Abstract
Heparin-induced thrombocytopenia (HIT) is a life- and limb-threatening thrombotic disorder that develops after exposure to heparin, often in the setting of inflammation. We have shown previously that HIT is associated with antibodies to complexes that form between platelet factor 4 and glycosaminoglycan (GAG) side chains on the surface of platelets. However, thrombosis can occur in the absence of thrombocytopenia. We now show that platelet factor 4 binds to monocytes and forms antigenic complexes with their surface GAG side chains more efficiently than on platelets likely due to differences in GAG composition. Binding to monocytes is enhanced when the cells are activated by endotoxin. Monocyte accumulation within developing arteriolar thrombi was visualized by situ microscopy. Monocyte depletion or inactivation in vivo attenuates thrombus formation induced by photochemical injury of the carotid artery in a modified murine model of HIT while paradoxically exacerbating thrombocytopenia. These studies demonstrate a previously unappreciated role for monocytes in the pathogenesis of arterial thrombosis in HIT and suggest that therapies targeting these cells might provide an alternative approach to help limit thrombosis in this and possibly other thrombotic disorders that occur in the setting of inflammation.
Introduction
Platelet factor 4 (PF4) is a cationic chemokine with high affinity for unfractionated heparin (UFH) and other large, negatively charged molecules.1 PF4 is stored in platelet α-granules, released upon activation, when it then binds rapidly to glycosaminoglycan (GAG) side chains expressed on the surface of platelets2 and other vascular cells, with little remaining free in the circulation.3
Heparin-induced thrombocytopenia (HIT) is an iatrogenic complication of heparin therapy caused by antibodies that recognize complexes of human (h) PF4 with heparin or other GAGs.4,5 In solution, formation of antigenic complexes between PF4 and heparin is critically dependent on their molar ratio, with loss of antibody binding when the optimal ratio is disrupted by an excess of either component.6,7 Antigen formation on the platelet surface also follows a bell-shaped curve as PF4 concentration is increased, with maximal binding of antibody seen at an exogenous PF4 concentration of 50 μg/mL.8
Chondroitin sulfates (CSs) are the predominant GAG side chains expressed on platelets.9,10 We have shown that the binding of the HIT-like monoclonal antibody KKO11 to platelets is abrogated by chondroitinase ABC,8 indicating that HIT antibodies bind to PF4/CS complexes on this cell type. Therapeutic concentrations of UFH disrupt antibody binding in part by eluting PF4 from the platelet surface, which reduces formation of antigenic complexes and the potential for platelet activation8 through platelet FcγRIIA.12 Thus, variation in the expression of platelet-derived PF4 (or CS) might help to explain why only a small percentage of patients who generate antibodies to PF4/UFH develop HIT.13
Although it has been generally accepted that thrombosis in HIT is mediated through antibody-mediated platelet activation,14,15 extensive thrombosis affecting 1 or more vessels often develops in the setting of moderate thrombocytopenia and may precede its occurrence or even appear after the platelet count returns to normal. The risk of new thromboembolic events extends well beyond the time required for platelet recovery. Thus, the pathophysiology of the thrombocytopenia and the reliance on platelet activation to develop thrombosis remain unclear.
The possibility that additional cell types are involved in the pathogenesis of thrombosis is suggested by the prevalence of HIT in the setting of local or systemic inflammation16 characterized by trauma to the vasculature, such as coronary bypass surgery.17 Leukocyte-platelet aggregates and leukocyte activation has been identified in the circulation of affected patients,18-20 and HIT antibodies have been shown to induce elaboration of tissue factor (TF) in several cell types,21 including monocytes.22,23 Yet, the involvement of monocytes in the pathogenesis of thrombosis has not been demonstrated directly.
Participation of monocytes in the pathogenesis of HIT may be mediated through their proclivity to bind PF4 released from activated platelets. Monocytes, unlike platelets, also express GAG side chains composed of dermatan sulfate (DS) and heparan sulfate (HS) as well,24,25 both of which bind PF4 with higher affinity than CS,1 making the bound PF4 more resistant to elution by heparin. Moreover, monocytes express hypersulfated GAGs when induced to differentiate to macrophages, which may further enhance PF4 binding.26
In this paper, we demonstrate that monocytes bind PF4 with greater affinity than platelets, at least in part because of differences in the composition of cell surface GAGs. Monocytes develop HIT-like complexes at lower levels of PF4 than are required to generate antigenic complexes on platelets, and these complexes are more resistant to dissociation by UFH. Monocytes are activated by HIT antibodies at lower PF4 concentrations than are platelets, and activation of monocytes further enhances PF4 and antibody binding. In vivo, monocytes accumulate in arteriolar thrombi induced by HIT antibody in a HIT murine model, and depletion of monocytes attenuates thrombosis while, paradoxically, exacerbating thrombocytopenia. These studies implicate monocytes in the pathogenesis of HIT and suggest novel approaches to ameliorate the risk of thrombosis in this and possibly other related disorders.
Methods
Preparation of recombinant hPF4
Wild-type (WT) hPF4 in plasmid pT7-7 was expressed in BL21DE30 pLysS bacteria, purified, and characterized as described earlier.27 WT hPF4 was isolated from bacterial lysate supernatant by affinity chromatography using a HiTrap Heparin HP column (Amersham Bioscience) and then by fast protein liquid chromatography using a Resource RPC column (Amersham Bioscience). Protein purity was assessed on 15% (wt/vol) sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by silver staining. Total protein concentrations were determined using the bicinchoninic acid (BCA) protein assay (Pierce) per the manufacturer with bovine serum albumin (BSA) as the standard.
Monoclonal antibodies
KKO, the HIT like monoclonal antibody (moAb), and the isoimmune control TRA are both mouse immunoglobulin G2b moAbs.11 Both antibodies were fluorescein isothiocyanate (FITC) labeled using an E-Z FITC labeling kit (Pierce) per the manufacturer. Anti–human CD41a moAb conjugated to tandem conjugate system peridinin-chlorophyll-protein (PerCP) and cyanine 5.5 (Cy5.5; hCD41a-PerCP-Cy5.5), anti–human CD14 conjugated to allophycocyanin (APC; hCD14-APC), anti–hCD45-PerCP, anti–mouse CD45-PerCP (anti–mCD45-PerCP), anti–mouse CD41 conjugated to R-phycoerythrin (PE; mCD41-PE), and mouse anti–human CD64 (Clone 10.1) antibodies were from BD Pharmingen. Anti–human CD16 (Clone 3G8) was from BioLegend. Rat anti-mCD115 PE or APC and F4/80 APC moAbs were from eBioscience. Antigen-binding fragments from the rat anti–mouse CD41 antibody from BD Pharmingen were conjugated with Alexa488 using the Alexa Fluor Protein labeling kit (Molecular Probes). Mouse anti–human CD32 (IV.3) antibody was purified from supernatants of hybridoma cells (HB-217 ATCC) using recombinant protein G-agarose (Invitrogen) per manufacturer's instructions.
Platelet, monocyte, and macrophage preparation
Human samples.
Studies were performed using human platelets and peripheral blood mononuclear cells (PBMCs) as well as cultured primary macrophages. Human blood was collected after informed consent from healthy, aspirin-free volunteers in 0.129M sodium citrate (10:1, vol/vol) supplemented with 300nM carbacyclin. Samples were obtained under a protocol approved by the institutional review board for studies involving human subjects at the Children's Hospital of Philadelphia.
To isolate platelets, 10 mL of whole blood was centrifuged at 200g for 15 minutes at room temperature (RT) to obtain platelet-rich plasma (PRP). PRP was centrifuged at 800g for 10 minutes at RT, and the pellet washed and resuspended in modified Tyrode buffer (134mM NaCl, 3mM KCl, 0.3mM NaH2PO4, 2mM MgCl2, 5mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 5mM glucose, 12mM NaHCO3, 0.1% BSA; Sigma-Aldrich A7030, fatty acid-free).
To obtain monocytes/macrophages, PBMCs were isolated from 50 mL of blood by centrifugation over a Ficoll-Paque density gradient (GE Healthcare Biosciences) 45 minutes at 400g. Cells were removed from the upper band located at the interface between the plasma and resolving medium. Cells were then washed twice with Mg2+- and Ca2+-free phosphate-buffered saline (PBS) containing 1% BSA and 2mM EDTA (ethylenediaminetetraacetic acid; pH 7.4). Monocytes were obtained by magnetic sorting using CD14 magnetic beads (magnetic-activated cell sorting; Miltenyi Biotec) per the manufacturer's instructions. To obtain cultured monocytes/macrophages, PBMCs were resuspended in RPMI 1640 medium containing 10% fetal bovine serum and incubated in plastic 6-well cell culture dishes (Costar) at 2 × 106 cells per well. After incubation at 37°C for 1 hour, media were removed along with nonadherent cells, and adherent cells were maintained at 37°C in a 5% CO2 atmosphere in serum-free culture medium X-vivo 15 (Lonza) containing 10 ng/mL of human recombinant macrophage colony stimulating factor (M-CSF, Biosource). To generate differentiated macrophages, cells were cultured for 72 to 96 hours in 2 mL of serum-free medium containing 10 ng/mL M-CSF and 1 μg/mL lipopolysaccharide (LPS, Escherichia coli serotype 0111:B4, Sigma-Aldrich). Control wells containing unstimulated monocytes were maintained similarly in the absence of LPS. The purity of monocyte preparations was > 95% based on flow cytometric analysis of detached cells stained with monoclonal anti-CD14 and anti-CD11b antibodies (data not shown). To measure incorporation of sulfated side chains into surface GAG side chains, media from differentiated and undifferentiated cells were replaced with fresh media containing 50 μCi/mL 35S-sodium sulfate (PerkinElmer). After 24 hours, cells were washed thoroughly with PBS and detached gently by a 30 minute incubation with 5 mL of Mg2+- and Ca2+-free PBS containing 1% BSA and 2 mM EDTA. Surface HS and CS were then degraded by adding heparinase or chondroitinase, respectively, as described below. 35S radioactivity was measured in a Packard 1500 Tri-Carb scintillation counter in Ultima Gold liquid scintillation cocktail (Perkin Elmer).
Mouse samples.
Mouse PRP and washed platelets were prepared using blood collected from the inferior vena cava of 8-14-week-old mice in ACD (1:5 vol:vol), diluted immediately 1:3 (vol:vol) in modified Tyrode buffer containing prostaglandin E1 (final concentration, 1 μg/mL), and centrifuged at 200g for 4 minutes at RT. PRP was centrifuged at 800g for 10 minutes at RT, and the pelleted platelets washed and resuspended in modified Tyrode buffer.
Mouse bone marrow–derived macrophages were isolated from femoral and tibial bone marrow and propagated in the presence of recombinant mouse (m)M-CSF as described previously.28 Briefly, single-cell suspensions of bone marrow cells were obtained by flushing the femurs and tibias of mice. Cell suspensions (including erythrocytes) were cultured in 5-cm diameter culture plates at 2 × 106 leukocytes/mL in Iscove Modified Dulbecco Medium with Glutamax-I, 25 mM HEPES, 3.024 g/L sodium bicarbonate (Gibco) supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, 10% FCS (HyClone), and 10 ng/mL mM-CSF (Biosource). Three days later, nonadherent cells were removed and media replenished every second day. After 7 days, serum-free culture medium X-vivo 15 (Lonza) was supplemented with 10 ng/mL mM-CSF and 1 μg/mL LPS. Control wells containing undifferentiated cells were maintained in the absence of LPS. Bone marrow–derived macrophages were analyzed by flow cytometry. All studies involving mice were approved by the Institute Animal Care and Use Committee of Children's Hospital of Philadelphia.
HIT antigen expression
Washed platelets (105/μL) and/or monocytes (2 × 103/μL) were incubated with varying concentrations (0-400 μg/mL) of recombinant hPF4 in a final volume of 100 μL for 45 minutes at RT. In some experiments, UFH (0-200 μg, porcine intestinal mucosa, 212 USP/mg, Sigma-Aldrich) was added to the cells before the hPF4. Fc receptors (FcγRIIA–CD32 on platelets and monocytes and FcγRI–CD64 and FcγRIII–CD16 on monocytes) were blocked by adding corresponding moAb prior to PF4. FITC-labeled KKO (50 μg/mL) and PerCP and/or APC-labeled antibodies to identify platelets and/or monocytes were then added for an additional 15 minutes. Samples were diluted with Tyrode buffer and the cells enumerated immediately or fixed in 1% paraformaldehyde in PBS (1:10 vol/vol) and counted later. Binding of FITC-labeled KKO to platelets and monocytes was identified using a Becton Dickinson FACSscan daily calibrated for fluorescence and light scatter using manufacturer's standard beads (CaliBRITE; Becton Dickinson). The surface area of monocytes is approximately 50-fold larger than the surface area of platelets based on the known diameter and assuming spherical shape for monocytes and a discoid shape for platelets. The fluorescence intensity of monocytes, including background fluorescence, is correspondingly higher. The FITC fluorescence detector (FL1 PMT) was adjusted specifically for platelets and monocytes to bring the background fluorescence in the absence of PF4 to the same geometric mean fluorescence intensity (MFI) for both cell types, thus normalizing for this difference in surface area. The binding of KKO in the presence of PF4 was expressed as the ratio of MFI of antibody bound in the presence of a specific amount of KKO and PF4 to the MFI generated by background fluorescence of each cell type incubated with KKO in the absence of PF4. Human platelets were identified and gated according to side scatter and immunofluorescence with PerCP-Cy5.5 labeled anti-CD41a moAb. Monocytes were identified and gated by side scatter and immunofluorescence with anti–CD45-PerCP and anti–CD14-APC moAb. Monocyte differentiation into macrophages was estimated by increased expression of CD11b and a marker for differentiation F4/80.29
Role of GAGs in surface HIT antigen expression
Binding of hPF4 to surface GAGs.
Surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide) has been shown to block hPF4 binding to surface GAGs.30 Originally synthesized by the Open Chemical Repository in the Developmental Therapeutic Program at the National Cancer Institute (NSC12155), surfen was kindly provided by Dr Jeffrey Esko (Glycobiology Research and Training Center, La Jolla, CA). A stock solution of surfen (30mM) was prepared in dimethyl sulfoxide (DMSO) and stored in glass containers under nitrogen at −80°C in the absence of light. Fresh aqueous solutions were prepared as needed. Washed cells were incubated with 10μM surfen for 10 minutes in Tyrode buffer before being added to the various concentrations of hPF4 and stained with antibodies. Cells incubated with DMSO diluted in Tyrode buffer 1:3000 served as the control.
Enzymatic cleavage of surface GAGs.
In some studies, platelets and/or monocytes were incubated with 1 U/mL chondroitinase ABC (Sigma-Aldrich) or heparinase III (Sigma-Aldrich) at 37°C. After 30 minutes, aliquots containing equal numbers of cells were incubated with Tyrode buffer containing various concentrations of hPF4 (0-400 μg/mL, final concentration) for 60 minutes at RT. FITC-labeled KKO (50 μg/mL) was added for 15 minutes, samples were diluted 1/10 with Tyrode buffer, and antibody binding was measured by flow cytometry.
TF assay
Washed monocytes were adjusted to a concentration of 2 × 106 cells/mL and incubated with increasing amounts of hPF4 with or without 50 μg/mL of KKO for 3 hours with gentle agitation at 37°C in a 5% CO2 atmosphere in serum-free culture medium X-vivo 15. TF expression was measured by binding of PE-labeled anti-CD142 moAb using flow cytometry and TF activity by factor (F) Xa generation in monocyte suspensions after adding FVIIa (NovoSeven) and FX (purified from plasma, kind gift from R. Camire, University of Pennsylvania). Surface FXa generation was measured using chromogenic substrate S-2765, as previously described.22 Monocytes activated with LPS (10 μg/mL) for 3 hours served as the positive control in each experiment. Each test was performed in triplicate.
Transgenic mice
Transgenic mice expressing hPF4 in platelets (hPF4+) have been described previously.8,31 These mice were crossbred with mouse PF4 knockout (mPF4KO) mice32 and with mice expressing human FcγRIIA (generously provided by Steven McKenzie, Thomas Jefferson University).33 All murine lines had been previously backcrossed onto the C57BL/6J background more than 12 times. The genomic composition of mice was determined by polymerase chain reaction analysis using oligonucleotide primers described previously.32,33 Controls were littermates transgenic for hPF4 or FcγRIIA on mPF4KO background. As all mice studied were mPF4KO, this component of their genetic makeup is omitted hereafter. Mice were 6-10 weeks of age at the time of study.
Monocyte depletion in mice
Clodronate liposomes.
Clodronate (dichloromethylene diphosphonate)/PBS-encapsulated liposomes obtained from Encapsula Nanosciences have been previously shown to cause monocyte depletion after intravenous administration.34 Mice were anesthetized with isoflurane and injected intra-orbitally with 100 μL of PBS solution– or clodronate-containing liposomes. Complete blood counts were measured in 50 μL of whole blood obtained by retro-orbital puncture into Safe-T-Fill minicapillary blood collection tubes (Kabe Labortechnik, Nümbrecht-Elsenroth). Platelets and white blood cells (WBC) were enumerated using an automatic cell counter (HEMAVET; Drew Scientific). Absolute monocyte counts were calculated from total WBC counts and the proportion of CD115+ cells out of total CD45+ cells identified by flow cytometry.
Gadolinium chloride (GdCl3).
GdCl3 (Sigma-Aldrich) has previously been shown to block phagocytosis by liver Kupfer cells within 24 to 48 hours after intravenous infusion and to selectively eliminate large macrophages situated in the periportal zone of the liver acinus.35,36 Isofluorane anesthetized mice were injected intra-orbitally with GdCl3 (50 mg/kg) or an equal volume of 0.15 M NaCl, and platelets and monocytes were enumerated as above.
Photochemical carotid injury model
Carotid artery thrombosis was induced by photochemical injury.37 Adult male and female mice were anesthetized with ketamine and xylazine (80 mg/kg and 10 mg/kg, respectively), and the right carotid artery and left jugular vein were exposed by blunt dissection. A 3 mW, 540-nm laser beam (green) was applied to the artery from a distance of 5 cm. Rose bengal dye (50 mg/kg body weight; Sigma-Aldrich) was then injected into the left jugular vein, and blood flow in the artery was recorded using a small animal blood flow meter (model T106; Transonic Systems) for 90 minutes. Time to initial formation of a complete occlusive thrombus (occlusion > 10 minutes) was used as the end point. Thirty minutes before injecting rose bengal dye, some animals were injected intra-orbitally with 2 mg/kg of KKO in 100 μL PBS. To examine the effect of monocyte depletion on thrombosis, some animals were also injected with clodronate liposomes 24 hours before induction of the photochemical carotid injury.
Cremaster laser injury model
The intravital video-microscopy system used to study clot formation within the cremaster muscle blood vessels has been described previously.38,39 Briefly, each mouse was anesthetized using an intraperitoneal injection of pentobarbitol sodium (80 mg/kg; Abbott Laboratories) and maintained with the same anesthetic delivered via a catheterized jugular vein, as needed. Microvessels were studied using an Olympus BX61WI microscope (Olympus) equipped with a 40×/0.8 numeric aperture water-immersion objective lens. Labeled antibodies (CD41 Alexa488 and CD115 APC) were injected intravenously into the cannulated jugular vein 5 minutes before the onset of injury. Laser injury was induced using an SRS NL100 Nitrogen Laser system (Photonic Instruments) at 65% energy level. Blood vessels ranging in size from 20-40 μm were studied. Injuries to arterioles and venules were performed at the edge of the vessel wall. Visual confirmation of small extravasations was made for each blood vessel studied as an indicator of consistent injury. No more than 10 laser pulses were used to generate each injury. Data were collected over 4 minutes at 4 frames per second (f/s; 1000 frames per study). Up to 5 arteriolar and 5 venular injuries were studied per mouse. KKO was injected intravenously (2 mg/kg) into the cannulated jugular vein after the first 4 injuries (2 arteriole and 2 venule injuries), and the remaining vessels were injured 20-60 minutes later. Data were analyzed using Slidebook software Version 4.2 (Intelligent Imaging Innovations). Background fluorescence was removed by placing a high-sensitivity mask upstream of the growing clot and the intensity of the signal collected subtracted from the intensity of the clot signal in a frame-by-frame fashion.
Statistical analysis
KKO binding and time to occlusion were analyzed using the 2-tailed t test. Statistical analyses were performed using Microsoft Excel for Mac 2004 Version 11.5.1. Differences were considered significant at a P value of < .05.
Results
PF4 bound to the monocyte surface forms antigenic complexes
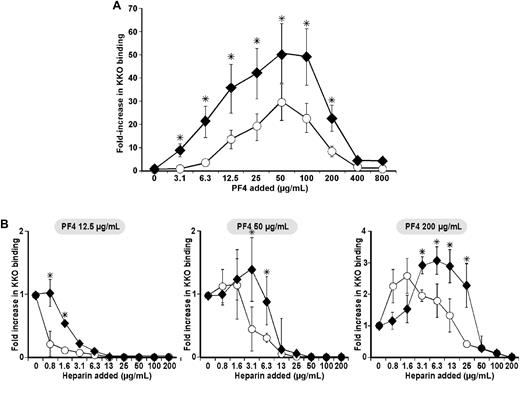
We have previously shown that the HIT-like moAb KKO and HIT immunoglobulin (IgG) share requirements for binding to purified hPF4/heparin11 and to platelets, including the effect of varying hPF4 concentration.8 To understand the possible participation of monocytes in thrombus development, we first examined formation of HIT-like antigenic complexes on the surface of monocytes exposed to exogenous hPF4. The binding of KKO to unstimulated human monocytes incubated with varying amounts of hPF4 followed a bell-shaped curve seen previously with platelets, with maximum binding to each cell type seen at an external hPF4 concentration of 50-100 μg/mL (Figure 1A). In the absence of PF4, the MFI of cells incubated with an isotype control mAb (TRA) was not significantly different from that with KKO (data not shown). Neither did the MFI of cells incubated with TRA change after addition of hPF4 (0-800 μg/mL; data not shown). However, the effect of hPF4 on binding of KKO to monocytes was quantitatively greater than on platelets, even taking the differences in surface area into consideration. Moreover, at suboptimal hPF4 concentrations (< 12.5 μg/mL), there was a significantly greater increase in KKO binding to monocytes than to platelets (P < .005). For example, at an hPF4 concentration of 3.1 μg/mL, little or no KKO binding to the platelets was observed, while an approximately 8-fold increase in KKO binding to monocytes above baseline was detected.
Surface PF4/GAG antigenicity on monocytes compared with platelets. (A) PF4/GAG complexes detected using the HIT-like monoclonal antibody KKO on human monocytes (♦) compared with simultaneously studied human platelets from the same blood sample (○) and (B) in the presence of increasing concentration of heparin (0.8-200 μg/mL ≈ 0.17-42.4 USP/mL) at 3 fixed PF4 concentrations: 12.5 μg/mL (left), 50 μg/mL (center), and 200 μg/mL (right). The graphs show the fold-increase in mean fluorescence intensity (MFI) of antibody binding in the presence of the concentrations of PF4 and heparin noted compared with MFI in its absence after the results were normalized for size based on equalizing the background MFI of monocytes and platelets. N ≥ 3 experiments, each done in duplicate. The mean ± 1 SE is shown. *P < .05, comparing the fold increase in MFI for monocytes to platelets at a specific PF4 concentration.
Surface PF4/GAG antigenicity on monocytes compared with platelets. (A) PF4/GAG complexes detected using the HIT-like monoclonal antibody KKO on human monocytes (♦) compared with simultaneously studied human platelets from the same blood sample (○) and (B) in the presence of increasing concentration of heparin (0.8-200 μg/mL ≈ 0.17-42.4 USP/mL) at 3 fixed PF4 concentrations: 12.5 μg/mL (left), 50 μg/mL (center), and 200 μg/mL (right). The graphs show the fold-increase in mean fluorescence intensity (MFI) of antibody binding in the presence of the concentrations of PF4 and heparin noted compared with MFI in its absence after the results were normalized for size based on equalizing the background MFI of monocytes and platelets. N ≥ 3 experiments, each done in duplicate. The mean ± 1 SE is shown. *P < .05, comparing the fold increase in MFI for monocytes to platelets at a specific PF4 concentration.
KKO binding in the presence of heparin
We had shown previously that heparin modulates recognition of hPF4 by KKO on the platelets surface depending on concentration of both reactants.8 PF4 has greater affinity for heparin than for platelet CS. Therefore, addition of heparin displaces hPF4 from CS, reducing surface concentration and shifting the binding curve of KKO to the right.8 We now compared the effect of hPF4 and heparin on KKO binding to monocytes and platelets from the same blood samples measured in parallel (Figure 1B). At all concentrations of hPF4 added, whether below (12.5 μg/mL), near (50 μg/mL), or above peak antigenicity (200 μg), KKO binding to monocytes followed the same pattern as seen on platelets, but monocytes retained more surface antigenic complexes at every concentration of heparin added. As a result, 2-8× higher concentrations of heparin were required to reduce KKO binding to monocytes to the same extent as on platelets.
The role of different surface hPF4/GAG complexes on monocytes
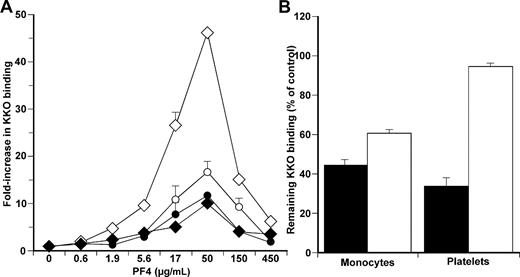
We hypothesized that this difference to KKO binding to monocytes and platelets at different concentrations of hPF4 and heparin relates to surface GAG side chain composition, which is almost exclusively confined to CS on platelets,9,10 but involves a more complex mixture of CS, HS, and DS on monocytes.24 To test this hypothesis, we measured KKO binding to monocytes and platelets at various concentrations of hPF4 in the presence of surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide). Surfen is a recently described antagonist of UFH, HS, and other GAGs. This compound binds more strongly to cellular HS than CS.30 Surfen, like PF4, interacts with HS through electrostatic interactions. Addition of surfen to cultured Chinese hamster ovary cells blocks fibroblast growth factor 2 binding and signaling that depends on cell surface HS.40 Preincubation of monocytes with surfen before addition of hPF4 markedly attenuated KKO binding (Figure 2A) compared with cells incubated with vehicle only or binding to platelets (Figure 2A), consistent with the lower affinity of surfen for CS than HS.
Studies of surface GAGs in KKO binding to cell surface. (A) KKO binding to monocytes (diamonds) and to platelets (circles) at increasing concentrations of PF4 as in Figure 1A but with either the vehicle DMSO (open symbols) or in the presence of 10 μM surfen (closed symbols). N = 5 experiments, each done in duplicate. The mean ± 1 SE is shown. (B) Monocytes and platelets pretreated with either chondroitinase ABC alone (■) or heparinase alone (□). Residual KKO binding compared with control (untreated) cells at the optimal PF4 concentration (50 μg/mL) is shown. N = 3 experiments, each done in duplicate. The mean ± 1 SE is shown.
Studies of surface GAGs in KKO binding to cell surface. (A) KKO binding to monocytes (diamonds) and to platelets (circles) at increasing concentrations of PF4 as in Figure 1A but with either the vehicle DMSO (open symbols) or in the presence of 10 μM surfen (closed symbols). N = 5 experiments, each done in duplicate. The mean ± 1 SE is shown. (B) Monocytes and platelets pretreated with either chondroitinase ABC alone (■) or heparinase alone (□). Residual KKO binding compared with control (untreated) cells at the optimal PF4 concentration (50 μg/mL) is shown. N = 3 experiments, each done in duplicate. The mean ± 1 SE is shown.
To examine the role of specific surface GAGs in greater detail, we pretreated both cell types with either chondroitinase ABC, which selectively removes CS, or heparinase, which removes HS and DS. Preincubation of platelets with chondroitinase ABC alone reduced KKO binding to 35% ± 5% of control (P < .05), whereas heparinase alone was ineffective (Figure 2B). In contrast, binding of KKO to monocytes was decreased by either chondroitinase ABC or heparinase (43.5% ± 3% and 63.5% ± 2.5%, respectively, both P < .05 compared with untreated cells), suggesting variation in the composition of surface GAGs contribute to the differences in hPF4 binding, effect of heparin, and amount of KKO binding seen on the 2 cell types.
Changes in surface properties on LPS-stimulated monocytes
Activation of monocytes is associated with an increase in sulfation of cell surface GAGs,24,26,41 and HIT often occurs on a background of systemic or local inflammation.13,16 Therefore, we asked whether activation of monocytes would increase binding of hPF4 and KKO. To test this hypothesis, primary human monocytes were incubated with LPS (1 μg/mL) for 72 hours. Total sulfation, as measured by 35S-incorporation, was increased 4-fold in LPS-stimulated compared with unstimulated monocytes (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Sulfation of surface CS as measured by treatment of cells with chondroitinase ABC increased approximately 2-fold, while sulfation of surface HS and DS as measured by treatment of cells with heparinase increased approximately 1.8-fold (supplemental Figure 1B).
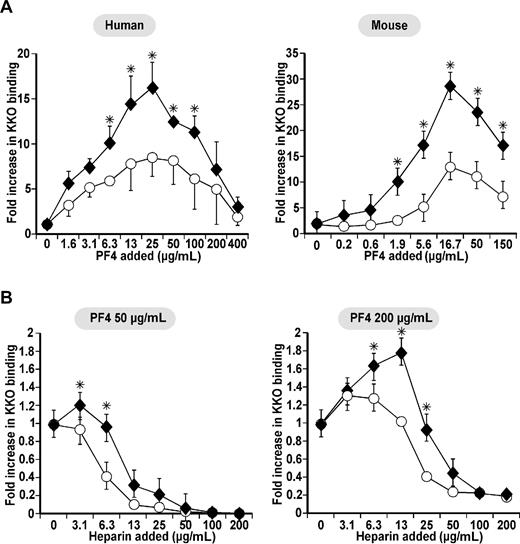
We next measured binding of KKO at different concentrations of hPF4 to human and mouse primary monocytes exposed to LPS (Figure 3A). Murine monocytes were obtained from mPF4KO mice to exclude PF4 synthesis.42 LPS activation increased KKO binding to both human and mouse LPS-stimulated monocytes at all concentrations of hPF4 added. At low hPF4 concentrations (eg, 1.9 μg/mL), LPS-stimulated murine monocytes bound approximately 9-fold more KKO while there was almost no change seen on control cells (Figure 3A). Even at hPF4 concentrations that are near peak (50 μg/mL) or above peak (200 μg/mL) binding to control cells, LPS increased KKO binding an additional 2-4-fold (Figure 3A). Moreover, LPS-stimulated monocytes were more resistant to loss of KKO binding by added heparin at every hPF4 concentration tested (Figure 3B). Thus, 2-4× more heparin was needed at every PF4 concentration to reduce KKO binding to activated macrophages compared with resting monocytes.
Surface KKO binding on monocytes compared with macrophages. (A) Human (left) and mouse (right) studies of relative KKO binding as in Figure 1A to cultured unstimulated monocytes (□) and concurrently studied LPS-stimulated monocytes (♦). The murine cells were isolated from a mPF4KO mouse. N ≥ 3, each in duplicate. Mean ± 1 SE are shown. *P < .05 for relative binding to stimulated cells versus resting cells. (B) mPF4KO mice cultured, unstimulated monocyte (○), and concurrently studied LPS-stimulated monocytes (♦) at 2 different PF4 concentrations and different heparin concentrations as in Figure 1B. N ≥ 3, each in duplicate. Mean ± 1 SE are shown. *P < .05 for relative binding to stimulated cells versus unstimulated cells.
Surface KKO binding on monocytes compared with macrophages. (A) Human (left) and mouse (right) studies of relative KKO binding as in Figure 1A to cultured unstimulated monocytes (□) and concurrently studied LPS-stimulated monocytes (♦). The murine cells were isolated from a mPF4KO mouse. N ≥ 3, each in duplicate. Mean ± 1 SE are shown. *P < .05 for relative binding to stimulated cells versus resting cells. (B) mPF4KO mice cultured, unstimulated monocyte (○), and concurrently studied LPS-stimulated monocytes (♦) at 2 different PF4 concentrations and different heparin concentrations as in Figure 1B. N ≥ 3, each in duplicate. Mean ± 1 SE are shown. *P < .05 for relative binding to stimulated cells versus unstimulated cells.
TF expression and activity
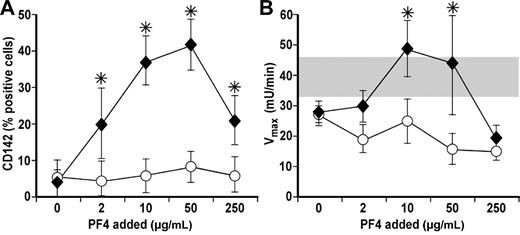
Binding of HIT antibodies to hPF4 on monocytes leads to expression of TF and acceleration of membrane-dependent coagulation.22,23 We asked if these effects triggered by KKO followed a bell-shaped curve that mirrored formation of hPF4/GAG complexes. We observed no increase in TF expression or TF activity when monocytes were incubated with 0-250 μg/mL hPF4 alone or with 50 μg/mL of KKO alone. When monocytes were incubated with hPF4 and KKO together, the elaboration of TF expression and activity followed a bell-shaped curve closely reflecting the pattern of KKO binding in the presence of varying PF4 concentration (Figure 4A-B).
Expression and activity of TF on monocytes in the presence of PF4 and KKO. (A) Expression of TF on monocytes at different PF4 concentrations in the absence (○) or presence of KKO (♦). N ≥ 3, each in duplicate. Mean ± 1 SE are shown. *P < .05 for relative binding in the absence versus presence of KKO. (B) Same as in panel A but for TF activity. Gray bar represents the range of TF activity after LPS stimulation. N ≥ 3, each in duplicate. Mean ± 1 SE are shown. *P < .05 for relative binding in the absence versus presence of KKO.
Expression and activity of TF on monocytes in the presence of PF4 and KKO. (A) Expression of TF on monocytes at different PF4 concentrations in the absence (○) or presence of KKO (♦). N ≥ 3, each in duplicate. Mean ± 1 SE are shown. *P < .05 for relative binding in the absence versus presence of KKO. (B) Same as in panel A but for TF activity. Gray bar represents the range of TF activity after LPS stimulation. N ≥ 3, each in duplicate. Mean ± 1 SE are shown. *P < .05 for relative binding in the absence versus presence of KKO.
Effect of monocyte depletion on HIT-induced thrombocytopenia and thrombosis
The studies described to this point suggest that monocytes are more susceptible than platelets to binding hPF4 and KKO, leading to elaboration of procoagulant activity. Therefore, we next examined the effect of monocyte depletion on the development of thrombosis and thrombocytopenia in a previously described murine model of HIT,8,43 where we had demonstrated that binding of KKO to endogenous hPF4:surface GAG complexes is sufficient to cause thrombocytopenia (hereafter “modified” murine HIT model).8,43 Two models for inducing monocyte depletion or inactivation were employed. The first involved the intravenous injection of liposome-encapsulated clodronate, an intracellular toxin taken up by monocyte/macrophages that has been used extensively to study the effects of monocyte depletion in mice.34,44 To study the contribution of monocytes in HIT, we depleted monocytes with clodronate liposomes in transgenic FcγRIIA+/hPF4+ mice. We used flow cytometry to measure the relative number of cells expressing the monocyte differentiation marker CD115 as a proportion of total WBC characterized by expression of CD45. Upon injection of clodronate liposomes, virtually all monocytes were depleted from the peripheral blood within 16 hours. Full recovery did not occur until day 3 (supplemental Figure 2A). The number of circulating lymphocytes, granulocytes, and platelets was unchanged (data not shown).
Thrombocytopenia induced by injection of HIT moAb KKO 12 hours after the clodronate liposomes was exacerbated in monocyte-depleted mice compared with controls injected either with PBS or with PBS-encapsulated liposomes (Figure 5A). Mice expressing high levels of PF4 in their platelets develop severe thrombocytopenia after KKO,8 and in our preliminary experiments some of them died when KKO at a the dose of 10 mg/kg intraperitoneally was given after the depletion of monocytes. Therefore we used a low KKO dose (2.5 mg/kg),8 which produced more modest thrombocytopenia in our model. The platelet count after depleting monocytes with clodronate liposomes continued to drop over the next several days, with recovery following the same time course as the recovery in monocytes.
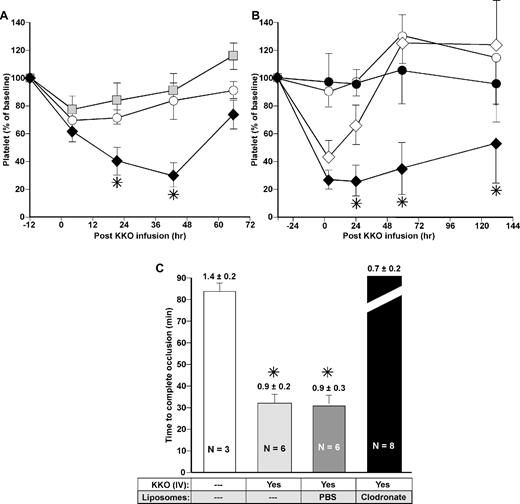
Effect of monocyte depletion on platelet count and thrombosis in the HIT model. (A) Platelet counts after induction of HIT in the FcγRIIA+/hPF4+ mice model. Clodronate- or PBS-laden liposomes were injected intravenously. Twelve hours later (Time 0), KKO 2.5 mg/kg was administered intraperitoneally. The first platelet count was measured 4 hours later. Animals receiving no liposomes (○) or PBS-laden liposomes (▩) had modest thrombocytopenia compared with the same mice receiving clodronate-laden liposomes (♦). N ≥ 3, each in duplicate. Mean ± 1 SE are shown. *P < .05 for platelet drop after KKO in mice with depleted monocytes versus control mice. (B) Same as panel A but animals received NaCl (open symbols) or GdCl3 (black symbols) 32 hours prior to the KKO 10 mg/kg intraperitoneal injection (diamonds) or controls without KKO (circles). N ≥ 3, each in duplicate. Mean ± 1 SE are shown. *P < .05 for platelet drop in mice treated with GdCl3 followed by KKO versus mice treated with NaCl followed by KKO. (C) Times to complete occlusion in a photochemical carotid artery model in FcγRIIA+/hPF4+ mice under conditions noted in the figure are shown as mean ± 1 SE. The platelet counts at the time of study are indicated above each bar and is per 109/mL. N values are noted in the figures. *P < .001 for time to occlusion relative to mice not receiving either KKO or liposomes.
Effect of monocyte depletion on platelet count and thrombosis in the HIT model. (A) Platelet counts after induction of HIT in the FcγRIIA+/hPF4+ mice model. Clodronate- or PBS-laden liposomes were injected intravenously. Twelve hours later (Time 0), KKO 2.5 mg/kg was administered intraperitoneally. The first platelet count was measured 4 hours later. Animals receiving no liposomes (○) or PBS-laden liposomes (▩) had modest thrombocytopenia compared with the same mice receiving clodronate-laden liposomes (♦). N ≥ 3, each in duplicate. Mean ± 1 SE are shown. *P < .05 for platelet drop after KKO in mice with depleted monocytes versus control mice. (B) Same as panel A but animals received NaCl (open symbols) or GdCl3 (black symbols) 32 hours prior to the KKO 10 mg/kg intraperitoneal injection (diamonds) or controls without KKO (circles). N ≥ 3, each in duplicate. Mean ± 1 SE are shown. *P < .05 for platelet drop in mice treated with GdCl3 followed by KKO versus mice treated with NaCl followed by KKO. (C) Times to complete occlusion in a photochemical carotid artery model in FcγRIIA+/hPF4+ mice under conditions noted in the figure are shown as mean ± 1 SE. The platelet counts at the time of study are indicated above each bar and is per 109/mL. N values are noted in the figures. *P < .001 for time to occlusion relative to mice not receiving either KKO or liposomes.
As a second model to determine whether the monocyte were indeed involved in exacerbation of thrombocytopenia, we used a method to selectively inactivate hepatic and other tissue macrophages by intravenous infusion of GdCl3.35,36 GdCl3 had no effect on the number of peripheral blood monocytes (supplemental Figure 2B). The duration of thrombocytopenia in mice injected with KKO 30 hours after administration of GdCl3 was significantly protracted compared with mice given KKO after receiving vehicle alone (Figure 5B).
We also examined whether monocyte depletion affects the prothrombotic state of mice in the modified murine HIT model. We depleted peripheral monocytes with clodronate in FcγRIIA+/hPF4+ mice 24 hours before inducing photochemical carotid artery injury using rose bengal. FcγRIIA+/hPF4+ mice injected with KKO 30 minutes before injury exhibited a shorter time to complete occlusion than mice injected with isotype control (35 ± 8 minutes vs 86 ± 10 minutes, P < .0005) notwithstanding the drop in platelet count in the KKO-treated mice at the time of study (Figure 5C). Mice injected with control PBS-liposomes developed complete occlusion as rapidly post-KKO as mice injected with KKO alone (33 ± 3 minutes). Depletion of monocytes using clodronate liposomes attenuated the prothrombotic state induced by KKO and prolonged the time to occlusion such that none of the 8 tested mice exhibited vascular occlusion by 90 minutes (Figure 5C). These monocyte-depleted mice developed significantly (P < .05) lower platelet counts than mice in either of the 2 KKO-infused control arms (Figure 5C). However, a drop in the platelet count to this level does not affect time to occlusion induced by KKO (data not shown). When studied at later time points, mice with normal numbers of monocytes still develop rapid vascular occlusion after injection of KKO even when their platelet count was as low as approximately 350 000/μL (data not shown).
Intravital microscopy of monocyte incorporation into thrombi
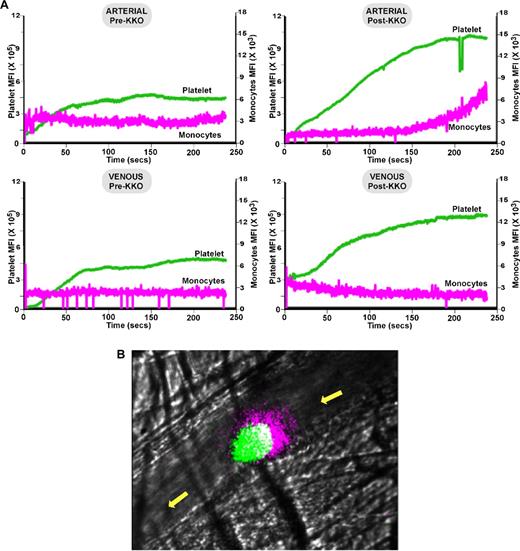
The studies described above show that depletion of monocytes prevents vascular occlusion induced by a HIT-like antibody. To determine whether this was due to a direct effect on monocytes entering the site of vascular damage, the studies were repeated in the cremaster laser injury model. After infusion of KKO, the rate and number of platelets that accumulate at the site of thrombosis increased both in small arterioles and venules (Figure 6A-B and supplemental Videos 1-4). Remarkably, CD115+ monocytes and/or monocyte-derived microparticles accumulated into growing thrombi after KKO infusion, but only on the arterial side and only in mice with HIT (Figure 6A-B and supplemental Videos 1-4). This accumulation of CD115+ monocytes at the upstream end of the developing arterial thrombus was delayed by approximately 2 minutes relative to the onset of platelet accumulation.
In situ laser injury studies in the murine HIT model. (A) Shown are cumulative information for platelets (green) and monocytes (pink) at a site of induced injury for ≥ 10 injuries per condition within the arterioles and venules and pre- and post-KKO as indicated. (B) Same as in panel A but still from video at 4 minutes from an arteriole injury showing the accumulation of CD115+-monocytes and/or microparticles at the upstream end of a growing thrombus. Platelets appear green, monocytes pink, and the overlap white. The yellow arrow denotes the direction of blood flow.
In situ laser injury studies in the murine HIT model. (A) Shown are cumulative information for platelets (green) and monocytes (pink) at a site of induced injury for ≥ 10 injuries per condition within the arterioles and venules and pre- and post-KKO as indicated. (B) Same as in panel A but still from video at 4 minutes from an arteriole injury showing the accumulation of CD115+-monocytes and/or microparticles at the upstream end of a growing thrombus. Platelets appear green, monocytes pink, and the overlap white. The yellow arrow denotes the direction of blood flow.
Discussion
Thrombocytopenia in HIT is often mild and is rarely associated with bleeding.17,45,46 Rather, patients with HIT often develop thrombosis, which has been attributed to the binding of HIT antibodies to platelet surface PF4/GAG complexes and subsequent platelet activation through its FcγRIIA receptors.8,15 The reason why anti-PF4/GAG, but not other potent platelet antibodies, lead to thrombosis rather than bleeding has not been explained satisfactorily. Furthermore, it is clear that thrombosis may develop in HIT in the absence of thrombocytopenia or long after platelet recovery.17
We believe that part of the explanation for the above observations lies in data presented in this paper: the surface of monocytes binds hPF4 and forms HIT antigenic complexes at lower concentrations than platelets in large part due to the presence of HS and DS in addition to CS, the dominant heparanoid side chain on the surface of platelets. PF4 complexed with HS and DS is more resistant to dissociation by heparin because of their higher natural affinity.1,47 More detailed biochemical studies will be required to identify the specific proteoglycans or glycopeptides involved in PF4 binding to monocytes, and it is entirely possible that the capacity of PF4 and antibody to interfere with their respective physiologic activities contributes to the pathogenesis of HIT. However, irrespective of the specific targets, formation of antibody binding complexes on the surface of monocytes at lower PF4 concentrations means they will become targets for HIT antibodies under some conditions when platelets are not. Moreover, the requirement for higher heparin concentrations to remove PF4 from the surface of monocytes implies they will remain targets for pathogenic antibodies at levels of heparin at which antigenic PF4/GAG complexes no longer remain on platelets.
HIT often occurs in complex medical settings characterized by inflammation with elaboration of cytokines that activate monocytes.13 We show that LPS stimulation of monocytes leads to hypersulfation of surface HS and CS, consistent with prior studies.24,26,41 The affinity of PF4 to GAGs depends directly on extent of sulfation,1 and LPS induces a selective increase in HS relative to other GAG side chains.48 Our demonstration that these changes increase surface PF4/GAG complexes at low levels of PF4 and in the presence of heparin may help to explain why HIT and thrombosis is more common in states associated with inflammation. Consistent with studies by others,22,23 we show that monocytes and macrophages with surface-bound PF4/GAGs are activated by HIT antibodies resulting in the expression of TF, which likely contributes to the observed thrombosis. In a prior study, monocyte activation and TF expression by HIT antibodies occurred with isolated monocytes when PF4 was added alone without the addition of heparin.23 When whole blood was studied, both PF4 and heparin had to be added to see activation by the HIT antibody. These studies are consistent with the data in Figure 1B, if there were released endogenous PF4 in addition to the exogenous PF4, in the whole blood samples.
Our in vivo studies provide additional support for these conclusions. Monocyte depletion attenuates the prothrombotic state as measured in a photochemical carotid artery injury model. In situ microscopy studies affirm the prothrombotic phenotype on both the arterial and venular sides of the circulation and demonstrated that CD115+-monocytes and/or monocyte-derived microparticles accumulate at the upstream end of the clot induced by a HIT-like moAb. Why this accumulation appears restricted to the arterial side is unclear, but in videos of vessels with slow arterial blood flow, monocytes accumulation was diminished or absent (data not shown), suggesting that attachment is shear dependent. Additional studies are needed to define the mechanism by which monocytes adhere to the arterial side of the circulation and their effect on thrombus growth.
Videomicroscopy of the cremaster vasculature shows HIT antibody increased platelet accumulation in venules as well as arterioles. On the arteriole side, platelet accumulation occurs immediately while monocyte accumulation is delayed by approximately 2 minutes. These studies suggest that the prothrombotic state in HIT may involve additional cell types, including the endothelium, which, like monocytes, express HS and DS within their luminal and abluminal matrices.49 Vascular expression of GAG side chains having greater affinity for PF4 than CS on platelets may make them especially important reservoirs for surface-bound PF4, binding of HIT antibody, and susceptibility to perturbation of their antithrombotic phenotype. Studies are in progress to assess the hypothesis that activation of the endothelium by HIT antibody contributes to venular injury where adherent monocytes are sparse, as well as arterial injury before monocyte incorporation becomes prevalent.
Why monocyte depletion or inactivation by 2 distinct techniques exacerbated thrombocytopenia in our modified murine HIT model is unclear. Indeed, we predicted that the opposite would have been seen, that is, depletion of monocytes would decrease clearance of antibody-coated platelets as in other immune platelet disorders. As treatment with clodronate liposomes or GdCl3 alone did not affect the platelet count, the more severe thrombocytopenia seen when either was given before KKO does not appear to represent an antibody-independent effect on platelets, such as an induction of disseminated intravascular consumption, nor have others reported this complication, but we have not systematically pursued other possibilities. One plausible explanation is that depletion of monocytes increases the availability of PF4 and KKO to bind to platelets, although how this affects platelet distribution or survival is unknown. Indeed, this observation highlights the fact that the mechanism(s) by which thrombocytopenia develops in patients with HIT is actually not defined, and the contribution of intravascular lysis or apoptosis, splenic, or hepatic clearance or incorporation into growing thrombi is unknown. It has also been reported that monocytes contribute to down-regulation of platelet activation in some settings.50
The involvement of monocytes in the prothrombotic state in HIT may have diagnostic and therapeutic implications. The greater affinity of monocytes to bind and retain PF4 to their surface may allow them to remain a target for HIT antibodies well after platelets are no longer targeted. The consequent activation of monocytes extends the prothrombotic state. Thus, patients may begin to develop HIT before antigenic complexes are detectable on platelets and may remain at risk long after platelet counts are normal, while evidence of monocyte or vascular activation (eg, expression of TF) is apparent. Current approaches to management with direct thrombin inhibitors fails to prevent recurrent thromboembolic events in a substantial proportion of patients. Efforts to understand the mechanism underlying monocyte (and endothelial) involvement in greater detail may provide new approaches to therapy. Last, the involvement of monocytes as a target cell may not be exclusive to HIT but may occur in other inflammatory disorders associated with thrombosis that are poorly controlled by conventional antiplatelet and anticoagulant managements including cancer, systemic lupus, and antiphospholipid syndrome, among others.
In summary, we have shown that monocytes, like platelets, bind hPF4 onto their surface and form HIT antigenic complexes. Optimal binding and antigen formation occurs over a specific narrow range of hPF4 concentrations. The presence of HS and DS, in addition to CS, increases the affinity of monocytes for hPF4 above platelets. Antigenic complexes are found on monocytes at lower PF4 concentrations than on platelets and are more resistant to dissociation by heparin. Activation of monocytes increases sulfation of surface GAGs, which further increases PF4 and antibody binding, which in turn initiates expression of TF. Monocytes or monocyte-derived particles are incorporated into thrombi, especially on the arterial side of the circulation, where they might contribute significantly to thrombus development. Appreciation of this previously pathophysiologic pathway may lead to novel approaches to prevent life-threatening thrombotic complications in HIT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Steven McKenzie and Michael Reilly for having generously shared the FcγRIIA mouse with our group; Dr Gowthami M. Arepally for the generous supply of KKO monoclonal antibody; Dr Rodney Camire for supplying factors VIIa and X; and Dr Jeffrey Esko for providing surfen.
This work was supported by American Heart Association grants 0735277N (L.R.) and 09GRNT2251065 (M.A.K.), and RO1 HL084006 (M.P.).
National Institutes of Health
Authorship
Contribution: L.R. was the primary investigator who designed and supervised or carried out the described studies and data analysis and interpretation, and wrote the manuscript. J.H. and T.G. performed cremaster laser injury model studies; L.Z. performed purification of WT and mutant PF4s; V.H. assisted in experimentation, focusing on the breeding and genotyping of the mice; M.A.K. assisted with the data analysis and interpretation and manuscript preparation; D.C. contributed to the design and interpretation of the described studies and helped with manuscript revision; and M.P. provided overall direction, supervised design and analysis of the studies, and contributed to manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lubica Rauova, The Children's Hospital of Philadelphia, 3615 Civic Center Blvd, ARC, Rm 316, Philadelphia, PA 19104; e-mail: lubica@email.chop.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal