Abstract
Investigations of bone marrow (BM) erythroblast development are important for clinical concerns but are hindered by progenitor cell and tissue availability. We therefore sought to more specifically define dynamics, and key regulators, of the formation of developing BM erythroid cell cohorts. A unique Kit−CD71highTer119− “stage E2” proerythroblast pool first is described, which (unlike its Kit+ “stage E1” progenitors, or maturing Ter119+ “stage E3” progeny) proved to selectively expand ∼ 7-fold on erythropoietin challenge. During short-term BM transplantation, stage E2 proerythroblasts additionally proved to be a predominantly expanded progenitor pool within spleen. This E1→E2→E3 erythroid series reproducibly formed ex vivo, enabling further characterizations. Expansion, in part, involved E1 cell hyperproliferation together with rapid E2 conversion plus E2 stage restricted BCL2 expression. Possible erythropoietin/erythropoietin receptor proerythroblast stage specific events were further investigated in mice expressing minimal erythropoietin receptor alleles. For a hypomorphic erythropoietin receptor-HM allele, major defects in erythroblast development occurred selectively at stage E2. In addition, stage E2 cells proved to interact productively with primary BM stromal cells in ways that enhanced both survival and late-stage development. Overall, findings reveal a novel transitional proerythroblast compartment that deploys unique expansion devices.
Introduction
Erythropoiesis in mouse and humans is ontogenically compartmentalized. Primitive yolk sac hematopoietic progenitor cells initially give rise to nucleated red cells (which can later enucleate) and also colonize the aorta-gonad-mesonephros and umbilical cord.1-3 In fetal liver (which may be seeded by yolk sac and aorta-gonad-mesonephros hematopoietic progenitor cells), red cell formation from committed erythroid progenitors becomes erythropoietin (EPO) dependent.4 Fetal liver erythropoiesis also relies on stromal cell interactions. Examples include erythroblast KIT and Eph4 binding to their stromal ligands (KIT-L and Ephrin-B2)4-6 as well as fetal liver erythroblast α4, β1 integrin effects.7,8 In perinatal and adult life, erythropoiesis (and hematopoiesis) shift to the bone marrow (BM) compartment. In humans, this remains the prime erythropoietic tissue, although under atypical conditions (eg, spherocytosis or inhibited vascular endothelial growth factor signaling) spleen and liver can become erythropoietic sites.8,9 In mouse, splenic erythropoiesis additionally can be readily induced, and experimentally such stress erythropoiesis can be a useful barometer of a compromised erythron.10,11 BM erythropoiesis is less studied in part because of low frequencies of erythroid progenitors and limited tissue per se. Genetic evidence also indicates that erythroblast development in BM differs in at least several basic ways from splenic, fetal liver, and yolk sac erythropoiesis. Examples include roles for Hedgehog plus BMP4 interplay in splenic but not BM erythropoiesis9 ; essential roles for stem cell leukemia during embryonic but not adult erythropoiesis12 ; and roles for EPO/EPO receptor (EPOR) action during definitive, but not yolk sac, erythropoiesis.4
In an aim to advance an understanding of BM erythroblast development, we have presently applied novel in vivo and ex vivo approaches, transcriptome analyses of purified developmental erythroblast cohorts, minimal EPOR allele mouse models, and coculture systems to reveal a previously uncharacterized proerythroblast population (as Kit−CD71highTer119− “stage E2” cells) as a uniquely dynamic and highly expandable cohort. One impetus for these studies concerns the issue of how circumscribed populations of colony-forming units-erythroid (CFUe; and burst-forming units-erythroid [BFUe]) might provide for more than pulsatile red cell production. In particular, we hypothesized that functional heterogeneity might exist within a developmental series of early- to late-stage erythroid cells. As a broad biologic problem, this can be compared (for example) to the stepwise development that occurs among developing lymphoid cells within B- and T-cell lineages.13,14
Beyond this, we speculated on the existence of a possible BM stromal cell niche that might support the expansion of intermediate-stage erythroblasts. Within BM, several key hematopoietic niches previously have been described. Hematopoietic stem cells frequently reside within an interacting osteoblastic endosteal region,15 whereas megakaryocytic progenitors occupy a vascularized niche in sinusoids (and respond to SDF1 and FGF4).16 Within the erythroid lineage, assemblages of macrophage-interacting erythroblasts originally were described by Bessis.17 As recently reviewed,18-20 these erythroblastic islands typically contain maturing erythroblasts around a central (and perhaps specialized) macrophage. Tethering occurs via at least 3 sets of factors (erythroblast-macrophage protein homotypic actions; VCAM1 plus α4, β1 integrin; and ICAM4 plus α5 integrin),21-25 and one major function of erythroblastic islands involves macrophage engulfment of reticulocyte-expelled nuclei.26 Related studies similarly have implicated stromal components as intriguing erythroblastic regulators. In analyses of human CD34- as well as hES cell-derived erythroblast development, Giarratana et al27 and Ma et al28 each have described essential roles for BM stromal cell components in supporting efficient erythroblast development. Eshghi et al7 further have observed that, during fetal erythroblast development ex vivo, EPO-dependent expansion is followed by a secondary expansion phase. Within fetal liver, this latter phase appears to be mediated (at least in part) by stromal adhesion and α4, β1 integrin action. Present findings are consistent with the notion that stromal cells can markedly affect erythroblast development and bring this concept to bear for a new uniquely expandable proerythroblast population.
Methods
Preparation and isolation of a developmentally staged primary murine BM erythroid series
BM from C57BL/6 mice (7-12 weeks) was gently expelled (from cleaned femurs and tibiae) into Iscove modified Dulbecco medium (IMDM; Invitrogen) containing 2% bovine serum (Invitrogen, no. 26170-035). After passage through 21-gauge needles (3 times) and a 40-μm strainer, cells were resuspended in 1 mL of phosphate-buffered saline (PBS; Invitrogen, no. 14190-144) and exposed for 2 minutes to 9 mL of buffered 0.8% ammonium chloride (StemCell Technologies, no. 07850); 10 times PBS then was added (1.1 mL), and cells were centrifuged at 450g for 6 minutes through 20 mL of 50% bovine serum in PBS. Cells were then washed twice in IMDM, and cultures were initiated at 1.8 × 106 cells/mL in StemPro-34 base medium (Invitrogen) supplemented with 2.5 U/mL EPO (Epoietin-alpha, Amgen), 100 ng/mL murine stem cell factor (PeproTech), 0.5μM dexamethasone, 1.5μM β-estradiol, 75 μg/mL h-transferrin (Sigma-Aldrich, no. T-0665), 0.5% bovine serum albumin (BSA, StemCell Technologies, no. 9300), 0.1mM 2-mercaptoethanol, 1.5mM l-glutamine (Invitrogen) and 100 U/mL penicillin G, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B (Invitrogen, ie, “SP34-ex” medium). At 24 hours, a half-volume of fresh SP34-ex medium was added. At 48 hours, cells were collected and returned to culture in 10% residual expansion media plus 90% of fresh SP34-ex medium. At day 3 of expansion, stage E1, E2, and E3 cells were isolated via a combination of selective depletion retrieval and procedures. E3 cells were first isolated by Ter119+ selection (with stringent washing). Lineage depletion (StemCell Technologies, biotinylated CD5, CD45R/B220, CD11b, Ter119, and Ly6G antibodies) was then used to enrich E1 and E2 cohorts. Stage E1 cells next were isolated via stringent CD117 magnetic-activated cell separation (MACS; Miltenyi Biotec). Remaining stage E2 cells then were isolated via CD71 MACS retrieval.
Preparation and analyses of splenic erythroid cells after EPO dosing, or short-term BMT
Post-EPO dosing (1250 U/kg at 1 and 24 hours) spleens were gently mechanically disrupted in IMDM, 2% fetal bovine serum, and 5mM Na2 ethylenediaminetetraacetic acid and passed through 18-gauge needles and a 40-μm cell strainer. Cells then were collected, washed in IMDM, counted (total, and nucleated), and then analyzed by flow cytometry. Spleens from BM transplantation (BMT) recipients were processed similarly at days 11.5 and 14.5 after BMT. BMT was performed as detailed by Menon et al.29
Flow cytometry
BM preparations and/or cultured cells were washed (1 × 106) and resuspended directly in 0.2 mL PBS, 0.1% BSA. Rat IgG (15 μg, Jackson ImmunoResearch Laboratories) was used as a blocking agent (15-minute incubation, 4°C). Cells were then stained with allophycocyanin-CD117 (2 μg/mL), fluorescein isothiocyanate (FITC)–CD71 (5 μg/mL), and phycoerythrin (PE)–Ter119 (5 μg/mL; BD Biosciences) for 30 minutes at 4°C. PBS-washed cells were then analyzed (as equivalent numbers of gated events) using a BD Biosciences FACSCalibur flow cytometer and Cellquest software Version 3.3. Cell cycle distributions (ModFIT software Version 3.2) were analyzed by depleting Ter119+ erythroblasts from total BM preparations, staining with PE-CD117 (2 μg/mL) and FITC-CD71 (7.5 μg/mL; BD Biosciences), and fixing, permeabilizing, and staining with 7-amino-actinomycin D.
Transcriptome profiling and RT-PCR
Purified stage E1, E2, and E3 cells were lysed directly in Trizol reagent (Invitrogen). RNA then was isolated (RNeasy Mini Kit, QIAGEN). For transcriptome analyses, total RNA (4 μg) was used to synthesize biotin-cRNA. Hybridizations were to Affymetrix 430-2.0 arrays. GeneChip scanner 3000 and GCOS software Version 1.2 (Affymetrix) was used to process primary data, and ArrayAssist software Version 4.0 (Stratagene) was used in initial data analyses. In analyses of EPO/EPOR response factors, purified stage E1, E2, and E3 cells were cultured for 6 hours in IMDM containing 0.5% BSA, 50 μg/mL transferrin, 15 ng/mL insulin (Invitrogen), and 0.1mM 2-mercaptoethanol. Cells were then exposed to EPO (± 5 U/mL) for the intervals indicated, and RNA was extracted. Reverse transcription (Superscriptase III, Invitrogen) and real-time quantitative polymerase chain reactions (iQ SYBR Green, i-Cycler, Bio-Rad) were performed as previously described.30-32 Primer pairs were from Superarray Bioscience.
Western blotting
For direct analyses of BCL-2 and BCL-XL, purified stage E1, E2, and E3 cells were lysed as detailed previously.29,31,32 Alternatively, purified stage E1, E2, and E3 cohorts were cultured for 6 hours in IMDM containing 0.5% BSA, 50 μg/mL transferrin, 15 ng/mL insulin (Invitrogen), and 0.1mM β-mercaptoethanol and were subsequently exposed to EPO (± 5 U/mL) for 2.5 hours and 7.5 hours before lysate preparations. Cleared extracts were assayed for protein content, denatured, electrophoresed, transferred to polyvinylidene difluoride membranes, and blotted. Antibodies used were anti–BCL-XL (Cell Signaling Technology, no. 3881), anti-BCL2 (Cell Signaling Technology, no. 2876), and anti–β-tubulin (Cell Signaling Technology, no. 2128). Horseradish peroxidase-conjugated antibodies were from Jackson ImmunoResearch Laboratories. For enhanced chemiluminescence, Super-Signal West-Dura reagent (Pierce Biotechnology) was used. Quantitative analysis of Western blot signals was via ImageJ software Version 1.42j (http://rsb.info.nih.gov/ij).
EPOR-H and EPOR-HM mouse models, and EPO dosing
Mice expressing knocked-in EPOR-H and EPOR-HM alleles29 (and congenic controls) were maintained as a C57BL/6J-129/Ola line and were used at 8 to 12 weeks. EPO (1200 U/kg) was injected intraperitoneally (in saline) at 1 and 24 hours (hours, or days, after EPO injection refer to the first 1-hour EPO injection point). Hematocrits and reticulocyte levels were determined by capillary microcentrifugation and thiazole-orange staining (via flow cytometry). These and all procedures used were ethical, and approved by the Institutional Animal Care and Use Committee of the Maine Medical Center Research Institute.
BM stromal cell preparations, erythroid cell interaction assays, and fluorescent microscopy
Stromal cells were prepared from lineage-depleted (and F4-80 macrophage-depleted) BM preparations. After depletions, cells were resuspended in IMDM, 5% fetal bovine serum, 0.05mM 2-mercaptoethanol, and 1 × P cell-stimulating factor and plated (at 1 × 106 cells/mL) to 4-chamber slides (Thermo Scientific, no. 154526). Medium was changed at 4-day intervals; and at 90% confluence, cells were passed at 1:3. In analyses of erythroid cell-stromal cell interactions, stromal cells were cultured for 1 day in advance in stromal medium plus SP34-ex medium (1:1). For interaction assays, freshly prepared stage E2 and E1 cells (5 × 105) were stained with limiting concentrations of PE-CD71 and FITC-CD71, (respectively), washed, and cocultured for 4 hours with Lin− F4-80− BM stromal cells (BMSCs). After uniform washing (3 times with 0.5 mL 1 × PBS), bound erythroid cells were scored by fluorescent microscopy (Axiovert 200 microscope, Carl Zeiss). For survival, growth, and differentiation analyses, E2 proerythroblasts were cocultured with BMSCs for 72 hours in SP34-ex medium. Cell growth and viability were determined via ViCell counting. Erythroblast enucleation was analyzed via flow cytometry using DRAQ5 (10μM; Alexis Biochemicals) and Ter119 staining.
Results
In vivo analyses of EPO's effects on BM erythroid cell development reveal a novel Kit−CD71highTer119− population with unique expansion capacities
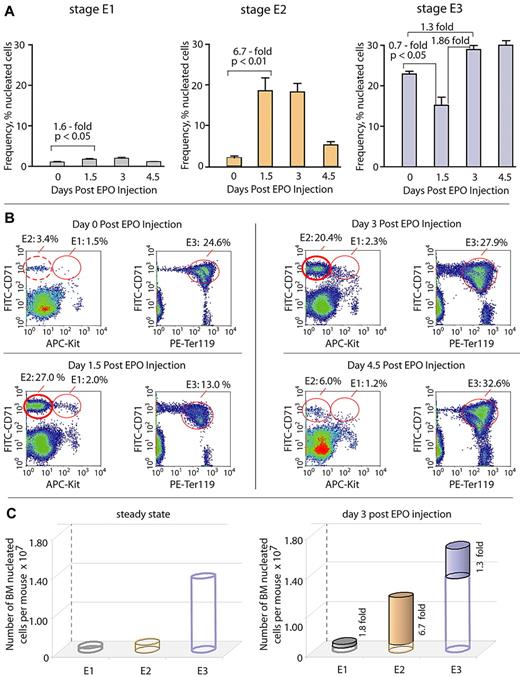
To assess EPO's in vivo actions on BM erythroid progenitor pools, we first developed flow cytometric procedures for the assay of cells expressing KIT, CD71 (transferrin receptor), and Ter119 markers within freshly isolated total BM preparations (while carefully gating off reticulocytes and red blood cells; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This procedure was then used to analyze BM cell populations from mice at steady state, as well as at days 1.5, 3, and 4.5 after EPO dosing. Under each condition, 3 discrete erythroid cohorts were apparent as Kit+CD71highTer119−, Kit−CD71highTer119−, and Kit−CD71highTer119+ pools (designated here as stages E1, E2, and E3, respectively; Figure 1). At steady state, stage E3 erythroblasts were represented as ∼ 20% of nucleated cells. At day 1.5 after EPO dosing, E3 cells were depleted by 30%, but by day 3 E3 levels were restored to ∼ 130%. Stage E1 cells expectedly were less frequent (∼ 1.5%) but somewhat unexpectedly also remained relatively static after EPO dosing and increased only 1.8-fold. Stage E2 cells, in contrast, dramatically expanded in response to EPO. At days 1.5 and 3.0, this included increases from a baseline of ∼ 3%, to ∼ 19.5%, and ∼ 18%, respectively. On average, this represents up to a 6.7-fold selective expansion of stage E2 cells. To determine whether any portion of this E2 cell pool might be compromised, viability was assayed. Among E2 cells, less than 1% stained with annexin-V or YoPro1.
BM erythroid cells develop in vivo as discrete Kit+CD71highTer119− “E1,” Kit−CD71highTer119− “E2,” and Kit− CD71highTer119+ “E3” cohorts, among which stage E2 proerythroblasts compose a uniquely expandable pool. (A-B) Defining of stage E1, E2, and E3 BM cohorts and their EPO-induced expansion potentials. At 1 and 24 hours, mice were dosed with saline (control) or EPO (1200 U/kg). At days 1.5, 3, and 4.5, femoral BM cells were isolated and analyzed for KIT, transferrin receptor (CD71), and Ter119 marker expression (via flow cytometry). Kit+CD71highTer119− cells are designated as stage “E1,” Kit−CD71highTer119− as stage “E2,” and Kit−CD71highTer119+ cells as stage “E3.” (A) At days 1.5 to 3, levels of CFUe-like stage E1 cells increased ∼ 1.6-fold. Stage E2 progenitors, in contrast, expanded up to 6.7-fold, whereas frequencies of stage E3 erythroblasts (∼ 30%) were modulated 1.3- to 1.8-fold. Values are mean frequencies ± SE (n = 3). (B) Representative flow cytometric analyses. (C) Dynamics of E1, E2, and E3 pools at steady state and as affected by EPO (at day 3) are summarized.
BM erythroid cells develop in vivo as discrete Kit+CD71highTer119− “E1,” Kit−CD71highTer119− “E2,” and Kit− CD71highTer119+ “E3” cohorts, among which stage E2 proerythroblasts compose a uniquely expandable pool. (A-B) Defining of stage E1, E2, and E3 BM cohorts and their EPO-induced expansion potentials. At 1 and 24 hours, mice were dosed with saline (control) or EPO (1200 U/kg). At days 1.5, 3, and 4.5, femoral BM cells were isolated and analyzed for KIT, transferrin receptor (CD71), and Ter119 marker expression (via flow cytometry). Kit+CD71highTer119− cells are designated as stage “E1,” Kit−CD71highTer119− as stage “E2,” and Kit−CD71highTer119+ cells as stage “E3.” (A) At days 1.5 to 3, levels of CFUe-like stage E1 cells increased ∼ 1.6-fold. Stage E2 progenitors, in contrast, expanded up to 6.7-fold, whereas frequencies of stage E3 erythroblasts (∼ 30%) were modulated 1.3- to 1.8-fold. Values are mean frequencies ± SE (n = 3). (B) Representative flow cytometric analyses. (C) Dynamics of E1, E2, and E3 pools at steady state and as affected by EPO (at day 3) are summarized.
The aforementioned findings were of interest in 3 basic ways. First, CFUe-like stage E1 cells recently have been shown to be highly EPO responsive31,32 but (as summarized in Figure 1) proved to be limited in their expansion capacity. Second, pools of E3 erythroblasts similarly did not markedly expand or accumulate subsequent to EPO dosing, despite their nature as a penultimate precursor to reticulocytes and red blood cells. Third, E2 progenitors (as a Kit− and presumably E1-derived cohort), in contrast, selectively expanded markedly in vivo.
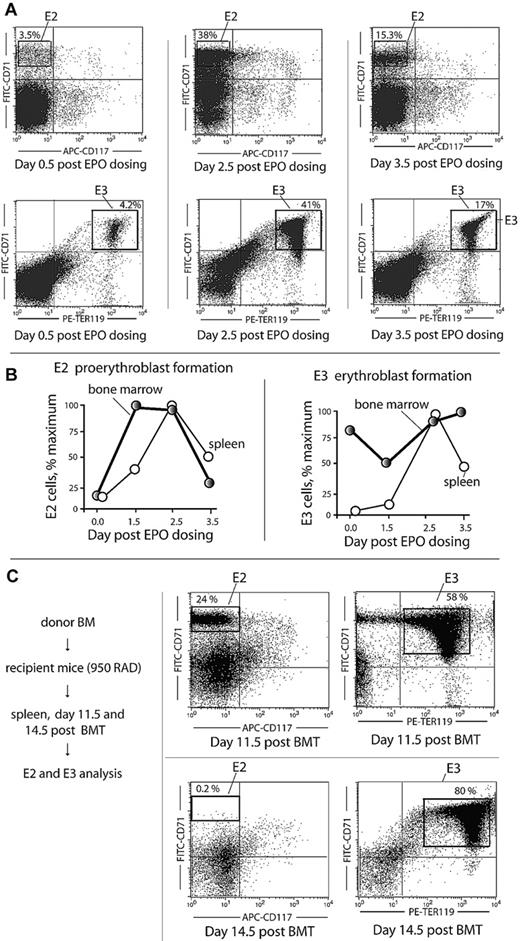
In mouse, spleen also can contribute significantly to erythropoiesis (especially stress erythropoiesis).33 Experiments therefore were performed to examine possible representation in spleen of the aforementioned initially defined transitional “E2” proerythroblast population. Here, splenic E2 and E3 cell formation was analyzed at days 0.5, 2.5, and 3.5 after EPO injection. E2 cells were induced at levels up to 38% of total nucleated cells (Figure 2A). Time courses of EPO-induced E2 and E3 cell formation in BM versus spleen also were compared. As shown in Figure 2B, E2 (and E3) cell expansion initiated within the BM compartment. In addition, EPO-induced E2, E3, and reticulocyte and red cell populations within BM, spleen, and blood compartments were assessed overall (supplemental Figures 2-3). This again revealed early contributions of the BM to erythron expansion. Finally, the extent to which a transitional E2 proerythroblast population might be generated in spleen on BMT was determined. At day 11.5, a predominant E2 donor population clearly expanded during short-term erythron reconstitution (Figure 2C).
Stage E2 proerythroblasts expand within spleen after EPO dosing or BMT. (A) At day 2.5 to 3.5 after EPO dosing (1250 U/kg at 1 and 24 hours), E2 cells were observed to form in spleen at frequencies of more than 30% among total nucleated cells. (B) Within BM, E2, and E3 cell formation precedes that in spleen. In these analyses, frequencies of E2 and E3 cells generated in BM versus spleen after EPO dosing were determined at the indicated intervals. (C) After BMT at day 11.5, E2 cells represent up to 24% of total nucleated cells in spleen. In these studies, possible E2 cell formation in BMT recipient spleens was assessed at days 11.5 and 14.5 after BMT.
Stage E2 proerythroblasts expand within spleen after EPO dosing or BMT. (A) At day 2.5 to 3.5 after EPO dosing (1250 U/kg at 1 and 24 hours), E2 cells were observed to form in spleen at frequencies of more than 30% among total nucleated cells. (B) Within BM, E2, and E3 cell formation precedes that in spleen. In these analyses, frequencies of E2 and E3 cells generated in BM versus spleen after EPO dosing were determined at the indicated intervals. (C) After BMT at day 11.5, E2 cells represent up to 24% of total nucleated cells in spleen. In these studies, possible E2 cell formation in BMT recipient spleens was assessed at days 11.5 and 14.5 after BMT.
BM erythroid cell series formation in vitro recapitulates in vivo stepwise E1→ E2 → E3-stage development
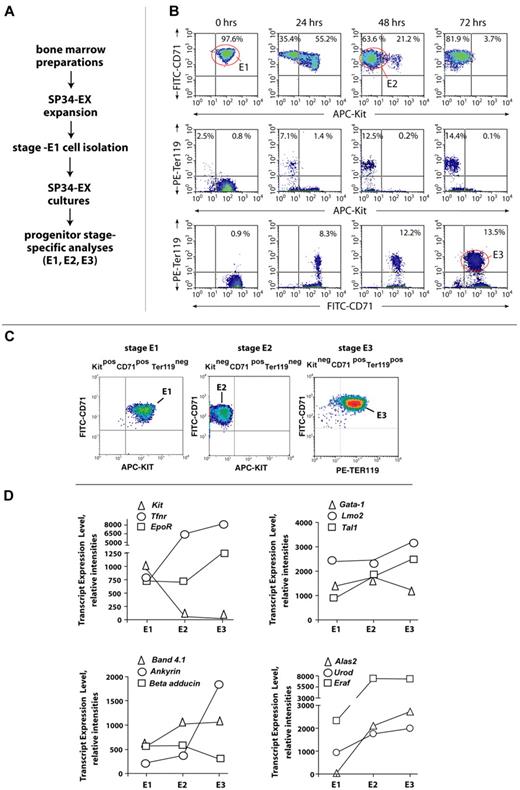
To enable further investigations of stage-specific BM erythroid cell formation, a system for the ex vivo development (and isolation) of stage E1, E2, and E3 cohorts next was established. Here, total BM progenitors were expanded initially for 3 days in a serum-free SP34-ex system, which our laboratory recently has optimized.31,32 Kit+CD71highTer119− stage E1 progenitors were then isolated (via Lin+ depletion plus Kit+ selection), and this cohort of E1 cells was returned to culture (Figure 3A). By 48 hours, more than 50% of E1 cells converted to stage E2 proerythroblasts (Figure 3B top panels) and a limited number further converted to Ter119+ stage E3 cells (Figure 2B bottom panels). By 72 hours, 13.5% of cells advanced to stage E3 (with the balance essentially represented by stage E2 cells). The case that E2 cells compose a discrete and transitional developmental compartment was supported further by an essential absence of observed Kit+ plus Ter119+ copositive cells (Figure 3B middle panels).
BM erythroid progenitors develop ex vivo sequentially as stage E1, E2, and E3 cohorts. (A) Outlined are ex vivo culture conditions for BM preparations and for the synchronous development of a murine BM erythroid series. (B) Time course and frequencies of stage E1 → E2 → E3 cell formation ex vivo. Here, stage E1 cell isolation (before return to culture) was via Lin+ depletion and subsequent MACS-based Kit+ selection. (C) Representative flow cytometric analyses of purified BM-derived stage E1, E2, and E3 cells. (D) Initial transcript profiling of select receptors (Kit, Tfrn, and Epor), transcription factors (Gata1, Lmo2, and Tal1), cytoskeletal components (Band 4.1, Ankyrin, and beta-adducin), and heme synthetic factors (Alas2, Urod, and Eraf) within purified stage E1, E2, and E3 cohorts. The potential occurrence of alternate splice forms is not accounted for via 430 2.0 array scanning.
BM erythroid progenitors develop ex vivo sequentially as stage E1, E2, and E3 cohorts. (A) Outlined are ex vivo culture conditions for BM preparations and for the synchronous development of a murine BM erythroid series. (B) Time course and frequencies of stage E1 → E2 → E3 cell formation ex vivo. Here, stage E1 cell isolation (before return to culture) was via Lin+ depletion and subsequent MACS-based Kit+ selection. (C) Representative flow cytometric analyses of purified BM-derived stage E1, E2, and E3 cells. (D) Initial transcript profiling of select receptors (Kit, Tfrn, and Epor), transcription factors (Gata1, Lmo2, and Tal1), cytoskeletal components (Band 4.1, Ankyrin, and beta-adducin), and heme synthetic factors (Alas2, Urod, and Eraf) within purified stage E1, E2, and E3 cohorts. The potential occurrence of alternate splice forms is not accounted for via 430 2.0 array scanning.
To initially characterize E1, E2, and E3 cohorts, each next was purified to more than 99% (via optimized MACS depletion and selection procedures; Figure 3C). RNA (and cDNA) were then prepared, and transcript levels were analyzed initially for select cell surface receptors, transcription factors, cytoskeletal components, and heme synthetic enzymes (Figure 3D). Among receptors (and as predicted), Kit transcripts were extinguished at stage E2, whereas transferrin receptor (Tfnr) levels increased multifold at stages E1 and E2. EpoR expression (at least at the transcript level) somewhat unexpectedly was sustained at stages E2 and E3. Among transcription factors, Scl and Lmo2 increased 1.4- to 2.1-fold by stage E3, whereas Gata1 levels were sustained in E1, E2, and E3 cells. For cytoskeletal components, band 4.1 increased ∼ 2-fold at stage E2, whereas ankyrin increased at stage E3 ∼ 4-fold. At stages E2 and E3, transcript levels for Alas2, Urod, and Eraf also increased 2- to more than 10-fold. Several markers of advanced erythroid differentiation therefore are significantly up-modulated by stage E3.
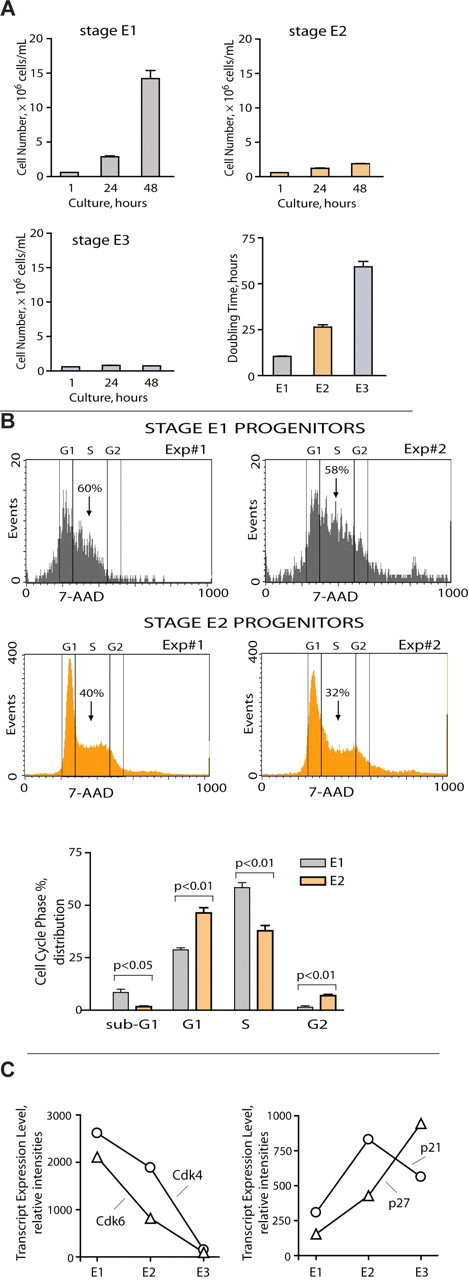
Next, to functionally assess basic properties of isolated E1, E2, and E3 cells, each was placed in SP34-ex culture and assessed for growth and doubling times (Figure 4A). Stage E1 cells proliferated rapidly with an average doubling time of ∼ 10 hours (supplemental Figure 4). By direct comparison, rates of E2 proerythroblast doubling were ∼ 25 hours, whereas E3 cells required more than 2 days to double. As a follow-up to in vivo findings, we also advanced flow cytometric procedures to provide for basic cell cycle analyses of E1 versus E2 progenitors as prepared directly from BM of EPO-dosed mice. As analyzed at day 1.5 after EPO, E1 progenitors proved to enter S-phase at high frequencies (ie, up to 60%). This differed significantly from coanalyzed stage E2 progenitors, which exhibited ∼ 36% S-phase frequencies (on average; Figure 4B). In addition, when plated in CFUe assays, E1 cells formed CFUe-like colonies in ∼ 2 days at a plating efficiency of ∼ 65% to 70%. By direct comparison, E2 cells did not efficiently give rise to hemoglobinized 4- to 8-cell colonies. To define basic molecular features that distinguish E1 from E2 cells, levels of CDK and CDK-i factors for fluorescence-activated cell sorter-purified populations also were assessed (Figure 4C). At the transcript level, Cdk6 and Cdk4 were selectively elevated several-fold in E1 cells (left panel), whereas p21 and p27 were several-fold elevated in stage E2 progenitors (right panel). This at least suggested a functional compartmentalization of BM proerythroblasts in which E1 cells rapidly divide but then rapidly give rise to an expandable E2 proerythroblast cohort. Finally, to provide for histochemical characterizations, stage E1, E2, and E3 cells were purified and processed as May-Grünwald-Giemsa cytospin preparations (supplemental Figure 5A). For E1 cells, colony-forming assays underline their nature as CFUe-like cells, and observed morphologies were consistent with this property (large 8- to 10-μm cells with large nuclei). For E2 cells, May-Grünwald-Giemsa staining was similar to E1 cells, but the size (diameter) of E2 cells is clearly decreased. For E3 cells, it is first noted that Ter119+ cells can be a heterogeneous population. Therefore, by design, we focused our attention on an early E3 subpopulation. As illustrated in cytospins, E3 cells are further decreased in size and also have somewhat contracted nuclei. As a further point of comparison, we also fluorescence-activated cell sorter purified and examined Ter119high erythroblasts. As shown in supplemental Figure 5C, these late-stage erythroblasts are smaller yet and exhibit further condensed nuclei. Therefore, stage E1 cells are CFUe-like progenitors, and stage E3 cells are Ter119+ erythroblasts. Stage E2 cells are an intermediate for which we therefore prefer to reserve the term “proerythroblasts.”
Stage E1 progenitors hyperproliferate, whereas E2 proerythroblasts limit this expansion mechanism. (A) Primary stage E1, E2, and E3 BM cells were isolated, returned to culture, and analyzed for proliferation and doubling times. (B) E1 and E2 cell cycle distributions also were determined based on 7-amino-actinomycin D analyses using fresh bone preparations (and Kit, CD71, plus Ter119 costaining and gating). Stage E1 cells were distributed at ∼ 60% in S-phase. (C) During stage E1 → E2 → E3 cohort development, levels of Cdk4 and Cdk6 decrease, whereas p21 and p27 increase multifold. Values are mean ± SE for triplicate transcriptome profiling experiments.
Stage E1 progenitors hyperproliferate, whereas E2 proerythroblasts limit this expansion mechanism. (A) Primary stage E1, E2, and E3 BM cells were isolated, returned to culture, and analyzed for proliferation and doubling times. (B) E1 and E2 cell cycle distributions also were determined based on 7-amino-actinomycin D analyses using fresh bone preparations (and Kit, CD71, plus Ter119 costaining and gating). Stage E1 cells were distributed at ∼ 60% in S-phase. (C) During stage E1 → E2 → E3 cohort development, levels of Cdk4 and Cdk6 decrease, whereas p21 and p27 increase multifold. Values are mean ± SE for triplicate transcriptome profiling experiments.
Stage-selective survival mechanisms are deployed by E1 and E2 erythroid cell cohorts
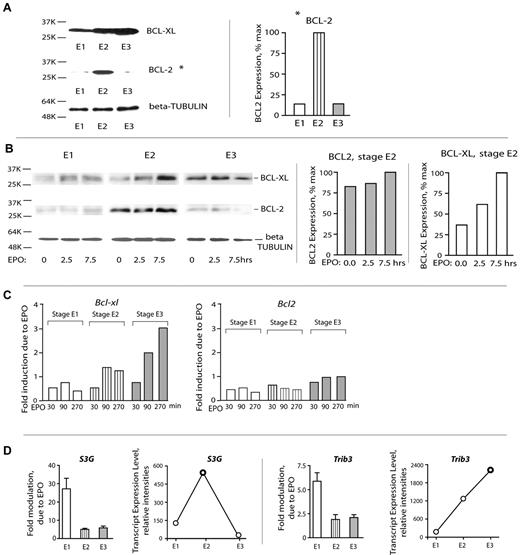
The above analyses (“Results,” first two subsections) indicated that stage E1 cells respond sharply to EPO but that this includes an apparently strong proliferative response. This raised basic related questions concerning the nature of survival factors that are used by expanding BM erythroid cohorts. To initially address this, BCLXL and BCL2 levels in purified E1, E2, and E3 cells were assayed. Expectedly, BCLXL levels increased as erythroblasts matured. In contrast, and unexpectedly, BCL2 proved to be expressed at substantial levels selectively in stage E2 proerythroblasts (Figure 5A). The extent to which BCL-XL and BCL2 might be EPO/EPOR-regulated was next studied. As analyzed via Western blotting, BCLXL expression was first detectably induced by EPO in stage E2 proerythroblasts (but only on extended exposure; 7.5 hours). BCL2 expression, in contrast, was activated selectively within stage E2 cells and appeared to be EPO-independent (Figure 5B). Similarly, when analyzed at the transcript level, Bcl-xL was most clearly induced by EPO in later-stage erythroblasts, whereas Bcl2 transcript levels were not EPO-modulated at E1, E2, or E3 stages (Figure 5C).
Stage E2 proerythroblasts deploy BCL2, whereas BCL-XL expression is activated subsequently at stage E3. (A) Stage E2 proerythroblasts (but not E1 or E3 cells) express elevated BCL2. Stage E1, E2, and E3 cells were purified from primary SP34-ex cultures, and lysates were directly prepared. Levels of BCL2 and BCL-XL were then assayed by Western blotting. (B) BCL-XL and BCL-2 expression in stage E1, E2, and E3 BM erythroid cohorts. Stage E1, E2, and E3 cells were isolated and cultured for 6 hours in the absence of hematopoietic cytokines. Cells were then exposed to EPO (5 U/mL) for 2.5 and 7.5 hours. Cell lysates were prepared and analyzed for BCL-XL, BCL-2, and β-tubulin expression via Western blotting (left panel). Band intensities of BCL-XL and BCL2 were determined using ImageJ software Version 1.42j (right panel). (C) EPO/EPOR signals regulate Bcl-xL (but not Bcl2) in late-stage E3 erythroblasts. Purified primary BM stage E1, E2, and E3 cells were cultured for 6 hours in the absence of hematopoietic growth factors and then challenged with EPO. At 30, 90, and 270 minutes, cells were lysed in Trizol, and RNA and cDNA were prepared. Bcl-xL and Bcl2 levels then were determined via quantitative RT-PCR (with beta-actin as an internal control). (D) Stage-predominant EPO induction and up-modulation of select EPO response factors, such as S3G and Trib3. Assays of transcripts were performed via quantitative RT-PCR. Values are mean ± SE.
Stage E2 proerythroblasts deploy BCL2, whereas BCL-XL expression is activated subsequently at stage E3. (A) Stage E2 proerythroblasts (but not E1 or E3 cells) express elevated BCL2. Stage E1, E2, and E3 cells were purified from primary SP34-ex cultures, and lysates were directly prepared. Levels of BCL2 and BCL-XL were then assayed by Western blotting. (B) BCL-XL and BCL-2 expression in stage E1, E2, and E3 BM erythroid cohorts. Stage E1, E2, and E3 cells were isolated and cultured for 6 hours in the absence of hematopoietic cytokines. Cells were then exposed to EPO (5 U/mL) for 2.5 and 7.5 hours. Cell lysates were prepared and analyzed for BCL-XL, BCL-2, and β-tubulin expression via Western blotting (left panel). Band intensities of BCL-XL and BCL2 were determined using ImageJ software Version 1.42j (right panel). (C) EPO/EPOR signals regulate Bcl-xL (but not Bcl2) in late-stage E3 erythroblasts. Purified primary BM stage E1, E2, and E3 cells were cultured for 6 hours in the absence of hematopoietic growth factors and then challenged with EPO. At 30, 90, and 270 minutes, cells were lysed in Trizol, and RNA and cDNA were prepared. Bcl-xL and Bcl2 levels then were determined via quantitative RT-PCR (with beta-actin as an internal control). (D) Stage-predominant EPO induction and up-modulation of select EPO response factors, such as S3G and Trib3. Assays of transcripts were performed via quantitative RT-PCR. Values are mean ± SE.
Recently, 2 new candidate survival factors have been reported to be induced in primary BM Kit+ cells: the intracellular serpin Serpina 3G31 and pseudokinase TRB3. It therefore was of interest to at least initially examine expression patterns for each in developing E1, E2, and E3 cells and in each ± EPO challenge (Figure 5D). Interestingly, S3g was predominantly EPO-induced at stage E1 but peaked in expression at stage E2. Trb3, in contrast, was EPO-induced more than 5-fold in E1 cells but remained more than 2-fold inducible at stage E2 and E3 and furthermore accumulated more than 12-fold by stage E3. These data therefore again point to interesting stage differential use of EPO-modulated signal transduction factors.
Erythropoiesis as supported by a hypomorphic EPOR allele is skewed within BM selectively at an E2 proerythroblast stage
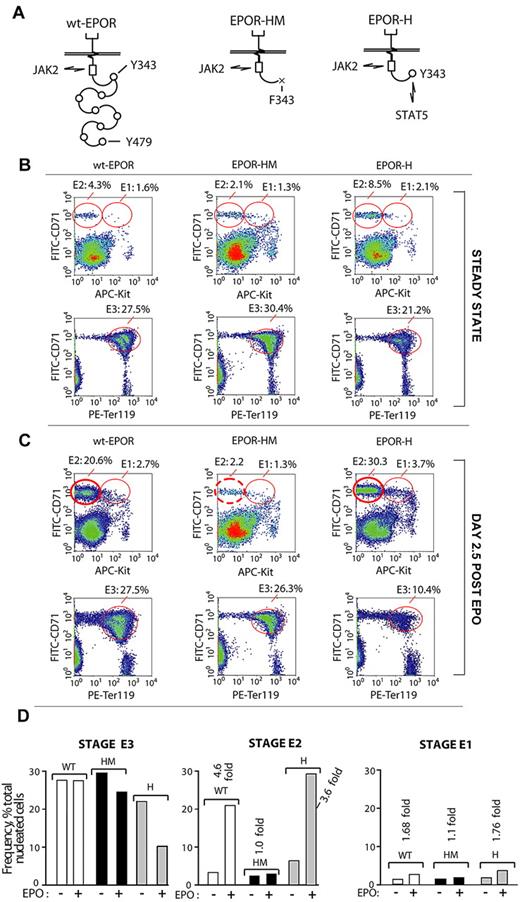
Experiments next returned to in vivo analyses and examined representations of E1, E2, and E3 cohorts in mice expressing the minimal knocked-in EPOR alleles, EPOR-HM or EPOR-H (Figure 6A). EPOR-HM lacks 8 cytoplasmic PY sites for the coupling of several signal transduction factors (including p85-α, SHP2, STAT5, and LNK)34-38 and is a hypomorphic allele.29,39 In EPOR-H, a single cytoplasmic PY-binding site for STAT5 (PY343) is restored, and EPOR-H is hypermorphic.39 For BM preparations from each line, E1, E2, and E3 populations were analyzed both at steady state and after EPO challenge (Figure 6B-D). At steady state, E1, E2, and E3 cell profiles for EPOR-H and EPOR-HM mice were somewhat unexpected in 2 regards. First, although EPOR-H mice typically exhibit elevated hematocrits,39 their late-stage E3 BM erythroblast pools were under-represented (ie, 21% on average vs 27.5% and 30.4% in wt-EPOR and EPOR-HM BM, respectively). Second, EPOR-HM mice exhibited essentially wild-type levels of E3 erythroblasts (despite their phenotype of at least moderate anemia). Additional unexpected yet informative results were generated when the erythron was challenged by EPO. Within EPOR-HM BM, E2 proerythroblasts faltered markedly in their expansion (∼ 10-fold deficit compared directly with wt-EPOR and EPOR-H mice). In addition, the apparently limited production of E3 erythroblasts by EPOR-H mice at steady state became marked after EPO challenge (10.4% on average, vs 27.5% and 26.3% for wt-EPOR and EPOR-HM mice). Another relevant observation is that viability was uniformly high for E1, E2, and E3 cell populations among wt-EPOR, EPOR-H, and EPOR-HM mice (as assessed by staining with both annexin V, and YoPro1; data not shown). Taken together, these findings point further to stage E2 proerythroblasts as a key transitional and uniquely expandable BM component of the adult erythron.
Disruption of EPOR/JAK2/STAT5 signaling disrupts BM erythropoiesis selectively at an E2-stage of BM proerythroblast development. (A) Diagrammed are the wild-type EPOR (wt-EPOR); the PY-null allele, EPOR-HM; and a related EPOR-H allele in which a PY343 site for STAT5 is selectively restored. (B-C) EPOR-HM mice exhibit a major selective defect in the expansion of BM stage E2 proerythroblasts. In mice harboring wt-EPOR, EPOR-HM, or EPOR-H alleles, frequencies of stage E1, E2, and E3 cells were analyzed at steady state and at day 2.5 after EPO dosing. In EPOR-HM mice, and on EPO challenge, levels of stage E3 erythroblasts unexpectedly were near-normal. Their stage E2 progenitors, however, failed to undergo the more than or equal to 5-fold expansion observed within wt-EPOR and EPOR-H BM. For EPOR-H BM erythroid cells, also note an enhanced expansion of stage E2 proerythroblasts (and a > 2-fold deficit in EPOR-H stage E3 erythroblast formation). (D) Effects of EPOR-HM (and EPOR-H) allele signaling on E1, E2, and E3 BM cell formation are summarized.
Disruption of EPOR/JAK2/STAT5 signaling disrupts BM erythropoiesis selectively at an E2-stage of BM proerythroblast development. (A) Diagrammed are the wild-type EPOR (wt-EPOR); the PY-null allele, EPOR-HM; and a related EPOR-H allele in which a PY343 site for STAT5 is selectively restored. (B-C) EPOR-HM mice exhibit a major selective defect in the expansion of BM stage E2 proerythroblasts. In mice harboring wt-EPOR, EPOR-HM, or EPOR-H alleles, frequencies of stage E1, E2, and E3 cells were analyzed at steady state and at day 2.5 after EPO dosing. In EPOR-HM mice, and on EPO challenge, levels of stage E3 erythroblasts unexpectedly were near-normal. Their stage E2 progenitors, however, failed to undergo the more than or equal to 5-fold expansion observed within wt-EPOR and EPOR-H BM. For EPOR-H BM erythroid cells, also note an enhanced expansion of stage E2 proerythroblasts (and a > 2-fold deficit in EPOR-H stage E3 erythroblast formation). (D) Effects of EPOR-HM (and EPOR-H) allele signaling on E1, E2, and E3 BM cell formation are summarized.
Stage E2 BM proerythroblasts selectively and productively interact with primary BM stromal cells
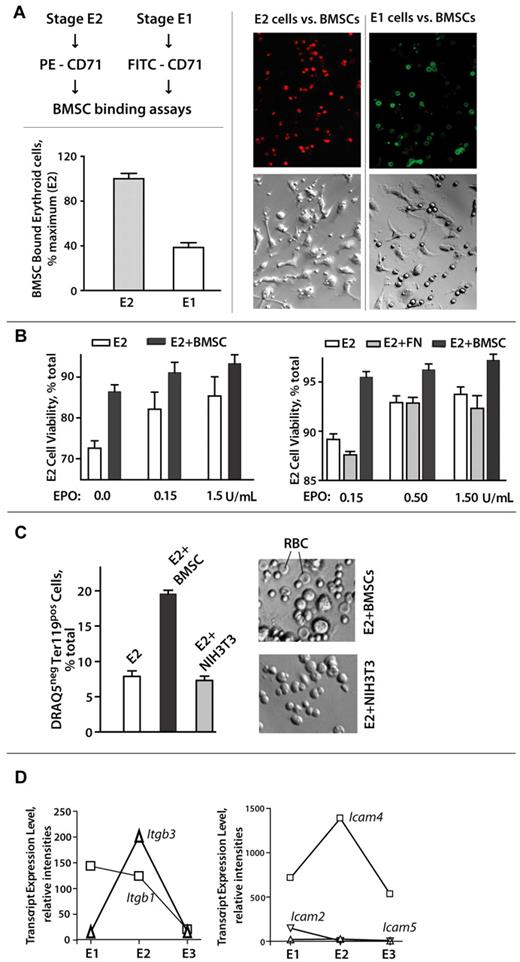
The selective ability of stage E2 proerythroblasts to markedly expand within BM prompted a concept that E2 cells might engage and use stromal cells as a supporting functional niche. Support for this concept also was provided by recent reports that CD34-derived erythroid progenitors27,28 as well as those from fetal liver7,40 can advance in their development via interactions with stromal cell populations. Therefore, primary murine BMSCs were prepared as Lin-depleted, F4-80-depleted populations, and assessed for possible interactions with BM E1, E2, and E3 cells. E3 erythroblasts essentially failed to bind such primary BMSCs; E1 cells detectably bound BMSCs, whereas stage E2 proerythroblasts avidly bound BMSCs. To assist visualizations, E2 and E1 cells were stained (at limiting levels) with PE–anti-CD71 (stage E2) or FITC–anti-CD71 (stage E1) and then cultured (in equal numbers) with primary BMSCs. Stage E2 cells showed clearly enhanced BMSC interactions (Figure 7A; supplemental Figure 3), and this was reproducibly observed in 6 independent experiments.
Stage E2 BM proerythroblasts selectively and productively interact with primary BM stromal cells. (A-B) Stage E1 and E2 cells were purified from SP34-ex cultures and assessed for interactions with primary BMSCs. When cultured in parallel (at equal numbers, and cells per milliliter) with BMSCs, E2 proerythroblasts bound BMSCs at ∼ 250% increased levels over E1 cells (Axiovent 200, 160× overall, 1.6 NA, Vectashield, Axiocam MR, Illustrator 10). (C) E2 proerythroblast-BMSC interactions can also promote maturation to DRAQ5− reticulocytes and apparent erythrocytes. On continued coculture (2-3 days), primary Lin− BMSCs supported reticulocyte and apparent red cell formation as assessed by DRAQ5 staining (via flow cytometry) and apparent morphology (∼ 5-μm-diameter cells with apparent (bi)concave shape). (D) Expression of adhesion factors Itgb3 and Icam-4 peaks in stage E2 BM proerythroblasts. Expression of cell surface adhesion factors was assessed initially via analyses of triplicate transcriptome profiles for purified BM E1, E2, and E3 cell populations. For the integrins and Icams illustrated, relative expression levels also were assayed via quantitative RT-PCR (with beta-actin as an internal control).
Stage E2 BM proerythroblasts selectively and productively interact with primary BM stromal cells. (A-B) Stage E1 and E2 cells were purified from SP34-ex cultures and assessed for interactions with primary BMSCs. When cultured in parallel (at equal numbers, and cells per milliliter) with BMSCs, E2 proerythroblasts bound BMSCs at ∼ 250% increased levels over E1 cells (Axiovent 200, 160× overall, 1.6 NA, Vectashield, Axiocam MR, Illustrator 10). (C) E2 proerythroblast-BMSC interactions can also promote maturation to DRAQ5− reticulocytes and apparent erythrocytes. On continued coculture (2-3 days), primary Lin− BMSCs supported reticulocyte and apparent red cell formation as assessed by DRAQ5 staining (via flow cytometry) and apparent morphology (∼ 5-μm-diameter cells with apparent (bi)concave shape). (D) Expression of adhesion factors Itgb3 and Icam-4 peaks in stage E2 BM proerythroblasts. Expression of cell surface adhesion factors was assessed initially via analyses of triplicate transcriptome profiles for purified BM E1, E2, and E3 cell populations. For the integrins and Icams illustrated, relative expression levels also were assayed via quantitative RT-PCR (with beta-actin as an internal control).
Effects of BMSC interactions on E2 cell survival and development also were examined (Figure 7B-C). When cocultured with primary BMSCs, survival rates for stage E2 BM proerythroblasts were significantly enhanced (Figure 7B left panel). This was most obvious at low EPO concentrations (and was not supported by fibronectin per se; Figure 7B right panel). On continued coculture (ie, 2-3 days), BMSCs also promoted the production of DRAQ5− cells, as well as morphologically apparent erythrocytes (Figure 7C). This effect was not supported by NIH-3T3 cells. Finally, to begin to consider what cell surface factors might mediate selective E2 proerythroblast-BMSC interactions, we initially mined our E1, E2, and E3 BM erythroid cell series transcriptome profiles and identified 2 candidate factors with clear peak expression at stage E2, integrin beta3, and Icam4 (Figure 7D).
Discussion
The present studies reveal interesting new complexities in the stepwise development of a BM erythroid cell series. This includes an advanced defining of how developing cohorts transition (in response to EPO) in growth and survival contexts to more mature stages with distinct survival mechanisms. In addition, evidence is provided to support the existence of a proposed functional niche for the selective expansion of transitional stage E2 proerythroblasts, especially when the erythron is challenged.
One basic component for discussion concerns the nature of distinct survival mechanisms that appear to be differentially used by stage E3 Kit−CD71highTer119+, stage E2 Kit−CD71highTer119−, and stage E1 Kit+CD71highTer119− cells. Previously, BCL-XL has been proposed to be a central (and perhaps necessary and sufficient) explanation for EPO's prosurvival effects.40 However, studies of BCL-XL-null erythroblasts, high-level BCL-XL expression, and cell survival effects were restricted to relatively late-stage erythroblasts.41 Follow-up studies argued further that BCL-XL might largely be uncoupled from EPO's actions. Present analyses are consistent with these latter concepts in that, for highly EPO-responsive (and EPO-dependent) stage E1 cells, significant EPO induction of Bcl-xl was not detected (Figure 5C). Rather, this response did not become obvious until BM erythroblasts developed to stage E3. Late-stage BCL-XL expression therefore might further depend on previously proposed reinforcing roles for GATA1 plus GFI-1B.42 GATA1, in addition, recently has been shown to stimulate LRF (leukemia/lymphoma-related factors) expression43 ; and in late-stage erythroblasts, this transcription factor further can enforce survival by repressing BIM (a proapoptotic BH3-only factor).43 As noted in “Results,” and for stage E2 cells, prolonged exposure to EPO (7.5 hours) did lead to detectably increased BCL-XL levels (Figure 5B). Recently, EPO has been demonstrated to stimulate BIM phosphorylation44 ; phosphorylation at S65 has been shown to down-modulate BIM45 ; and BIM down-modulation has been shown to stabilize BCL-XL.46 Therefore, possible S65 phosphorylation of BIM could account for enhanced BCL-XL levels in EPO-exposed E2 proerythroblasts. On exiting from stage E1, earlier stage E2 proerythroblasts, in contrast, are shown to uniquely deploy stage-specific expression of BCL2 (Figure 5). This transient boost in BCL2 is proposed to assist a transition from EPO dependence. In other hematopoietic lineages, BCL2 is frequently used as an important antiapoptotic factor.47 Reports of BCL2 utilization in erythroid cells, however, are limited (perhaps because of the presently defined small window of BCL2 activation).
For early-stage E1 cells, the specific nature of EPO/EPOR regulated targets that act as survival factors is less clear but is a subject of considerable interest. Via recent transcriptome profiling analyses of EPO/EPOR response circuits in such primary BM erythroid cells,30,31 2 candidate effectors have been suggested to function as survival factors, TRIB3 and Serpina-3G (S3G). TRIB3 is a pseudokinase and emerging E3 ubiquitin ligase adaptor,48 whereas S3G is an atypical intracellular serpin31 which in memory T cells may inhibit lysosomal cathepsin-induced cell death.49 On ectopic expression, each was demonstrated to attenuate erythroid progenitor cell death.31 In the present studies, Trib3 was EPO-induced primarily in stage E1 cells as well as stage E2 and E3 cells. Profiling of purified E1, E2, and E3 cells further revealed a more than or equal to 12-fold increase in maturing E3 erythroblasts. This suggests potential broad roles for TRIB3 during erythroid development (and this suggestion is supported further by initial analyses of novel TRIB3-knockout mice).50 S3g, in contrast, is EPO-induced predominantly in E1 cells, peaks at stage E2, but is markedly diminished at stage E3. This points to possible prime positive roles for S3G in earlier stage cells. Such analyses also raise an additional interesting prospect that cohorts at various stages of development may be programmed to differentially respond to EPO. Additional EPO/EPOR-modulated regulators, of course, may also exist as mediators of stage E1 and E2 cell survival. Recent examples include GAS6 as a growth-arrest specific cytokine, which can synergize with EPO,51 and LIAR as a novel partner of LYN.52
An additional set of findings concerns observed dynamics of erythroid cell residency within stage E1, E2, and E3 pools. In vivo, stage E1 cells expectedly were relatively infrequent at steady state but, in addition, did not markedly accumulate after EPO dosing. This indicated not only rapid proliferation but also rapid conversion to stage E2 proerythroblasts. Consistent with this concept, EPOR ligation in stage E1 cells suppressed inhibitory cyclin G2 (and induced cyclin D2 ∼ 4-fold, data not shown). In addition, when stage E1 cells were purified and returned to culture, stage E2 proerythroblasts were rapidly generated. Indeed, the numbers of stage E2 cells accumulated in an EPO-dependent fashion up to 50-fold over a 3-day period (supplemental Figure 4). This raised a central question regarding whether select stages of BM proerythroblast development might be specifically affected in mice harboring a compromised PY-null EPOR allele (EPOR-HM).29,39 Interestingly, in EPOR-HM mice, stage E2 proerythroblast formation proved to be dramatically compromised (Figure 6). It is noted, however, that this also could involve effects on E1 proliferation as well as E2 expansion. In a broader hematopoietic framework, these results are interesting to compare with the stepwise process of B-cell development and studies of roles of the IL7R and STAT5. Here, IL7R deficiency leads to failed development selectively at a pre-pro B-cell stage.53 Recent studies point further to duality in action. Specifically, for pre-pro progenitors, the IL-7R may predominantly enforce early B-cell factor transcription factor levels54 but subsequently may engage STAT5 to promote proliferation (and to perhaps inhibit Igk transcription).55 It is therefore provocative to speculate that EPO/EPOR action may likewise involve such stage-specific effects.
One final interesting observation concerns the selective propensity of stage E2 cells to bind to, and productively interact with, primary BMSCs (Figure 7; supplemental Figure 6). One basic point to be made is that, based on the ability of E2 cells to form in liquid culture, such BMSCs interactions may be nonessential for E1 → E2 → E3 development. This, however, may not lessen the potential in vivo importance of such interactions. Specific factors that mediate E2 cell-BMSC binding are presently unresolved. Stage-specific transcriptome analyses at least initially point to Itgb3, and Icam4 as stage E2 predominant cell surface adhesion proteins (Figure 7). More work, however, is needed to functionally test the relative importance of candidate tethering factors. Observed effects of E2 cell-BMSC interactions are 2-fold. First, BMSCs provide significant survival advantages (especially when EPO is limiting). Second, E2 cell development to stage E3 erythroblasts, reticulocytes, and erythrocytes is stimulated. At present, this aspect of the current report is somewhat descriptive, but nonetheless interesting in that it may reflect a need to use stromal cell lines to facilitate the development of primary erythroblasts from both BM and ES cell sources.27,28 A closing notion is that BMSCs also may provide a compartmentalized surface for rapid expansion, given the observation that E2 BM proerythroblasts may comprise up to 20% of total nucleated BM cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grants R01 HL44491 and R01 DK089439; principal investigator D.M.W.), Maine Medical Center Research Institute core facilities in Flow Cytometry and Progenitor Cell Analysis, and Bioinformatics (P20RR018789; principal investigator D.M.W.).
National Institutes of Health
Authorship
Contribution: A.D. and J.F. performed analyses of EPO effects on erythroid progenitor pools, gene profiling, RT-PCR, Western blotting, and experiments on erythroblast-stromal cell interaction assays; P.S. performed ex vivo erythroblast expansions, gene profiling, and experiments on erythroblast-stromal cell interaction assays; A.P. and C.E. performed ex vivo expansion of erythroblasts and experiments on erythroblast-stromal cell interaction assays; D.M.W. designed and directed these investigations and contributed prime efforts in manuscript writing and construction; and all authors contributed to both bench investigations and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Don M. Wojchowski, Center of Excellence in Stem Cell Biology and Regenerative Medicine, Maine Medical Center Research Institute, 81 Research Dr, Scarborough, ME 04074; e-mail: wojchd@mmc.org.
References
Author notes
A.D. and J.F. contributed equally to this study and are co–first authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal