In this issue of Blood, Boussetta et al provide novel insights into how TNFα primes human neutrophils and report that a specific conformational change switches the phagocyte NADPH oxidase into a more productive state.1
The phagocyte NADPH oxidase generates an array of reactive oxidants that synergize with granule contents and circulating host proteins to mediate efficient antimicrobial activity in the neutrophil phagosome. In resting neutrophils, the phagocyte oxidase is unassembled and inactive, as the individual components are spatially segregated in naive phagocytes. Upon exposure to suitable stimuli, agonist-dependent changes in the cytoplasmic oxidase components p47phox and Rac2 result in their translocation to the phagosomal membrane and culminate in the assembly of an active oxidase complex.2
However, activation of the phagocyte NADPH oxidase has nuances beyond simply toggling from resting to activated status; neutrophils can adopt a “primed” phenotype, whereby suboptimal concentrations of typical agonists or exposure to agents that do not directly stimulate resting neutrophils can elicit robust oxidase activity. For example, pretreatment of neutrophils with tumor necrosis factor α (TNFα), an agent that does not directly trigger NADPH oxidase activity, renders neutrophils responsive to suboptimal concentrations of formylated peptides, such as the formylated tripeptide, formyl-methionylleucylphenylalanine (fMLF). Agents that have the capacity to prime neutrophils include cytokines, such as TNFα, microbial components, β2 integrin agonists, extracellular matrix proteins, and several pharmacologic agents (reviewed in El-Benna et al3 ). Not only have a variety of agents that prime neutrophils been identified, but several phenotypic changes in primed neutrophils have also been described, including transcriptional changes, increases in membrane flavocytochrome b558 expression, augmented G-protein activity, reorganization of plasma membranes, and partial phosphorylation of p47phox.
The El-Benna laboratory previously demonstrated that phosphorylation of 345Ser in p47phox is required for TNFα-dependent priming of the phagocyte NADPH oxidase.4 In this issue, the same laboratory now reports that Pin1, a peptidyl prolyl cis-trans isomerase (PPI), catalyzes a conformational change in p47phox that is required for oxidase priming by TNFα. Appreciation of the beauty of this novel insight requires some background information.
Because of its ringed structure, proline can profoundly alter protein conformation, depending on whether it is in the cis or trans configuration with respect to the peptide bond. Cis-trans isomerization of proline occurs at a very slow rate, on the order of minutes, unless catalyzed by a PPI. The conformational switch provided by prolyl cis-trans isomerization participates in modulation of a wide range of cellular processes.5,6 The subfamily of PPI represented by Pin1 is unique in its ability to promote efficient isomerization of prolines adjacent to phosphorylated Ser or Thr residues,7 thereby coupling Pin1 binding and activity to regulation of signal transduction systems that rely on proline-directed phosphorylation.
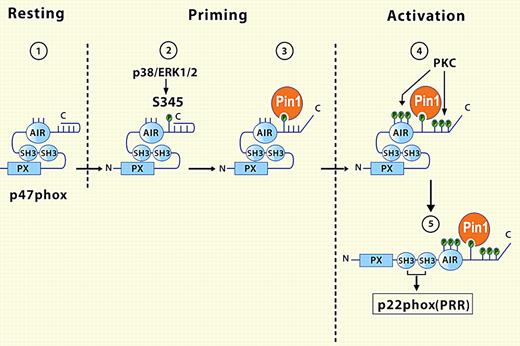
Boussetta et al demonstrate for the first time that Pin1 is present in the cytoplasm of human neutrophils and is required for TNFα priming of the NADPH oxidase. Furthermore, 345Ser, phosphorylated in response to TNFα, binds Pin1, which in turn triggers conformational changes in p47phox, rendering neighboring serine residues susceptible to phosphorylation by protein kinases (see figure). The subsequent serine phosphorylations elicit another conformational change, which exposes otherwise cryptic SH3 motifs that then dock at the membrane with proline-rich regions of p22phox, the light subunit of flavocytochrome b558, resulting in stable assembly of the oxidase. Overall, the enlistment of Pin1 by TNFα treatment changes the configuration of p47phox and the phenotype of the NADPH oxidase, thereby amplifying its responsiveness to subsequent agonist challenge.
In the cytoplasm of resting neutrophils (1), the conformation of p47phox renders cryptic its SH3 and PX domains, regions that have the potential to interact with targets on the phagosomal or plasma membrane. TNFα-mediated activation of p38/ERK (1/2) phosphorylates 345S in the autoinhibitory region (AIR) of p47phox (2), allowing the prolyl isomerase Pin1 to bind and catalyze a conformational change in p47phox (3), with subsequent phosphorylation of neighboring serines by protein kinase C (PKC). Now revealed after Pin1-induced conformational rearrangement, SH3 domains of p47phox can associate with the membrane-bound p22phox and complete the assembly and activation of the NADPH oxidase (5). See the complete figure in the article beginning on page 5795.
In the cytoplasm of resting neutrophils (1), the conformation of p47phox renders cryptic its SH3 and PX domains, regions that have the potential to interact with targets on the phagosomal or plasma membrane. TNFα-mediated activation of p38/ERK (1/2) phosphorylates 345S in the autoinhibitory region (AIR) of p47phox (2), allowing the prolyl isomerase Pin1 to bind and catalyze a conformational change in p47phox (3), with subsequent phosphorylation of neighboring serines by protein kinase C (PKC). Now revealed after Pin1-induced conformational rearrangement, SH3 domains of p47phox can associate with the membrane-bound p22phox and complete the assembly and activation of the NADPH oxidase (5). See the complete figure in the article beginning on page 5795.
The most obvious consequence of Pin1-dependent cis-trans prolyl isomerization is increased oxidase activity. Whether the augmented output reflects more efficient oxidase assembly, increased specific activity of the assembled oxidase, or both is not yet known. Application of the broken cell superoxide-generating system to this question may provide insights into the underlying mechanism. In addition, it is worth considering that increased oxidase activity is not the only means by which more oxidants could be produced. Termination of oxidase activity, an event both incompletely understood and characterized, may be delayed or inhibited by TNFα treatment. If dephosphorylation of one or more serines in p47phox normally contributes to termination of oxidase activity, a conformational shift from trans to cis might render the site(s) resistant to phosphatase action, as some phosphatases dephosphorylate exclusively trans configuration of phosphorylated Ser/Thr,8 and thereby stabilize the oxidase complex assembled and active at the membrane. Like increased activation, delayed termination of the NADPH oxidase would result in greater oxidant production.
The report by Boussetta et al adds to our understanding another critical step in phagocyte oxidase activation that rests heavily on conformational changes in the adaptor protein p47phox. Do other agents, host factors akin to TNFα as well as microbial components such as endotoxin, share in their dependence on Pin1 activity for the capacity to prime NADPH oxidase activity? As several innate immune responses to microbial and endogenous danger signals overlap in other situations, it may be informative to determine the extent to which unrelated priming agents share mechanistic pathways. Likewise, examination of Pin1-dependent responses may elucidate fundamental elements of the excessive inflammatory activity seen in clinical settings such as adult respiratory distress syndrome, ventilator-associated pneumonia, or in pulmonary infections in patients with cystic fibrosis. Perhaps patients with excessive inflammatory responses that seem disproportionate to the type or magnitude of the offending agent, a clinical situation commonly seen by practicing allergists and immunologists, have abnormal Pin1 or aberrant Pin1 activity underlying their unchecked phagocyte activation.
As is always the case with novel work, the exciting findings reported by Boussetta et al not only provide important mechanistic insights into the immediate objective of their research (that is, the basis of TNFα priming of the phagocyte NADPH oxidase), but also inspire many provocative questions that extend to more general aspects of phagocyte biology and inflammation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal