Neutropenia remains a major risk factor in the development of invasive aspergillosis (IA) after HSCT. In this issue of Blood, Hartigan and colleagues illustrate that lack of expression of CCR7 on hematopoietic and progenitor cells alters their proliferation and differentiation potential and the cytokine milieu of the lungs leading to a significant reduction in the susceptibility of HSCT recipients to IA.1
IA is an acute and life-threatening fungal disease characterized by severe pneumonia and intense inflammation that affects primarily immunocompromised individuals such as patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT). In these patients, mortality from IA can be as high as 80%-90%. Given that anti-IA immunity is predominantly mediated by cells of myeloid origin (although lymphoid-derived cells have been also recognized as effector cells), many attempts have been made to either engineer the stem cell graft to impact the disease or to accelerate myeloid cell differentiation and enhance effector cell production after allogeneic HSCT.2
The role of chemokines in regulating many fundamental processes of hematopoietic cells—including survival, proliferation, differentiation, and migration—is well recognized.3 Chemokine receptors are expressed on a large number of primitive hematopoietic cells as well as differentiated and specialized cells such as T cells.4 These receptors—including CCR7, the target of the investigations by Hartigan et al—have been detected on murine cells and on different classes of human stem and progenitor cells from different hematopoietic tissues.5 Many chemokines mediate both in vivo and in vitro myelosuppressive effects including CCL19 and CCL21, the ligands of the receptor CCR7.6 Therefore, it stood to reason that CCL19- or CCL21-mediated myelosuppression might be reduced or eliminated if their receptor was not expressed on hematopoietic stem cells (HSCs). This prompted Hartigan and colleagues to examine the reconstitution potential of CCR7−/− HSCs in the context of a transplantation regimen involving the cotransplantation of more committed myeloid progenitors such as common myeloid progenitors (CMPs) and granulocyte-monocyte progenitors (GMPs).
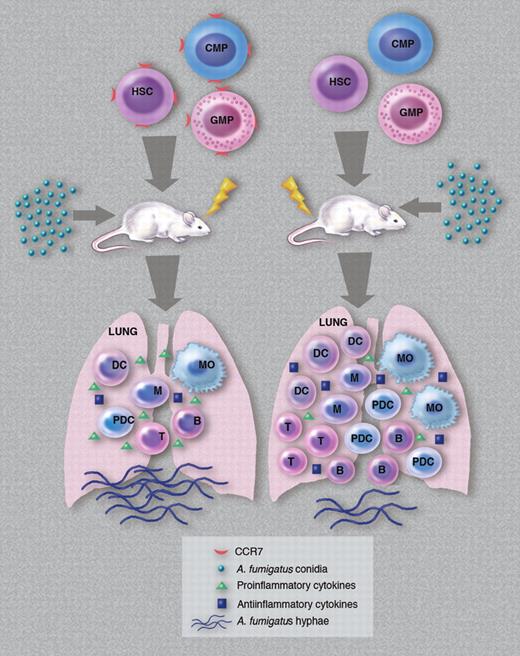
Clearly, 14 days after transplantation, CCR7−/− HSCs reconstituted myeloid effector cells in the spleen and lung of transplanted recipients more efficiently than HSCs from wild-type (WT) donors. Interestingly, however, transplanted myeloid progenitor cells (a mix of CMPs and GMPs) were a minor contributor to the overall number of reconstituting myeloid cells at this time point, suggesting that direct stem cell differentiation was responsible for the appearance of these cells in circulation and in these organs. In their experimental design, Hartigan et al challenged transplanted mice with Aspergillus fumigatus conidia 14 days after transplantation, and assessed the presence of donor-derived hematopoietic cells in the lungs of recipient mice (with and without challenge) 2 days later. Before the A fumigatus challenge, and compared with control mice that received transplants with a mix of WT HSCs, CMPs, and GMPs, mice receiving all 3 groups of cells from CCR7−/− mice had significantly higher numbers of total cells, myeloid dendritic cells, macrophages, plasmacytoid dendritic cells, T cells, and B cells. Following the challenge, all these cell types (except T and B cells) increased in number in recipients of CCR7−/− cells and remained significantly higher than in their counterparts receiving transplants with WT cells. Most importantly, 75% of mice receiving transplants with CCR7−/− cells survived the challenge (compared with 100% mortality among recipients of WT cells), and had a less prominent inflammatory response and reduced fungal growth in the lungs. In addition to this altered cellular reconstitution and infiltration into the lungs, recipients of CCR7−/− cells also had a distinct cytokine profile in the lungs after conidial challenge. Analysis revealed that the levels of several proinflammatory cytokines (at both the mRNA and protein levels) were significantly reduced in recipients of CCR7−/− cells while the levels of anti-inflammatory cytokines in these mice were elevated. In head-to-head competition in the same recipient, CCR7−/− cells outcompeted WT cells in the production of dendritic cells in both the lung and spleen. Collectively, these data demonstrate that in the absence of signaling through CCR7, transplanted stem and progenitor cells generate a microenvironment conducive to better control of IA via the production of larger numbers of myeloid progeny and reduced levels of proinflammatory cytokines (see figure).
How cotransplantation of different classes of CCR7-null hematopoietic cells contribute to a cellular and cytokine microenvironment in the lungs of host animals that can efficiently respond to an A fumigatus infection. HSC indicates hematopoietic stem cells; CMP, common myeloid progenitors; GMP, granulocyte-monocyte progenitors; DC, dendritic cells; M, monocytes; MO, macrophages; pDC, plasmacytoid DC; T, T cells; and B, B cells. (Professional illustration by Marie Dauenheimer.)
How cotransplantation of different classes of CCR7-null hematopoietic cells contribute to a cellular and cytokine microenvironment in the lungs of host animals that can efficiently respond to an A fumigatus infection. HSC indicates hematopoietic stem cells; CMP, common myeloid progenitors; GMP, granulocyte-monocyte progenitors; DC, dendritic cells; M, monocytes; MO, macrophages; pDC, plasmacytoid DC; T, T cells; and B, B cells. (Professional illustration by Marie Dauenheimer.)
These interesting and exciting results emphasize the need for both CCR7−/− CMPs and GMPs in mediating the observed protection against IA after HSCT. The exact reason why these classes of progenitor cells are required for the observed pattern of myeloid reconstitution is not apparent. Given the unattainable task of using CCR7−/− hematopoietic cells in clinical transplantation, Hartigan and colleagues examined the outcome of neutralizing CCL19 or CCL21 in recipients of WT cells to achieve similar results. A profile of cellular reconstitution similar to that seen in recipients of CCR7−/− cells was detected in mice treated with anti-CCL19 antibody and challenged with A fumigatus conidia, illustrating that interference with CCR7-mediated signaling promotes faster hematopoietic reconstitution. Because CCR7 plays an important role in lymphocyte homing to lymph nodes,7 it is important to determine whether the increased number of T cells in the lung is somehow related to or caused by a dysregulation in the homing of these cells to lymph nodes.
So the question then arises as to whether such an approach is clinically feasible. The fact that CCR7 is expressed on human hematopoietic cells from different tissues suggests that interfering with the binding of CCR7 to its cognate ligands may modify the kinetics of myeloid reconstitution in a manner similar to what was described by Hartigan and colleagues. That anti-CCL19 antibody treatment recapitulated the major results of the work with CCR7−/− cells is quite encouraging in as far as the clinical utility of such an intervention. What remains perhaps problematic is the need to transplant CMPs and GMPs to achieve the results described in this report. However, it is quite likely that cells used for clinical HSCT contain different classes of progenitor cells in addition to reconstituting stem cells and that such a graft may already contain the phenotypically defined mix of cells described in this work. Translation of these results to clinical HSCT may be well within reach.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal