Abstract
Erythropoietin (Epo) is required for erythroid progenitor differentiation. Although Epo crosslinking experiments have revealed the presence of Epo receptor (EpoR)–associated proteins that could never be identified, EpoR is considered to be a paradigm for homodimeric cytokine receptors. We purified EpoR-binding partners and identified the type 2 transferrin receptor (TfR2) as a component of the EpoR complex corresponding to proteins previously detected in cross-linking experiments. TfR2 is involved in iron metabolism by regulating hepcidin production in liver cells. We show that TfR2 and EpoR are synchronously coexpressed during the differentiation of erythroid progenitors. TfR2 associates with EpoR in the endoplasmic reticulum and is required for the efficient transport of this receptor to the cell surface. Erythroid progenitors from TfR2−/−mice show a decreased sensitivity to Epo and increased circulating Epo levels. In human erythroid progenitors, TfR2 knockdown delays the terminal differentiation. Erythroid cells produce growth differentiation factor-15, a cytokine that suppresses hepatic hepcidin production in certain erythroid diseases such as thalassemia. We show that the production of growth differentiation factor-15 by erythroid cells is dependent on both Epo and TfR2. Taken together, our results show that TfR2 exhibits a non hepatic function as a component of the EpoR complex and is required for efficient erythropoiesis.
Introduction
Erythropoiesis is mainly regulated by the kidney-produced hormone erythropoietin (Epo), which is absolutely required for the survival and proliferation of erythroid progenitors and their terminal differentiation to red cells.1 The Epo receptor (EpoR) is a type 1 transmembrane protein that belongs to the class 1 cytokine receptor family. Its expression level in erythroid cells is low whatever the differentiation stage and even the most Epo-sensitive cells, colony-forming units erythroid (CFU-Es), or proerythroblasts express less than 1000 EpoRs per cell at their surface.2
In the absence of Epo, EpoR is believed to be homodimeric, in which each dimer protein is constitutively associated with a Janus kinase-2 (Jak-2) tyrosine-kinase molecule. Epo binding modifies the organization of the receptor complex leading to Jak-2 activation. Association between Jak-2 and the EpoR occurs during the receptor maturation process, most likely before EpoR leaves the endoplasmic reticulum, and is essential for expression of the receptor at the cell surface.3 However, the mechanisms that control the maturation and the transport of the EpoR to the cell surface remain largely unknown. Indeed, the number of cell surface EpoRs is not related to the synthesis level of this protein and most of these molecules accumulate in the endoplasmic reticulum in EpoR-overexpressing cells.4 Both functional and direct evidence also suggests that unidentified proteins are associated with EpoR on the cell surface. In particular, chemical cross-linking of radiolabeled Epo to cell surface erythroid cells has frequently revealed the presence of 2 additional proteins with apparent molecular masses of 85 and 100 kDa in the EpoR complex.2,5-8 Unfortunately, because of the low expression level of the EpoR, these proteins could never be identified up to now. The dramatic progress that has been recently introduced by the application of mass spectrometry methods to protein analysis led us to address the question of the identity of the EpoR-associated proteins.
Here, we have purified the EpoR complex from UT7 erythroleukemic cells that express endogenous EpoRs to identify EpoR-associated proteins. Mass spectrometry analysis of the purified proteins revealed the presence of the type 2 transferrin receptor (TfR2) in the EpoR complex.
Mammals express 2 transferrin receptors that share similar overall structures but possess specific functions. Most cells including erythroid precursors internalize iron from plasma diferric transferrin through the ubiquitously expressed transferrin receptor 1 (TfR1). A Tfr1 gene deletion is lethal in mice with severe anemia and is not compensated by TfR2.9 In contrast, TfR2 expression is tissue-restricted with high expression in the liver10 where it plays a key role in iron metabolism regulation. Indeed, TfR2 contributes to the adaptation of hepcidin production to the needs of the body by sensing the circulating iron bound to transferrin.11 Familial inactivating or non-sense mutations in the TfR2 gene are responsible for hemochromatosis in humans.12 Moreover, mice containing a targeted deletion of the TfR2 gene in the liver also develop iron overload.13-15
We show here that TfR2 and EpoR are coexpressed during the differentiation process of erythroid progenitors. TfR2 associates with EpoR in the endoplasmic reticulum and is required for the efficient cell surface expression of this receptor. Erythroid progenitors from TfR2−/−mice show a decreased sensitivity to Epo that is corrected by increased circulating Epo levels. In human erythroid progenitors, TfR2 knockdown delays the terminal differentiation. Finally, the production of the candidate hepcidin regulator cytokine growth differentiation factor (GDF-15)16 by erythroid cells is dependent on both Epo and TfR2.
Methods
Reagents and antibodies
Recombinant human Epo was a gift of Dr M. Brandt (Roche Penzberg). Anti-EpoR (C-236) used in the immunoprecipitation experiments has been described previously.17 The anti-EpoR (C-20, batch K-200) antibodies used for Western blotting were purchased from Santa Cruz Biotechnology. We have previously shown that these antibodies recognized the EpoR with high specificity.18,19 Anti-TfR2 antibodies (9F8C11) were purchased from Santa Cruz Biotechnology. Human GDF15 enzyme-linked immunosorbent assay (ELISA) kit was obtained from R&D Systems.
Purification of the EpoR complex
Two purifications were performed using either Brij 98 (polyoxyethylene 20 oleyl ether) or NP-40 (nonyl phenoxypolyethoxylethanol) as the detergent (Figure 1). UT7 cells (500 × 106) were Epo-deprived overnight and incubated for 10 minutes with 10 U/mL Epo which had been biotinylated on sialic acid residues.18 The cells were solubilized in 5 mL solubilization buffer (25mM Tris/HCl, 150mM NaCl, 5mM EDTA [ethylenediaminetetraacetic acid], 10% glycerol, pH7.5) containing Complete protease inhibitors (Roche Diagnostics) and either 1% NP40 or 1% Brij 98. Cell extracts were cleared by centrifugation (20 minutes, 27 000 × g) and separated by chromatography on 160 μL UltraLink monomeric avidin beads (Pierce Thermoscientific). After extensive washing with solubilization buffer containing 0.1% detergent, bound proteins were eluted with 1.2 mL of the same buffer containing 5mM biotin. The eluted material was first incubated for 1 hour with 50 μL anti–glutathione S-transferase affinity beads and then with 50 μL anti-EpoR affinity beads. After incubation with biotin-eluted extracts, affinity beads were washed 6 times with solubilization buffer containing 0.1% detergent and twice with 50mM ammonium bicarbonate. Proteins were then digested on the beads with trypsin and the produced peptides were analyzed by mass spectrometry using a LTQ-Orbitrap mass spectrometer (Thermo Scientific).
Purification and amplification of human erythroid progenitors
Cluster of differentiation (CD)34+ cells were obtained from human donors who gave informed consent in accordance with the Declaration of Helsinki Principles. CD34+ cells were purified from the peripheral blood after cytapheresis by positive selection using an immunomagnetic procedure (MACS CD34 isolation Kit; Miltenyi Biotec). CD34+ cells were cultured for 7 days in Iscove Dulbecco Modified Eagle Medium containing 15% BiT (bovine serum albumin, insulin, and transferrin) 9500 (StemCell Technologies), 100 ng/mL stem cell factor (SCF), 10 ng/mL interleukin-6 (IL-6), and 10 ng/mL IL-3. After 7 days of culture, CD36+ cells corresponding to a highly purified population of human erythroid progenitors were obtained by selection on immunomagnetic beads as previously reported.20 CD36+ cells were then cultured in the presence of 2 U/mL Epo, 100 ng/mL SCF, and 10 ng/mL IL-3 up to 12 days for erythroid differentiation.
Mice
Mice of a pure sv129 background were used to produce the germinal and liver restricted TfR2 knockout animals.21 The mice were housed in the barrier facility at the Department of Clinical and Biological Sciences of the University of Torino, Italy and maintained on a standard diet. All procedures were carried out in compliance with the guidelines of Institutional Animal Care and Use Committee of Torino University. Experiments were performed on these animals at 4 weeks of age and wild-type sibling pairs were used as controls.
CFU-E assay
An aliquot of 2 × 105 bone marrow cells obtained from the femurs and tibias of 2 TfR2−/− or 2 wild-type mice were cultured in methylcellulose medium according to the manufacturer's recommendations (Methocult M3234; StemCell Technologies). The medium was supplemented by increasing Epo concentration. Cells were plated in duplicates. Colonies were scored after 48 hours of culture.
Production and use of lentiviral constructs
Lentiviral constructs for control (SHC001) and TfR2 (A: TRCN0000063631 or B: TRCN0000063632) shRNAs were purchased from Sigma-Aldrich (NM_003227.3). Infections of human CD34+ cells with lentiviruses were performed on days 5 and 6 of culture in the presence of IL-3, SCF, and IL-6. CD36+ cells from these different populations were then isolated on day 7 and cultured as described in the “Purification and amplification of human erythroid progenitors” section.
ATP measurement
Adenosine-triphosphate (ATP) measurement was determined with Cell Titer Glo (Promega).
Analysis of TfR2, GPA, and CD71 expression by flow cytometry
Cell labeling was carried out as previously described.22 Briefly, cells were labeled with mouse anti–human TfR2 antibody or irrelevant mouse immunoglobulin G1 (IgG1) antibody, washed and then labeled with a goat Fab'2 anti–mouse IgG1-phycoerythrin (PE) antibody (IM0855; Beckman Coulter). Anti–glycophorin A (GPA)–PE antibodies (MHGLA04) and irrelevant mouse IgG1-PE were from Caltag. Anti-CD71–FITC antibodies (IM0483) and irrelevant mouse IgG1-FITC were from Beckman Coulter.
Statistical analysis
Data are expressed as mean values plus or minus SDs. To allow comparison between experiments, absolute values measured in cells transfected with a control shRNA were generally set to 100 in each experiment and other values were expressed relative to this control. Statistical significance of differences observed between groups was determined using Student t test. When appropriate, a single asterisk means that a statistical difference exists with P < .05, 2 asterisks with P < .01, and 3 asterisks with P < .001.
Results
Association between TfR2 and EpoR
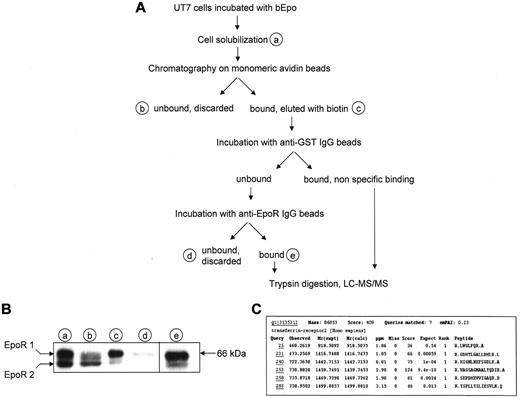
To answer longstanding question regarding the identity of unidentified proteins associated to cell surface EpoR, we developed a 2-step method that enables the purification of EpoR complex from UT7 erythroleukemia cells expressing the endogenous receptor23 using Epo biotinylated on sialic acid residues (bEpo) that retains full biological activity.18 Epo-starved UT7 cells were incubated with bEpo and solubilized. Because the association between membrane proteins is often sensitive to detergents and can be disrupted by some of them, 2 different detergents, NP40 and Brij 98, were used in 2 separate experiments. After centrifugation, solubilized proteins were separated by chromatography on monomeric avidin beads and bound material was eluted with biotin (Figure 1A). This method allowed the quantitative recovery of the cell surface form of the EpoR (Figure 1B). Biotin-eluted proteins were first cleared by incubation with anti–glutathione S-transferase antibodies bound on protein G beads and EpoR complexes were purified with anti-EpoR antibodies. The purified proteins were digested with trypsin on the beads before analysis by mass spectrometry, leading to the identification of proteins already known to belong to the activated EpoR complex such as Epo, EpoR, Jak2, and β-transducin repeat-containing protein (data not shown). In addition, TfR2 was identified in each purification experiment using NP40 or Bri98 (Figure 1C).
Identification of TfR2 as an EpoR binding partner. (A) Strategy for identification of EpoR partners. At the end of the purification process, proteins were directly digested on the beads by trypsin, peptides were recovered and analyzed by mass spectrometry using a LTQ-Orbitrap. (B) Detection of the EpoR at each step of the purification process by Western blotting. Material corresponding to 5 × 105 cells from purification steps indicated in panel A was analyzed by Western blot using anti-EpoR antibodies. EpoR 1: cell surface form of the EpoR; EpoR 2: precursor form of the EpoR. (C) Results of Mascot analysis of the mass spectrometry data. Peptides matching with TfR2 are listed.
Identification of TfR2 as an EpoR binding partner. (A) Strategy for identification of EpoR partners. At the end of the purification process, proteins were directly digested on the beads by trypsin, peptides were recovered and analyzed by mass spectrometry using a LTQ-Orbitrap. (B) Detection of the EpoR at each step of the purification process by Western blotting. Material corresponding to 5 × 105 cells from purification steps indicated in panel A was analyzed by Western blot using anti-EpoR antibodies. EpoR 1: cell surface form of the EpoR; EpoR 2: precursor form of the EpoR. (C) Results of Mascot analysis of the mass spectrometry data. Peptides matching with TfR2 are listed.
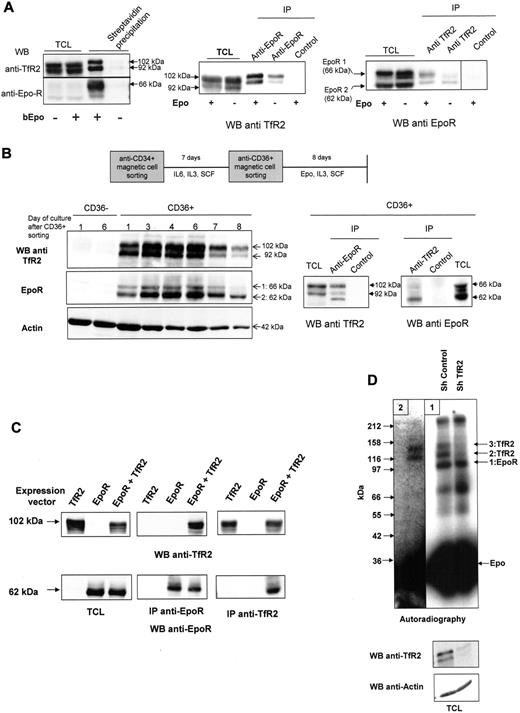
The association of TfR2 with EpoR was confirmed in UT7 cells: TfR2 coprecipitated with EpoR using either bEpo (Figure 2A left panel) or anti-EpoR antibodies (Figure 2A middle panel). Reciprocally, EpoR was also precipitated by anti-TfR2 antibodies (Figure 2A right panel). In these experiments, we constantly observed 2 bands corresponding to the TfR2 protein with apparent molecular masses of 102 and 92 kDa. The same 10-kDa difference was observed after deglycosylation, showing that these 2 bands did not correspond to different glycosylation levels (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Only the 102-kDa protein was detectable in human embryonic kidney 293 (HEK293) cells transfected with TfR2 expression vectors encoding the full-length TfR2 protein (Figure 2C), suggesting alternative splicing as already described.24 Alternatively, the lower molecular weight form could be the result of proteolytic events that only occur in some cell types, such as erythroblasts in the case of TfR1.25,26 The association between EpoR and TfR2 was found to be constitutive, although association of TfR2 with the cell surface form of EpoR was always slightly elevated in Epo-stimulated cells (Figure 2A), suggesting that Epo binding could strengthen the association between both proteins. EpoR western blots further revealed 2 bands that have been already thoroughly characterized and that differ in size due to glycosylation. The lower band (EpoR2) corresponds to the precursor form present in the endoplasmic reticulum and the upper band (EpoR1) is the cell surface form of EpoR.4,27 The association of the EpoR with TfR1 was never detected in UT7 cells, despite the very high level of TfR1 expression in these cells (data not shown).
EpoR interacts with TfR2 in hematopoietic cells. (A) EpoR and TfR2 interact in UT7 cells. Left panel, UT7 cells were stimulated or not with biotinylated Epo (bEpo; 10 U/mL) for 10 minutes. After solubilization, the proteins were precipitated with streptavidin beads. The precipitated proteins and total cell lysates were analyzed by Western blotting using anti-TfR2 and anti-EpoR antibodies. Middle panel, UT7 cells were stimulated or not by Epo. After solubilization, proteins were immunoprecipitated using anti-EpoR or control antibodies and analyzed by Western blotting using anti-TfR2 antibodies. Right panel, UT7 cells were stimulated or not by Epo (10 U/mL). After solubilization, proteins were immunoprecipitated using anti-TfR2 or control antibodies and analyzed by Western blotting using anti-EpoR antibodies. (B) Association between EpoR and TfR2 in human erythroid progenitors. Primary human erythroid progenitors were produced from circulating hematopoietic progenitors using a 2-step culture protocol. Left panel, determination of TfR2 and EpoR expression in CD36− cells and during erythroid differentiation of the CD36+ population. Right panel, on day 6 after CD36 cell sorting, CD36+ cells were harvested and solubilized. Proteins were immunoprecipitated using anti-EpoR antibodies or anti-TfR2 antibodies and then analyzed by Western blotting. (C) Association between EpoR and TfR2 in transfected HEK293 cells. HEK293 cells were transfected by expression vectors encoding TfR2, EpoR, or both. Expression of TfR2 and EpoR was analyzed by Western blotting using total cell lysates. After solubilization, proteins were immunoprecipitated using anti-EpoR or anti-TfR2 antibodies and analyzed by Western blot. (D) Cross-linking of 125I-Epo in UT7 cells with or without sh TfR2 expression. UT7 cells expressing control shRNA or TfR2 shRNA were stimulated for 10 minutes with 125I-Epo and treated with DSS to cross-link the proteins. After solubilization, EpoR was immunoprecipitated (panel 1). Immunoprecipitates from sh control cells were then submitted to denaturation by boiling in electrophoresis sample buffer, followed by a second immunoprecipitation using TfR2 antibodies (panel 2). Proteins were subsequently analyzed by polyacrylamide gel electrophoresis and autoradiography.32 The exposure times were 24 hours and 10 days for panels 1 and 2, respectively.
EpoR interacts with TfR2 in hematopoietic cells. (A) EpoR and TfR2 interact in UT7 cells. Left panel, UT7 cells were stimulated or not with biotinylated Epo (bEpo; 10 U/mL) for 10 minutes. After solubilization, the proteins were precipitated with streptavidin beads. The precipitated proteins and total cell lysates were analyzed by Western blotting using anti-TfR2 and anti-EpoR antibodies. Middle panel, UT7 cells were stimulated or not by Epo. After solubilization, proteins were immunoprecipitated using anti-EpoR or control antibodies and analyzed by Western blotting using anti-TfR2 antibodies. Right panel, UT7 cells were stimulated or not by Epo (10 U/mL). After solubilization, proteins were immunoprecipitated using anti-TfR2 or control antibodies and analyzed by Western blotting using anti-EpoR antibodies. (B) Association between EpoR and TfR2 in human erythroid progenitors. Primary human erythroid progenitors were produced from circulating hematopoietic progenitors using a 2-step culture protocol. Left panel, determination of TfR2 and EpoR expression in CD36− cells and during erythroid differentiation of the CD36+ population. Right panel, on day 6 after CD36 cell sorting, CD36+ cells were harvested and solubilized. Proteins were immunoprecipitated using anti-EpoR antibodies or anti-TfR2 antibodies and then analyzed by Western blotting. (C) Association between EpoR and TfR2 in transfected HEK293 cells. HEK293 cells were transfected by expression vectors encoding TfR2, EpoR, or both. Expression of TfR2 and EpoR was analyzed by Western blotting using total cell lysates. After solubilization, proteins were immunoprecipitated using anti-EpoR or anti-TfR2 antibodies and analyzed by Western blot. (D) Cross-linking of 125I-Epo in UT7 cells with or without sh TfR2 expression. UT7 cells expressing control shRNA or TfR2 shRNA were stimulated for 10 minutes with 125I-Epo and treated with DSS to cross-link the proteins. After solubilization, EpoR was immunoprecipitated (panel 1). Immunoprecipitates from sh control cells were then submitted to denaturation by boiling in electrophoresis sample buffer, followed by a second immunoprecipitation using TfR2 antibodies (panel 2). Proteins were subsequently analyzed by polyacrylamide gel electrophoresis and autoradiography.32 The exposure times were 24 hours and 10 days for panels 1 and 2, respectively.
This interaction was tested in primary erythroid progenitors. The expression of TfR2 expression in erythroid cells is controversial. Although TfR2 mRNA is strongly expressed in murine and human erythroid progenitors, particularly at the early stages of differentiation,28-30 the TfR2 protein has not been detected in erythroid cells.31 To elucidate this further, we amplified CD36+ human primary erythroid progenitors using a 2-step culture method (see “Purification and amplification of human erythroid progenitors” section and Figure 2B). These cells were found to express EpoR and TfR2 proteins in identical patterns, whereas cells from other hematopoietic lineages (CD36− cells), expressed none of these receptors (Figure 2B left panel). The association between TfR2 and EpoR was confirmed in CD36+ erythroid progenitors by coimmunoprecipitation (Figure 2B right panel). Due to the down-regulation of cell surface EpoR by the continuous presence of Epo, which is required to inhibit apoptosis of erythroid progenitors,27 the precursor form of EpoR was mainly detected in these cells. A third band was detected for TfR2 in EpoR immunoprecitates from erythroid progenitors which likely corresponds to the endoplasmic reticulum form of TfR2 (see Figure 3F). Finally, the association between both proteins was evaluated in transfected HEK293 cells that expressed neither TfR2 nor EpoR endogenously. By coimmunoprecipitation, we found that TfR2 and EpoR also associate in HEK cells cotransfected with expression vectors encoding these 2 proteins (Figure 2C). Furthermore, this experiment demonstrated that the TfR2 and EpoR antibodies do not cross-react.
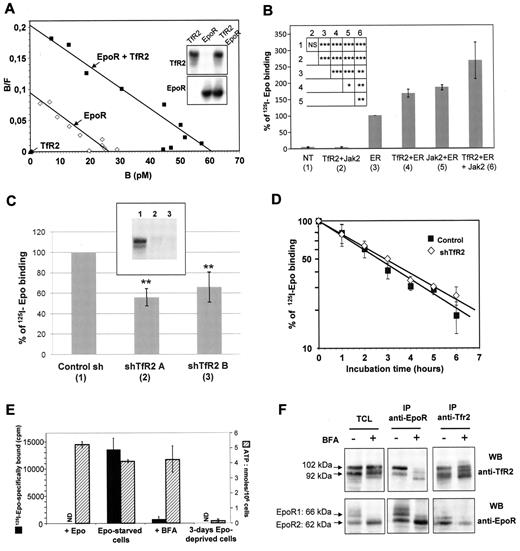
The expression of TfR2 increases the number of EpoR molecules at the cell surface. (A) Analysis of 125I-Epo binding to HEK293 cells transfected with expression vectors for TfR2 (filled triangles), EpoR (empty squares) or both (filled squares) by the Scatchard method as previously described.2 The efficient expression of TfR2 and EpoR was verified by Western blotting (insert). (B) 125I-Epo binding to HEK293 cells transfected or not (NT) with plasmids encoding TfR2, Jak2, and/or EpoR (ER). Cells were incubated for 10 minutes with 125I-Epo and specifically bound radioactivity was measured. The histogram shows the means ± SD from 5 independent experiments. Insert presents the results of Student t test analysis: NS: not significant, *P < .05, **P < .01, ***P < .001. (C) TfR2 knockdown via shRNA reduces Epo binding to EpoR in UT7 cells. Aliquots of 2 × 106 UT7 cells expressing control shRNA, TfR2 shRNA A, or TfR2 shRNA B were incubated for 10 minutes with 125I-Epo and specifically bound radioactivity was then measured. The presented results correspond to 6 independent experiments; 100% correspond to 125I-Epo specific binding in cells expressing a control shRNA and ranged from 11 450 cpm to 19 372 cpm in the 6 experiments. The inhibition of TfR2 expression was verified by Western blotting. Insert, (1) = sh Control; (2) = sh TfR2 A; (3) = sh TfR2 B. ** indicate a significant difference (P < .01) relative to cells transfected with the control shRNA. Difference between cells expressing shTfR2 A and shTfR2 B was not significant (P > .35). (D) Stability of the cell surface EpoR in UT7 cells expressing either a control or TfR2 shRNA construct. Epo-starved UT7 cells were incubated with 500μM cycloheximide for the indicated times and cell surface EpoRs were quantified after a 10-minute incubation with 1nM 125I-Epo. (E-F) TfR2 and EpoR proteins interact at an early stage of the maturation process. UT7 cells were Epo-starved and incubated in the absence or presence of 5 μg/mL BFA for 15 hours. Cell viability was tested by measuring cellular ATP levels after that deprivation period and UT7 cells deprived for 3 days of Epo were used as control. EpoR cell surface expression was also tested using radiolabeled Epo in viable Epo-deprived cells (ND: not done; 3E). UT7 cells were solubilized, proteins were immunoprecipitated using anti-EpoR or anti-TfR2 antibodies, and analyzed by Western blotting (3F).
The expression of TfR2 increases the number of EpoR molecules at the cell surface. (A) Analysis of 125I-Epo binding to HEK293 cells transfected with expression vectors for TfR2 (filled triangles), EpoR (empty squares) or both (filled squares) by the Scatchard method as previously described.2 The efficient expression of TfR2 and EpoR was verified by Western blotting (insert). (B) 125I-Epo binding to HEK293 cells transfected or not (NT) with plasmids encoding TfR2, Jak2, and/or EpoR (ER). Cells were incubated for 10 minutes with 125I-Epo and specifically bound radioactivity was measured. The histogram shows the means ± SD from 5 independent experiments. Insert presents the results of Student t test analysis: NS: not significant, *P < .05, **P < .01, ***P < .001. (C) TfR2 knockdown via shRNA reduces Epo binding to EpoR in UT7 cells. Aliquots of 2 × 106 UT7 cells expressing control shRNA, TfR2 shRNA A, or TfR2 shRNA B were incubated for 10 minutes with 125I-Epo and specifically bound radioactivity was then measured. The presented results correspond to 6 independent experiments; 100% correspond to 125I-Epo specific binding in cells expressing a control shRNA and ranged from 11 450 cpm to 19 372 cpm in the 6 experiments. The inhibition of TfR2 expression was verified by Western blotting. Insert, (1) = sh Control; (2) = sh TfR2 A; (3) = sh TfR2 B. ** indicate a significant difference (P < .01) relative to cells transfected with the control shRNA. Difference between cells expressing shTfR2 A and shTfR2 B was not significant (P > .35). (D) Stability of the cell surface EpoR in UT7 cells expressing either a control or TfR2 shRNA construct. Epo-starved UT7 cells were incubated with 500μM cycloheximide for the indicated times and cell surface EpoRs were quantified after a 10-minute incubation with 1nM 125I-Epo. (E-F) TfR2 and EpoR proteins interact at an early stage of the maturation process. UT7 cells were Epo-starved and incubated in the absence or presence of 5 μg/mL BFA for 15 hours. Cell viability was tested by measuring cellular ATP levels after that deprivation period and UT7 cells deprived for 3 days of Epo were used as control. EpoR cell surface expression was also tested using radiolabeled Epo in viable Epo-deprived cells (ND: not done; 3E). UT7 cells were solubilized, proteins were immunoprecipitated using anti-EpoR or anti-TfR2 antibodies, and analyzed by Western blotting (3F).
To test whether TfR2 corresponded to the EpoR-associated proteins previously observed in crosslinking experiments,2,5-8 iodinated Epo (125I-Epo) was bound to UT7 cells infected with lentiviral vectors that expressed either a control shRNA or a TfR2 shRNA. After crosslinking with disuccinimidyl suberate (DSS) and solubilization, proteins were immunoprecipitated using anti-EpoR antibodies and analyzed by gel electrophoresis and autoradiography (Figure 2D). As previously described, 3 bands with a high molecular mass were observed in control cells. Band 1 that migrated with an apparent molecular mass of 100 kDa had already been demonstrated to correspond to the crosslinking of Epo (34 kDa) with the EpoR itself (66 kDa).27,32 Proteins crosslinked with Epo in bands 2 and 3 had never previously been identified but their apparent molecular masses (85 and 100 kDa) are similar to those of the TfR2 isoforms observed in Figure 2. Furthermore, these bands were absent in TfR2 knockdown cells, strongly suggesting that these proteins are indeed the TfR2 isoforms (Figure 2D panel 1). To confirm this hypothesis, Epo-crosslinked proteins were first precipitated using anti-EpoR antibodies and immunoprecipitates were denatured before immunoprecipitation with anti-TfR2 antibodies. The TfR2 antibodies successfully precipitated both bands 2 and 3, thus establishing that these proteins were indeed TfR2 isoforms (Figure 2D panel 2).
TfR2 increases the expression of cell surface EpoR
These results of the crosslinking experiments show that in the EpoR complex the TfR2 molecule and the Epo molecule are close enough to be covalently linked by short length chemical crosslinkers. This suggested that TfR2 may participate to Epo binding. To examine this possibility, HEK293 cells were transfected with EpoR and/or TfR2 expression vectors and 125I-Epo binding to these cells was analyzed using a Scatchard plot (Figure 3A). However, TfR2 neither conferred the Epo binding capacity in HEK293 cells in the absence of EpoR, nor modified the affinity of EpoR for Epo. In contrast, TfR2 did increase the number of cell surface Epo binding sites when coexpressed with EpoR. It has been previously shown that Jak2 enhances the surface expression of EpoR.3 We observed that Jak2 and TfR2 showed a similar potency for this enhancement in HEK293 cells, with both molecules having an additive effect (Figure 3B). To rule out the possibility that this effect was an artifact of the artificial high expression of heterologous proteins in HEK293 cells, UT7 cells that express endogenous EpoR were infected with lentiviral vectors containing 2 different shRNAs that dramatically decrease TfR2 expression (Figure 3C, insert). Surface EpoR expression was tested by 125I-Epo binding. Six experiments were performed using 2 different TfR2 shRNA in each experiment. 125I-Epo binding was found to be decreased by around 50% in TfR2-knockdown cells (Figure 3C, P < .01 for each TfR2 shRNA expressing cells compared with control cells). Hence, our results show that TfR2 expression is required for the efficient surface expression of EpoR.
We studied the mechanism by which TfR2 increased the cell surface expression of EpoR. The data shown in Figure 3D revealed that TfR2 did not increase the number of cell surface EpoRs by increasing their stability at the cell surface. Coprecipitation of the precursor form of EpoR (EpoR2) with TfR2 suggested that both proteins can associate in the endoplasmic reticulum during the maturation process (Figure 2A right panel). To verify this possibility, UT7 cells were treated with brefeldin A (BFA), a molecule which blocks membrane traffic out of the endoplasmic reticulum by inhibiting ADP-ribosylation factors.33 BFA treatment results in Golgi complex disintegration and inhibition of the surface expression of various receptors. We verified by trypan blue staining that Epo deprivation and BFA treatment for 15 hours did not decrease cell survival (data not shown). Moreover, intracellular ATP levels were not significantly modified by these treatments.125I-Epo binding was not detected in BFA-treated UT7 cells (Figure 3E). However, association between EpoR and TfR2 was still detected by coimmunoprecipitation (Figure 3F). In agreement with the expected effects of BFA that disrupts the Golgi apparatus, this association involved the forms of lowest molecular masses corresponding to the endoplasmic reticulum forms of EpoR and TfR2. Thus, both proteins associate early in the maturation process and we propose that this association facilitates the maturation and cell surface expression of EpoR.
Role of TfR2 in human and murine erythropoiesis
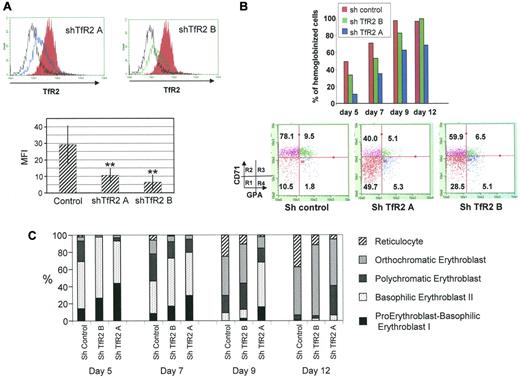
To further explore the role of TfR2 in erythropoiesis, human erythroid progenitors were infected with lentiviruses expressing control or TfR2-targeted shRNAs. The capability of these shRNAs to efficiently decrease the cell surface expression of TfR2 was confirmed by flow cytometry (Figure 4A). The TfR2 knockdowns caused a constant delay in the hemoglobinization of erythroid progenitors (Figure 4B top panel), particularly during early erythroid differentiation. After this period, hemoglobinization appeared to be equivalent whether or not the cells expressed TfR2, showing that the lack of TfR2 delays but does not inhibit hemoglobinization. Four successive differentiation steps labeled R1 to R4 can be distinguished during erythroid differentiation according to the TfR1 (CD71) and GPA expression levels.34 After 4 days of culture, TfR2 knockdown erythroid progenitors were less differentiated with more cells appearing in the R1 compartment compared with control cells (Figure 4B). Morphological examination confirmed that TfR2-knockdown delays the appearance of differentiated cells (Figure 4C). This delay of differentiation led to a slight increase of total cell number after 12 days of cell culture (data not shown).
A TfR2 knockdown retards human erythroid progenitor differentiation. (A) Flow cytometric analysis of cell surface TfR2 expression in CD36+ cells at day 4 of the culture expressing a control shRNA (solid red), TfR2 shRNA A (blue line), TfR2 shRNA B (green line). The black line in the top panels corresponds to an isotypic control labeling. The top panels show a typical experiment, the bottom panel presents the results of 4 independent experiments showing the decrease of mean fluorescence intensity due to TfR2 knockdown. (B) Top panel, percentage of hemoglobinized cells determined by benzidine staining of day 5, 7, 9, and 12 cultures after CD36+ cell sorting. Bottom panel, expression levels of TfR1 (CD71) and GPA measured by flow cytometric analysis of control and TfR2 knockdown cells on culture day 4. R1, R2, R3, and R4 populations were determined as described previously.34 (C) Evaluation of erythroid differentiation on days 5, 7, 9, and 12 of culture after CD36+ cell sorting. Cell classifications were established after May-Grunwald Giemsa staining of cytospin preparations.
A TfR2 knockdown retards human erythroid progenitor differentiation. (A) Flow cytometric analysis of cell surface TfR2 expression in CD36+ cells at day 4 of the culture expressing a control shRNA (solid red), TfR2 shRNA A (blue line), TfR2 shRNA B (green line). The black line in the top panels corresponds to an isotypic control labeling. The top panels show a typical experiment, the bottom panel presents the results of 4 independent experiments showing the decrease of mean fluorescence intensity due to TfR2 knockdown. (B) Top panel, percentage of hemoglobinized cells determined by benzidine staining of day 5, 7, 9, and 12 cultures after CD36+ cell sorting. Bottom panel, expression levels of TfR1 (CD71) and GPA measured by flow cytometric analysis of control and TfR2 knockdown cells on culture day 4. R1, R2, R3, and R4 populations were determined as described previously.34 (C) Evaluation of erythroid differentiation on days 5, 7, 9, and 12 of culture after CD36+ cell sorting. Cell classifications were established after May-Grunwald Giemsa staining of cytospin preparations.
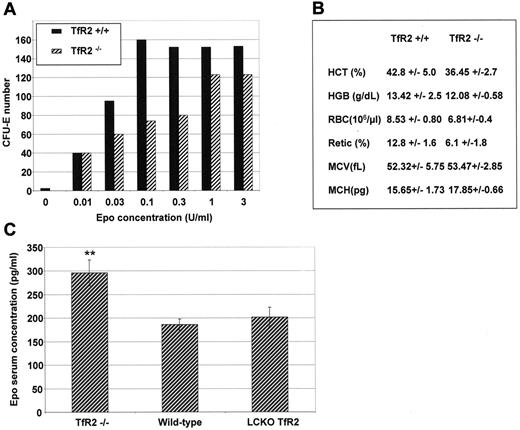
To confirm the role of TfR2 in erythroid differentiation, the ability of TfR2−/− murine erythroid progenitors to form hemoglobinized colonies (CFU-E) in vitro was determined in the presence of increasing concentrations of Epo. Four-week-old mice were used in these experiments because older TfR2−/− mice develop hemochromatosis that could modify the erythroid cell physiology. Erythroid colony formation was reduced in the TfR2−/− mice at physiological Epo concentrations (from 0.03-0.3 U/mL, Figure 5A). At higher doses of Epo the ability of erythroid progenitors to form CFU-E was roughly identical between TfR2−/− and wild-type mice (Figure 5A). Using an optimal Epo concentration (3 U/mL), 133 ± 29 and 128 ± 14 CFU-E developed from 2 × 105 bone marrow cells of wild-type and TfR2−/− mice, respectively (N = 4 mice tested in duplicate for each group). The hematological parameters of TfR2−/− mice did not significantly differ from those of wild-type mice (Figure 5B), a surprising finding given the decreased sensitivity to Epo observed in our in vitro experiments. Enhanced circulating Epo levels is a common compensation mechanism to a deficient erythropoiesis and indeed, we observed that circulating Epo levels in TfR2−/− mice were significantly increased compared with wild-type mice of the same age (Figure 5C). In contrast, mice with a liver-targeted deletion of TfR2 presented normal circulating Epo levels.
Erythropoiesis in TfR2−/− mice. (A) CFU-E assay of TfR2−/− and wild-type mice. Bone marrow cells (2 × 105) from the femurs and tibias of 2 TfR2−/− and 2 wild-type mice at 4 weeks of age were cultured in methylcellulose medium dedicated to CFU-E growth (Methocult M3234, StemCell Technologies) supplemented by the indicated Epo concentrations. Colonies were scored after 48 hours of culture. The results are representative of 2 independent experiments performed in duplicate. (B) Hematological values from TfR2−/− and wild-type littermates at 4 weeks of age. HCT, hematocrit; HGB, hemoglobin; RBC, red blood cells; Retic, reticulocytes; MCV, mean cell volume; MCH, mean cell hemoglobin. (n = 6 for both TfR2−/− mice and wild-type mice; values are the mean ± SD). (C) Epo concentrations in the serum of TfR2−/− (n = 4), sibling pairs of wild-type (n = 3) and liver-targeted deletion of TfR2 (TfR2 LCKO, n = 4) mice were determined by sandwich ELISA (R&D Systems). Student t test: P < .01 between TfR2−/− and wild-type mice and between TfR2−/− and LCKO mice, P > .15 (NS) between wild-type and LCKO mice.
Erythropoiesis in TfR2−/− mice. (A) CFU-E assay of TfR2−/− and wild-type mice. Bone marrow cells (2 × 105) from the femurs and tibias of 2 TfR2−/− and 2 wild-type mice at 4 weeks of age were cultured in methylcellulose medium dedicated to CFU-E growth (Methocult M3234, StemCell Technologies) supplemented by the indicated Epo concentrations. Colonies were scored after 48 hours of culture. The results are representative of 2 independent experiments performed in duplicate. (B) Hematological values from TfR2−/− and wild-type littermates at 4 weeks of age. HCT, hematocrit; HGB, hemoglobin; RBC, red blood cells; Retic, reticulocytes; MCV, mean cell volume; MCH, mean cell hemoglobin. (n = 6 for both TfR2−/− mice and wild-type mice; values are the mean ± SD). (C) Epo concentrations in the serum of TfR2−/− (n = 4), sibling pairs of wild-type (n = 3) and liver-targeted deletion of TfR2 (TfR2 LCKO, n = 4) mice were determined by sandwich ELISA (R&D Systems). Student t test: P < .01 between TfR2−/− and wild-type mice and between TfR2−/− and LCKO mice, P > .15 (NS) between wild-type and LCKO mice.
TfR2 expression is required for Epo-induced GDF15 expression
Tanno et al have shown that at the end of the differentiation process, erythroblasts produce GDF15, a cytokine that belongs to the TGFβ superfamily. In thalassemia and other erythroid diseases, GDF15 production is strongly increased and inhibits hepcidin production by hepatocytes. Thus, GDF15 could constitute a link between erythropoiesis and iron metabolism.16 We confirmed in our experiments that human erythroid cells produce high amounts of GDF15 at the end of the differentiation process (Figure 6A). Although Epo deprivation reduced only marginally cell survival at the end of their differentiation process, it reduced dramatically GDF15 production (Figure 6B). TfR2 knockdowns led also to a dramatic reduction of GDF15 production on day 9 of the culture (Figure 6C). Although most cells were strongly differentiated at that time, we could not rule out the possibility that the decreased GDF15 production was partly due to the delay in erythroid differentiation caused by the absence of TfR2. To address this question, a subline of UT7 cells that are unable to differentiate in the presence of Epo was employed; these cells also produce GDF15 in an Epo-dependent manner while the metabolic activity of cells is not affected as shown by intracellular ATP measurement (Figure 6D). GDF15 production in UT7 cells was fully abolished by a TfR2 knockdown (Figure 6E), thereby showing that the effect observed in primary cells was not due to the delay in erythroid differentiation and demonstrating that GDF15 production is directly dependent on the expression of TfR2 and cell stimulation by Epo. Because of the dramatic effect of TfR2 on Epo-stimulated GDF15 production, we tested whether TfR2 modifies EpoR signaling. Epo binding induces Jak2 activation that stimulates several intracellular signaling relays including signal transducer and activator of transcription 5 (STAT5), extracellular signal-regulated kinase (Erk), and Akt (see Constantinescu35 for review). TfR2 knockdown did not significantly modify STAT5, Erk, and Akt activation after Epo stimulation (supplemental Figure 2A). In agreement with these results, transferrin also did not modify EpoR signaling (supplemental Figure 2B).
GDF15 expression in erythroblasts requires TfR2, GDF15 production levels were measured in the supernatants of cultured cells. The data shown corresponds to the production of GDF-15 over 24 hours for 106 cells. (A) Determination of GDF15 levels secreted by erythroid progenitors on each day of the cell culture after CD36+ cell sorting. The results of 1 representative experiment from 3 independent cultures are shown. (B) Cells at day 9 of differentiation were incubated for 24 hours in the absence of Epo. The culture medium was then recovered and GDF15 concentration was determined by ELISA. (C) Determination of GDF15 secretion in control cells (Control Sh) and TfR2 knockdown cells (expressing shRNAs A or B). The data shown are the means ± SD determined on day 9 of 2 independent erythroid primary cultures (CD36+ cells). (D) Relationship between Epo concentrations in the culture medium and GDF15 production in UT7 cells. Cells were incubated for 24 hours with the indicated concentrations of Epo. After that time, GDF15 production was determined using the cell culture supernatants and intracellular ATP content of the cells was determined as described in “Methods.” (E) Determination of GDF15 secretion in UT7 control cells and in TfR2 knockdown cells expressing shRNAs A and B. Cells were cultured in the presence of 2 U/mL Epo. The data shown are the means ± SD from 4 independent experiments. **P < .01 relative to control.
GDF15 expression in erythroblasts requires TfR2, GDF15 production levels were measured in the supernatants of cultured cells. The data shown corresponds to the production of GDF-15 over 24 hours for 106 cells. (A) Determination of GDF15 levels secreted by erythroid progenitors on each day of the cell culture after CD36+ cell sorting. The results of 1 representative experiment from 3 independent cultures are shown. (B) Cells at day 9 of differentiation were incubated for 24 hours in the absence of Epo. The culture medium was then recovered and GDF15 concentration was determined by ELISA. (C) Determination of GDF15 secretion in control cells (Control Sh) and TfR2 knockdown cells (expressing shRNAs A or B). The data shown are the means ± SD determined on day 9 of 2 independent erythroid primary cultures (CD36+ cells). (D) Relationship between Epo concentrations in the culture medium and GDF15 production in UT7 cells. Cells were incubated for 24 hours with the indicated concentrations of Epo. After that time, GDF15 production was determined using the cell culture supernatants and intracellular ATP content of the cells was determined as described in “Methods.” (E) Determination of GDF15 secretion in UT7 control cells and in TfR2 knockdown cells expressing shRNAs A and B. Cells were cultured in the presence of 2 U/mL Epo. The data shown are the means ± SD from 4 independent experiments. **P < .01 relative to control.
Discussion
Overall, our data successfully address the longstanding question regarding the identity of the principal proteins that cross-link to Epo in erythroid cells. Our current results show that the structure of the EpoR is more complex than a simple homodimer as thought up to now and we demonstrate using several independent methods that TfR2 belongs to the EpoR complex. Both proteins were constantly coprecipitated by specific antibodies that recognize either EpoR or TfR2 (Figure 2). The specificity of anti-EpoR antibodies used in these experiments have been previously thoroughly established.18,19,27 Proteins recognized by anti-TfR2 antibodies disappeared in cells that have been infected with lentiviral vectors encoding different shRNA targeting TfR2 mRNA, showing that these antibodies recognized specifically the TfR2 proteins. Association between EpoR and TfR2 was also detected by coprecipitation with the Epo molecule bound to its receptor (Figure 2A) and our results show that after binding to EpoR, Epo can be crosslinked to TfR2 (Figure 2D). This strongly suggests that association between EpoR and TfR2 is direct. In agreement with this hypothesis, mass spectrometry analysis of EpoR-associated proteins did not reveal any protein already known to be associated with TfR2 such as HFE. Altogether, these results demonstrate unambiguously that TfR2 belongs to the EpoR complex and that association between both proteins is probably direct although we cannot formally exclude that another protein is required for this association. Nevertheless, although TfR2 seems to be very close to the Epo molecule bound to EpoR, Scatchard analysis shows that it is not involved in Epo binding (Figure 3A).
Like Jak2, TfR2 associates with the EpoR very early during the maturation process of the EpoR complex. Indeed, association of the precursor forms of both molecules can be detected, especially in BFA-treated cells showing that association occurs at the endoplasmic reticulum level (Figure 3F). We propose that TfR2 is an escort protein for the EpoR that facilitates the maturation and cell surface expression of EpoR (Figure 3B-C). A recent publication underscores the importance of the EpoR transport to the cell surface by showing that the intracellular pool of EpoR allows to replenish the cell surface EpoR pool during Epo stimulation and thereby to sense a broad range of Epo concentrations.36 Interestingly, another disulfide-bonded homodimeric protein, the envelop protein of the Friend spleen focus forming virus (gp55), also associates with EpoR in the endoplasmic reticulum.4 Gp55 activates the EpoR in the absence of Epo and is responsible for erythroid proliferation further leading to leukemia in mice. We previously showed by crosslinking experiments that EpoR either associated with Gp55 or with the 2 proteins now identified as TfR2 but not with both in the same complex.37 This suggests that physiological and pathological EpoR complexes should have similar structures: in both cases, EpoR proteins that are noncovalently bound are associated with covalently linked dimeric proteins; either the TfR2 or gp55. An attracting hypothesis would be that these covalently dimerized proteins could help dimerizing the EpoR itself.
We observed that TfR2 and the EpoR were simultaneously expressed during the differentiation of erythroid cells. These results are in agreement with several reports showing that the TfR2 mRNA is highly expressed in erythroid cells10,28 and that the TfR2 protein is expressed in K562 erythroleukemia cells.38 Interestingly, a recent meta-analysis of the linking of common genetic variants with hematologic parameters identified a signal centered on the TfR2 gene that is highly associated with red blood cell number.39 This suggested that TfR2 could play a direct role in erythropoiesis in addition to its well recognized role in iron metabolism and our present results could afford an explanation for this linkage. Functionally, we observed a delay in the differentiation of human erythroid progenitors lacking TfR2, but final differentiation up to the reticulocyte stage seems normal (Figure 4). We used TfR2−/− mice to assess the role of TfR2 in erythropoiesis in vivo. To separate potential effects of TfR2 deficiency on erythropoiesis from the effects on iron metabolism, we studied young mice that have not yet developed iron overload. TfR2−/− mice at this age have roughly normal haematological parameters except for a slightly decreased number of circulating reticulocytes, although erythroid progenitors from these mice exhibit a decreased sensitivity to Epo. The effects of TfR2 on erythroid maturation in both human and mouse differentiation models could hardly be explained by the contribution of TfR2 to iron uptake by erythroblasts. Indeed, the very high expression of TfR1 is likely sufficient to provide cells with adequate amount of iron to maintain efficient hemoglobin synthesis. In agreement with this hypothesis, erythrocytes of TfR2−/− mice are not microcytic (Figure 5B). The effects of TfR2 deficiency that we observed in mouse and human erythroid differentiation models (Figures 4 and 5) could probably be explained at least in part by the decreased cell surface expression of the EpoR in cells lacking TfR2. These effects are compensated by increased Epo levels in TfR2−/− mice (Figure 5C). These results are in agreement with the clinical observation that patients with TfR2 mutations do not present signs of anemia, even when the mutations lead to the complete lack of TfR2 expression.12,40 Instead they seem to tolerate well phlebotomy as the other hemochromatosis patients.41 It would be of interest to test the Epo sensitivity of erythroid cells from patients with TfR2-related hemochromatosis and to evaluate the circulating levels of Epo in these patients.
GDF15 is a divergent member of the TGFβ superfamily that is produced by erythroid cells at the end of the differentiation process. Serum GDF15 levels are increased in several erythroid disorders with ineffective erythropoiesis such as beta-thalassemia, type I congenital diserythropoietic anemia and refractory anemia with ring sideroblasts.16,42,43 In these diseases, GDF15 has been proposed to provide a link between erythropoiesis and iron metabolism as it could regulate hepcidin production by hepatocytes. Nevertheless, the relationship between serum GDF15 levels and erythropoietic activity is not straightforward because GDF15 levels have been found to be only slightly increased in other erythroid diseases such as sickle cell anemia. Moreover, GDF15 levels are normal in patients with increased effective erythropoiesis such as after bone marrow transplantation or Epo injection. It should be noticed that GDF15 is also produced by cells such as macrophages and the contribution of erythroid cell production to overall circulating GDF15 levels remains unclear (see Tanno et al for review44 ). Our results show that the production of GDF15 by erythroblasts and UT7 cells is fully dependent on both Epo stimulation and TfR2 expression, suggesting that TfR2 may specifically modify Epo intracellular signaling. In TfR2 knockdown cells, we did not observe any dramatic modifications of the Epo-induced activation of Erk, Akt. or STAT5, suggesting that more subtle modifications of these canonical Epo-activated intracellular relays could be involved in the knockdown effects, such as those recently reported for Erk activation by nerve growth factor or epidermal growth factor.45 Alternatively, other intracellular signaling pathways that have not been previously shown to be involved in Epo signaling could be responsible for mediating the downstream effects of TfR2. In liver cells, TfR2 associates with diferric transferrin and HFE to up-regulate hepcidin expression when transferrin saturation increases.11 However, how this membrane complex transduces signals to the nucleus that regulate the hepcidin gene remains to be determined. Because Epo injections strongly down-regulate hepcidin expression in mice46 and a direct action of Epo on liver cells has been suggested,47 the hypothesis that EpoR associates with TfR2 in liver cells was tested. Despite the high sensitivity of our anti-EpoR antibodies, we did not detect EpoR expression in several human hepatic cell lines (HuH7, HepG2, Hep3B) or in human primary hepatocytes by Western blot analysis, even after concentration attempts by immunoprecipitation using anti-EpoR antibodies (data not shown).
Erythropoiesis is by far the most iron-consuming process in mammals. TfR2 is recognized as a key player in iron metabolism through the regulation of hepcidin production by liver cells. Its involvement in erythropoiesis reveals a new connection between these 2 physiological processes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Carolin Enns and Olivier Bernard for generously donating the TfR2 and Jak2 expression vectors, respectively. We also thank Drs Jeroen Demmers and Sjaak Philipsen of the Erasmus University Medical Center, Rotterdam for mass spectrometry analysis. We are grateful to Dr Nabih Azar from la Pitié Salpétrière Hospital for providing the cytapheresis samples.
This work was supported by the Association pour la Recherche contre le Cancer, Ligue Nationale contre le Cancer, Agence National de la Recherche, and Telethon Grant GGP08089 to C.C. H F. was funded by the Eurythron Marie Curie Actions.
Authorship
Contribution: H.F., M.V., and M.L. performed research and analyzed and interpreted data; Y.Z. designed and performed research and analyzed and interpreted data; R.M.P., C.C., and A.R. provided essential materials (TfR2 knockout mice) and participated in the discussion; S.G. and M.G. contributed to CFU-E experiments; and C.L., P.M., and F.V. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Frederique Verdier, Institut Cochin, 27 rue du Faubourg Saint-Jacques, 75014 Paris, France; e-mail: frederique.verdier@inserm.fr; or Patrick Mayeux, Institut Cochin, 27 rue du Faubourg Saint-Jacques, 75014 Paris France; e-mail: patrick.mayeux@inserm.fr.
References
Author notes
M.V. and Y.Z. are co–second authors.
P.M. and F.V. are co–last authors.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal