide sequence but not to the nonphosphorylated peptide (Figure 4C). Thus, TNF-α induces Pin1 activation and its interaction with p47phox via phospho-Ser345 in human neutrophils.
Pin1 binding to phospho-Ser345-p47phox induces p47phox conformational changes
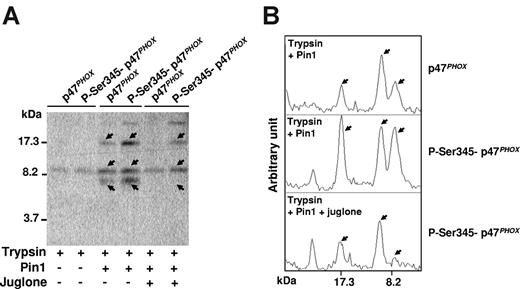
To determine whether Ser345-phosphorylated p47phox undergoes conformational change after interacting with Pin1, we examined the effect of Pin1 on p47phox sensitivity to trypsin proteolysis because conformational changes have been shown to affect trypsin sensitivity, presumably by exposing new cleavage sites or hiding existing cleavage sites in native proteins.37,38 The antibody used in this experiment was directed against the whole recombinant p47phox protein and thus recognizes multiple p47phox sequences. As shown in Figure 5A-B (arrows), the abundance of some digested peptides was higher in the presence of phosphorylated p47phox and active Pin1, suggesting that p47phox undergoes conformational changes. These apparent conformational changes were inhibited when Pin1 activity was abrogated by juglone, showing that Pin1 activity is required, and ruling out Pin1 interference with the protease. Pin1 also induced significant but lesser conformational changes with nonphosphorylated p47phox, which were inhibited by juglone. This could be the result of phosphorylation-independent Pin1 activity or to contamination of recombinant Pin1 with bacterial proline isomerase.
Pin1 induces conformational changes of p47phox via binding to phosphorylated Ser345. (A) p47phox was phosphorylated with p38MAPK, incubated with Pin1 in the presence or absence of juglone, and subjected to trypsin cleavage; peptides were analyzed with Tris-tricine gels and immunoblotting with an antibody directed against the whole recombinant p47phox protein. (B) Gels were scanned and peptides were quantified with Scion image Beta 4.03 for Windows 95 to XP software from the National Institutes of Health. Experiments are representative of 3.
Pin1 induces conformational changes of p47phox via binding to phosphorylated Ser345. (A) p47phox was phosphorylated with p38MAPK, incubated with Pin1 in the presence or absence of juglone, and subjected to trypsin cleavage; peptides were analyzed with Tris-tricine gels and immunoblotting with an antibody directed against the whole recombinant p47phox protein. (B) Gels were scanned and peptides were quantified with Scion image Beta 4.03 for Windows 95 to XP software from the National Institutes of Health. Experiments are representative of 3.
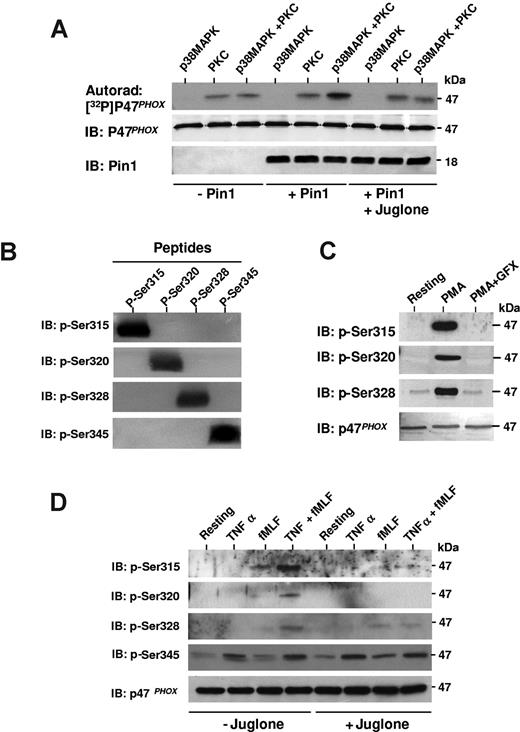
Pin1 facilitates p47phox phosphorylation by PKC on Ser315, Ser320, and Ser328
During neutrophil stimulation, it has been suggested that p47phox undergoes sequential phosphorylation.39 We thus studied the consequences of p38MAPKinase-dependent phosphorylation of p47phox on Ser345 and subsequent Pin1-induced conformational changes on PKC-mediated phosphorylation of p47phox at other sites in vitro. p47phox was first phosphorylated with p38MAPK; then Pin1 was added and p47phox was further phosphorylated with PKC in the presence of [32P]-ATP. p47phox phosphorylation by PKC was clearly enhanced when p47phox was first phosphorylated with p38MAPK in the presence of Pin1 (Figure 6A). Pin1 inhibition by juglone abrogated this effect, indicating that active Pin1 was required. Juglone did not inhibit p38MAPK or PKC activity (data not shown). To verify that Pin1 can effectively regulate PKC-dependent p47phox phosphorylation in intact neutrophils, we produced specific antibodies directed against the major PKC-phosphorylated sites of p47phox (Ser315, Ser320, and Ser328).9,15,39 These new antibodies specifically recognize each phosphorylated site (Figure 6B) and revealed PMA-induced p47phox phosphorylation in human neutrophils. In addition, PMA-induced phosphorylation of p47phox was inhibited by a selective PKC inhibitor, GF109203X (Figure 6C), as expected. We then treated neutrophils with TNF-α and fMLF, alone or sequentially, in the presence or absence of juglone, and analyzed Ser315, Ser320, and Ser328 phosphorylation status by SDS-PAGE and Western blotting with our phospho-specific antibodies (Figure 6D). TNF-α alone did not induce phosphorylation of these PKC sites (Ser315, Ser320, and Ser328), although it induced phosphorylation of Ser345, a MAPKinase site. fMLF (at 10−7M) alone induced only weak phosphorylation of Ser315, Ser328, and Ser345. However, priming with TNF-α followed by fMLF stimulation markedly increased p47phox phosphorylation on Ser315, Ser320, and Ser328, showing for the first time that phosphorylation of these sites is primed by TNF-α. Results also show that juglone inhibited this process. To analyze the effect of juglone on this priming, phosphorylated and total p47phox from 3 experiments were quantified by densitometry (using Scion image Beta 4.03 for Windows 95 to XP software) and phosphorylated p47phox corrected for the amount of p47phox. The results show that the TNF-α–induced increase in Ser315 and Ser320 phosphorylation was completely inhibited by the Pin1 inhibitor juglone (100% inhibition TNF-α + fMLF + juglone compared with TNF-α + fMLF; P < .01, n = 3). The TNF-α–induced increase in Ser328 phosphorylation was inhibited by 40.9% plus or minus 5.7% (P < .01, n = 3), whereas Ser345 phosphorylation was not inhibited. These results suggest that Pin1 mediates TNF-α–induced PKC-dependent hyperphosphorylation of p47phox in intact neutrophils, a phenomenon required for NADPH oxidase activation.8,14,39
Pin1 facilitates phosphorylation of p47phox by PKC, both in vitro and in intact neutrophils. (A) p47phox was phosphorylated with p38MAPK and then incubated with or without Pin1 preincubated with or without juglone. Where indicated, PKC was added in the presence of 32P-γ-ATP. Proteins were analyzed by SDS-PAGE, autoradiography, and immunoblotting (IB). (B) Phosphorylated p47phox peptides containing phospho-Ser315 (p-Ser315), p-Ser320, p-Ser328, or p-Ser345 were subjected to 16% SDS-PAGE, transferred to polyvinylidene difluoride membranes, and revealed with anti p-Ser315, p-Ser320, p-Ser328, or p-Ser345 antibodies. (C) Neutrophils were incubated with or without GF109203X (GFX) (5μM) for 15 minutes, stimulated with PMA, and proteins were analyzed with SDS-PAGE and Western blotting using anti–phospho-Ser315, anti–phospho-Ser320, anti–phospho-Ser328, and anti-p47phox antibodies. (D) Neutrophils were treated with TNF-α and fMLF, alone or together, in the absence or presence of juglone. Neutrophils were then lyzed, and proteins were analyzed with SDS-PAGE and Western blotting with anti–phospho-Ser315, anti–phospho-Ser320, anti–phospho-Ser328, anti–phospho-Ser345, and anti-p47phox antibodies. All experiments are representative of 3.
Pin1 facilitates phosphorylation of p47phox by PKC, both in vitro and in intact neutrophils. (A) p47phox was phosphorylated with p38MAPK and then incubated with or without Pin1 preincubated with or without juglone. Where indicated, PKC was added in the presence of 32P-γ-ATP. Proteins were analyzed by SDS-PAGE, autoradiography, and immunoblotting (IB). (B) Phosphorylated p47phox peptides containing phospho-Ser315 (p-Ser315), p-Ser320, p-Ser328, or p-Ser345 were subjected to 16% SDS-PAGE, transferred to polyvinylidene difluoride membranes, and revealed with anti p-Ser315, p-Ser320, p-Ser328, or p-Ser345 antibodies. (C) Neutrophils were incubated with or without GF109203X (GFX) (5μM) for 15 minutes, stimulated with PMA, and proteins were analyzed with SDS-PAGE and Western blotting using anti–phospho-Ser315, anti–phospho-Ser320, anti–phospho-Ser328, and anti-p47phox antibodies. (D) Neutrophils were treated with TNF-α and fMLF, alone or together, in the absence or presence of juglone. Neutrophils were then lyzed, and proteins were analyzed with SDS-PAGE and Western blotting with anti–phospho-Ser315, anti–phospho-Ser320, anti–phospho-Ser328, anti–phospho-Ser345, and anti-p47phox antibodies. All experiments are representative of 3.
Discussion
The molecular mechanisms by which TNF-α primes neutrophil ROS production are not fully understood. Here we show that Pin1, a unique cis-trans prolyl isomerase, is a previously unrecognized mediator of TNF-α–induced NADPH oxidase hyperactivation and ROS hyperproduction by human neutrophils. Inhibition of neutrophil Pin1 activity with juglone or with a specific Pin1-peptide inhibitor abrogated TNF-α–induced priming of human neutrophil ROS production. TNF-α induced both Pin1 activation and enhanced its translocation to the membrane fraction with p47phox, a cytosolic NADPH oxidase subunit in neutrophils. We also found that Pin1 binds to p47phox via phosphorylated Ser345, thereby inducing conformational changes that facilitate p47phox phosphorylation on other sites by PKC, and results in NADPH oxidase hyperactivation. Thus, Pin1-catalyzed prolyl isomerization is a novel mechanism regulating TNF-α–induced neutrophil NADPH oxidase priming.
It is clear from our data that basal Pin1 activity exists in the resting neutrophil cytosol. TNF-α and fMLF enhanced Pin1 activity through mechanisms that are under investigation in our laboratory. It has been reported that other agents, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and hyaluronic acid, can induce Pin1 activation in eosinophils and T lymphocytes.28,29,40 Subcellular fractionation experiments showed that fMLF stimulation increased the membrane localization of Pin1, whereas TNF-α stimulation did not (Figure 3A). However, TNF-α, contrary to fMLF, induced Pin1 binding to p47phox (Figure 4A). These results suggest that Pin1 translocation and Pin1 binding to p47phox may be controlled by different pathways. fMLF is a powerful Pin1 membrane translocation inducer but a weak Pin1-p47phox interaction inducer, whereas TNF-α is a weak Pin1 translocation inducer but a powerful Pin1-interaction inducer.
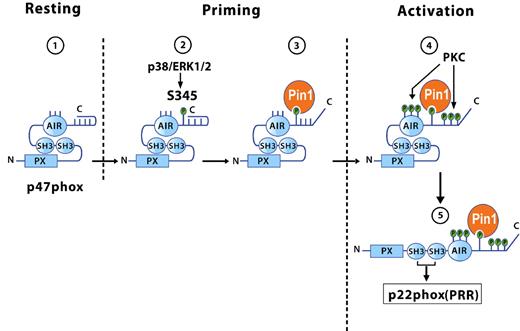
During human neutrophil stimulation, the NADPH oxidase organizer subunit p47phox is heavily phosphorylated.39 It is well known that, on phosphorylation, the p47phox isoelectric point (pI) shifts to the acidic range, giving rise to several phosphorylated isoforms corresponding to different phosphorylation states.41 When cells are stimulated with PMA, a PKC activator, 8 or 9 phosphorylation states of p47phox are observed in the cytosol and membranes, with the 2 most acidic forms being located in the membrane.41,42 These results suggest that p47phox undergoes sequential p47phox phosphorylation in the cytosol and membrane. Furthermore, we found that GM-CSF and TNF-α, which do not activate NADPH oxidase, induced partial phosphorylation of p47phox on Ser345 via ERK1/2 or p38MAPK in the cytosol of GM-CSF- and TNF-α–treated neutrophils, respectively, an event that primes NADPH oxidase assembly.16,21,32 Our results suggest that p47phox is first phosphorylated by a MAPKinase (ERK1/2 or p38MAPK) on Ser345 and that Pin1 then binds to this site, inducing conformational changes that facilitate subsequent phosphorylation of the remaining sites by PKC isoforms. Complete phosphorylation of p47phox allows its binding to p22phox (Figure 7). Phospho-Ser345 is located in a proline-rich region (—PX-phosphoSP–) that can exist in the cis or trans conformation, and proline isomerization has a regulatory effect involving subtle conformational changes. Prolyl cic/trans isomerization is a spontaneous process but can also be catalyzed by various enzymes. Pin1 is a unique prolyl isomerase that specifically recognizes phosphorylated serine or threonine residues located immediately N-terminal to a proline, and then isomerizes the peptide bond. The p38MAPKinase/p47phox(Ser345)/Pin1 axis could be essential for regulating the level of NADPH oxidase activation at inflammatory sites. It is noteworthy that this MAPKinase phosphorylation site (—SP—) is not conserved in murine p47phox, contrary to rat p47phox. Another MAPKinase site, Thr356, was recently identified in murine p47phox.43 Pin1 might target Th356 in mouse neutrophils and induce their priming.
Models of the different conformations of p47phox in resting, primed, and activated neutrophils: role of Pin1 and phosphorylation. In resting cells, p47phox is not phosphorylated and has a constrained conformation because of the tight interaction between SH3 domains and the autoinhibitory region (AIR). (1) During priming, p47phox is first phosphorylated by a MAPKinase (ERK1/2 or p38MAPK) on Ser345, and (2) activated Pin1 then binds to this site, (3) inducing the first conformational changes that allow PKC isoforms to phosphorylate p47phox on other sites during activation. (4) Phosphorylation of p47phox on several sites at its C-terminal tail prevents the SH3/AIR interaction, allowing the cryptic SH3 domains to bind to the proline-rich region (PRR) of p22phox (5) and NADPH oxidase hyperactivation.
Models of the different conformations of p47phox in resting, primed, and activated neutrophils: role of Pin1 and phosphorylation. In resting cells, p47phox is not phosphorylated and has a constrained conformation because of the tight interaction between SH3 domains and the autoinhibitory region (AIR). (1) During priming, p47phox is first phosphorylated by a MAPKinase (ERK1/2 or p38MAPK) on Ser345, and (2) activated Pin1 then binds to this site, (3) inducing the first conformational changes that allow PKC isoforms to phosphorylate p47phox on other sites during activation. (4) Phosphorylation of p47phox on several sites at its C-terminal tail prevents the SH3/AIR interaction, allowing the cryptic SH3 domains to bind to the proline-rich region (PRR) of p22phox (5) and NADPH oxidase hyperactivation.
In its nonphosphorylated state, p47phox has a constrained conformation because of the tight interaction between SH3 domains and the autoinhibitory region.44 Phosphorylation of p47phox at its C-terminal tail relaxes this interaction, allowing the cryptic SH3 domains to bind to the proline-rich region of p22phox. This implies that phosphorylation induces conformational changes of p47phox. Several in vitro experiments have shown that p47phox phosphorylation induces conformational changes of the protein.45,46 We now show that Pin1 is the enzyme that initiates and catalyzes this reaction in intact neutrophils, in a phosphorylation-dependent manner via phospho-Ser345.
Pin1 has a key role in some neurodegenerative diseases and cancers.26,27 Pin1 also regulates transforming growth factor-β and GM-CSF mRNA stability in eosinophils and T lymphocytes.28,29 In this study, we identify a key role of Pin1 in controlling the level of NADPH oxidase activation in TNF-α–primed neutrophils. ROS production by the phagocyte NADPH oxidase complex plays a major role in host defenses, but excessive ROS production can damage healthy bystander tissues, thereby contributing to inflammatory diseases. We have previously reported increased NADPH oxidase activity and excessive ROS generation through increased p47phox(Ser345) phosphorylation in neutrophils isolated from synovial fluid of rheumatoid arthritis patients, compared with those isolated from blood.21 The results presented here suggest that Pin1 could mediate neutrophil hyperactivation at inflammatory sites. Pharmacologic Pin1 targeting could thus dampen TNF-α–induced neutrophil hyperactivation and ROS production, a hallmark of inflammatory diseases.
TNF-α is a key cytokine in several inflammatory diseases. Here we show that Pin1 mediates TNF-α–induced neutrophil NADPH oxidase priming and ROS hyperproduction via specific binding to phosphorylated Ser345 of p47phox. Pin1 could mediate other TNF-α–induced processes and thus be an essential player in TNF-α–induced inflammatory diseases. Pin1 inhibitors might represent an important therapeutic advance for inflammation and other diseases associated with TNF-α overproduction.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by l'Agence Nationale de la Recherche, Arthritis Fondation Courtin, Vaincre la mucoviscidose, Comité Mixte de Coopération Universitaire franco-tunisien, Accord Inserm/DGRST, Inserm and Centre National de la Recherche Scientifique, and the National Institutes of Health (P30 HD03352, P01 HL088594, and R01 HL087950) (J.S.M.).
National Institutes of Health
Authorship
Contribution: T.B. performed experiments and analyzed the data; M.-A.G.-P., P.M.-C.D., and J.E.-B. designed the study, interpreted the results, and wrote the paper; G.H. designed the study and interpreted the results; S.C., H.R., R.A.D., Y.K., O.B., and X.Z.Z. performed experiments; and J.S.M., P.K.L., and A.B. designed the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jamel El-Benna, Inserm U773, CRB3, Faculté de Medecine Xavier Bichat, 16 rue Henri Huchard, 75018 Paris, France; e-mail: jamel.elbenna@inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal