Abstract
We show that the strength of T-cell stimulation determines the capability of human CD4+ T cells to become interleukin-17 (IL-17) producers. CD4+ T cells received either high- (THi) or low (TLo)–strength stimulation via anti-CD3/CD28 beads or dendritic cells pulsed with superantigen in the presence of pro-Th17 cytokines IL-1β, transforming growth factor β, and IL-23. We found that TLo, but not THi, stimulation profoundly promoted Th17 responses by enhancing both the relative proportion and total number of Th17 cells. Titration of anti-CD3 revealed that low TCR signaling promoted Th17 cells, but only in the presence of anti-CD28. Impaired IL-17 production in THi cells could not be explained by high levels of Foxp3 or transforming growth factor β–latency-associated peptide expressed by THi cells. Nuclear factor of activated T cells was translocated to the nucleus in both THi and TLo cells, but only bound to the proximal region of the IL-17 promoter in TLo cells. The addition of a Ca2+ ionophore under TLo conditions reversed the pro-Th17 effect, suggesting that high Ca2+ signaling impairs Th17 development. Although our data do not distinguish between priming of naive T cells versus expansion/differentiation of memory T cells, our results clearly establish an important role for the strength of T-cell activation in regulating Th17 responses.
Introduction
Differentiation of CD4+ T cells into different effector lineages depends on the activatory stimulus they receive and the cytokine milieu present.1 T-helper (Th)17 cells are a recently identified lineage of CD4+ T-helper cells, widely studied due to their important role in microbial host defense and autoimmune diseases.1-4 Th17 cells are characterized predominantly by the production of interleukin 17A (hereafter referred to as IL-17), a potent proinflammatory cytokine that induces neutrophil recruitment and production of further proinflammatory mediators, such as IL-1β, IL-8, matrix metalloproteinases 1 and 13, and prostaglandin E2.4
Th17-specific transcription factor retinoic acid receptor-related orphan receptor-γt (ROR-γt) is required for the expression of IL17, IL21, and IL22.4 In mouse T cells, it has been shown that Forkhead box p3 (Foxp3) can physically interact with RORγt along with Runx1, resulting in the inhibition of ROR-γt–mediated IL17 transcription.5 Both ROR-γt and Foxp3 require transforming growth factor β (TGF-β) for their expression.6 Another transcription factor involved in transcriptional regulation of IL-17 is nuclear factor of activated T-cells (NFAT)c1: it binds to conserved NFAT sites within both the human and murine IL-17 promoters and enhances IL-17 transcription.7,8
The generation of an in vitro population of Th17 cells is important for studying mechanisms of Th17 differentiation and for testing the effectiveness of therapeutics targeting Th17 cells. In mice, efficient in vitro differentiation toward a Th17 phenotype has been demonstrated in conditions incorporating IL-6 and TGF-β, resulting in up to 60% of Th17 cells.9 The requirement for TGF-β in human Th17 differentiation has been a matter of debate; however, TGF-β is now largely established as an essential factor for Th17 responses.10-12 IL-23 has been demonstrated to increase IL-17 production by stabilizing IL17 expression, although this cytokine alone is not sufficient to induce Th17 differentiation.13 In combination with TGF-β and IL-23, proinflammatory cytokines, such as IL-1β, IL-6, or IL-21, have also been suggested to be required for inducing Th17 development.11,14 However, despite a well-established pro-Th17 cytokine milieu, the efficiency of in vitro generation of human Th17 cells has remained poor in the majority of publications, not reaching the high proportions of Th17 cells achieved in mouse T-cell cultures.4,11,15,16
It has been demonstrated that for Th1/Th2 differentiation, strength of signaling through the T-cell receptor (TCR) regulates lineage development.17-19 Strength of T-cell stimulation may be altered via different means, for example, through the presence/absence of (co-)stimulatory signals through CD2 or CD28, or through variations in the affinity of the peptide/major histocompatibility complex (MHC) complex for the TCR, the total number of TCRs triggered, the number of antigen-presenting cells (APCs) available, or the duration over which interactions between T-cells and APCs occur. Th17 differentiation studies have, thus far, predominantly focused on the cytokine milieu, with little attention to TCR signaling or other pathways. Recently, it was reported that CD28 costimulation at high strength decreased the level of murine Th17 differentiation.20 Another recent study showed that varying potency of TCR signaling in mouse CD4+ T cells resulted in altered IL-17/IL-17F production ratios.8 We therefore sought to establish if the strength of T-cell stimulation would modulate human Th17 responses.
Here, we show that low-strength stimulation of human CD4+ T cells in a pro-Th17 cytokine milieu strongly favors Th17 responses. Thus, while the cytokine environment is important, the strength of T-cell stimulation is another vital factor that determines the ability of T cells to become IL-17 producers.
Methods
Isolation of CD4+ T cells
Human samples were obtained with informed consent in accordance with the Declaration of Helsinki and after approval by the Newcastle and North Tyneside Research Ethics Committee 2. CD4+ T cells were isolated from fresh blood or buffy coats using the RosetteSep human CD4+ T-cell enrichment kit (StemCell Technologies). CD4 enrichment was routinely > 90%, as determined by flow cytometry.
Activation of T cells by CD3/CD28 beads
CD4+ T cells were cultured in Iscove's modified Dulbecco medium (IMDM; Sigma-Aldrich), containing 10% (vol/vol) Serum Replacement (Invitrogen) and supplemented with 2mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. 1 × 106 CD4+ T cells were activated with CD3/CD28 T-cell expander Dynabeads (Invitrogen) at either a 1:1 bead:T cell ratio (THi; recommended by the manufacturer) or a 1:50 bead:T cell ratio (TLo) in the presence of the following pro-Th17 cytokine cocktail: IL-1β (10 ng/mL; Peprotech), IL-23 (10 ng/mL; R&D Systems), and TGF-β (10 ng/mL; Peprotech). Alternatively, T cells were activated with beads coated with different concentrations of anti-CD3 (40-2.5 μg/mL) ± anti-CD28 (10 μg/mL; Miltenyi Biotec) in the presence of the pro-Th17 cytokine cocktail. Cells were cultured for 6 days at 37°C, in 5% CO2, and IL-23 was refreshed on day 3 with the addition of 10 U/mL IL-2 (Proleukin, Novartis). Intracellular cytokine staining and enzyme-linked immunosorbent assay (ELISA) were performed as described “ELISA.” For selected cultures, the ALK5 inhibitor, SB505124 (Sigma-Aldrich), was added at different doses to THi/TLo cultures at days 0 and 3. To assess cytokine production from proliferating cells, CD4+ T cells were labeled with 0.5μM carboxyfluorescein succinimidyl ester (CFSE) at 37°C for 5 minutes, followed by serum quenching and washing before differentiation into Th17 cells.
Activation of T cells by dendritic cells
Peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood or buffy coats by density centrifugation on Lymphoprep (Axis-Shield Diagnostics). CD14+ monocytes were isolated by positive magnetic selection from PBMCs using anti-CD14 magnetic microbeads (Miltenyi). Monocytes were cultured at 0.5 × 106 cells/mL in RPMI 1640 (Sigma-Aldrich), supplemented with 10% fetal bovine serum (FBS; Gibco), 2mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin in the presence of IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF; 50 ng/mL each; Immunotools) for 6 days to generate monocyte-derived dendritic cells (DCs). Medium supplemented with cytokines was refreshed at day 3. In some experiments, DCs were treated with Staphylococcus aureus peptidoglycan (PGN; 10 μg/mL; InvivoGen) for 24 hours. 1 × 106 CD4+ T cells were cultured with 1 × 106 (1:1 DC/T cell ratio) or 2 × 104 (1:50 DC/T cell ratio) allogeneic PGN-treated DCs or with 1 × 105 autologous DCs pulsed with various concentrations of S. aureus enterotoxin B (SEB; 1000-0.1 pg/mL) in IMDM with 10% Serum Replacement for 6 days. Intracellular cytokine staining and ELISA was performed as described in “ELISA.”
Mouse Th17 cell generation
Male DBA/1 mice were purchased from Harlan and were kept in individually ventilated cages with water and food provided ad libitum. The work was performed under the terms of Animals (Scientific Procedures) Act of 1986 and was authorized by the Home Secretary, Home Office, United Kingdom. CD4+ T cells were isolated from the spleen using anti-CD4 microbeads according to the manufacturer's instructions (Miltenyi). Aliquots of 1 × 106 CD4+ T cells were stimulated for 4 days with anti-CD3/anti-CD28 expander beads (Dynal) at either a 1:1 or 1:50 bead/T cell ratio in the presence of TGF-β (10 ng/mL; R&D Systems), IL-6 (50 ng/mL; Peprotech), IL-23 (10 ng/mL; Peprotech), and 10 U/mL IL-2 (Hofmann La-Roche).
Intracellular flow cytometry
Human T cells were stimulated with phorbol myristate acetate (PMA; 10 ng/mL; Sigma-Aldrich) and ionomycin (1 μg/mL; Sigma-Aldrich), and, after 1 hour, Brefeldin A (10 μg/mL) was added for an additional 4 hours. Cells were harvested and fixed and permeabilized using appropriate buffers (eBioscience). To reduce background staining, the cells were blocked with 200 μg/mL mouse goat immunoglobulin (Ig; Sigma-Aldrich) for 15 minutes before the addition of antibodies. The following antibodies were used: interferon-γ (IFN-γ)–fluorescein isothiocyanate (FITC) (4S. B3; eBioscience) or IFN-γ-phycoerythrin (PE) (25 723.11; BD) or IFN-γ–Alexa Fluor 700 (B27; BD Pharmingen); IL-17–Alexa Fluor 647 (eBio64DEC17; eBioscience); latency-associated peptide (LAP)–PE (27 232; R&D Systems), Foxp3–Pacific blue (206 D; BioLegend), and RORγt-PE (AFKJS-9; eBioscience). Cells were incubated at 4°C for 30 minutes, then washed and resuspended in fluorescence-activated cell sorting (FACS) buffer. Data were collected using either a FACScan or FACSCanto II (Becton Dickinson) and analyzed using FlowJo Version 8.7.1 (TreeStar). For intracellular cytokine staining of mouse T cells, cells were stimulated with PMA (50 ng/mL) and ionomycin (500 ng/mL) for 5 hours. After 1 hour Brefeldin A (10 μg/mL; Sigma-Aldrich) was added. Cell-surface staining was performed with anti–CD4-PerCP-Cy5.5. Cells were fixed and permeabilized as described at the beginning of the paragraph. After fixation and permeabilization, cells were stained with anti–IFN-γ–FITC (XMG1.2) and anti–IL-17–PE (TC11-1H10.1; all from BD Pharmingen). After staining, the cells were analyzed using a FACScan or an LSR-II (Becton Dickinson).
ELISA
Cells were washed and replated at 1 × 106 cells/mL in IMDM with 10% (vol/vol) Serum Replacement and after 1 hour restimulated with PMA (10 ng/mL) and ionomycin (1 μg/mL) for 24 hours. Cytokine concentrations were assayed for IL-17 and IFN-γ by specific sandwich ELISA (IL-17, eBioscience; IFN-γ, BD PharMingen).
IPEX patient sample
Peripheral blood from a 4-month-old infant with IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome was gifted for research once diagnostic tests had been completed with informed parental consent. The patient was free of immunosuppressive treatments and had a mutation present in exon 10 (Iso346Thr) of FOXP3.
Western blotting
T cells were cultured under THi and TLo conditions for 6 days and were left untreated or were stimulated with PMA and ionomycin for 2 hours. Nuclear and cytoplasmic extracts were prepared with the NE-PER kit, according to the manufacturer's instructions (Thermo Scientific). Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride membranes, and blocked in 5% dried milk and 0.05% Tween in Tris-buffered salt solution. Membranes were probed with anti-NFATc1 monoclonal antibody (mAb; clone 7A6; Santa Cruz Biotechnology), followed by horseradish peroxidase (HRP)–conjugated anti–mouse antibody (Cell Signaling). Proteins were visualized using the Imobilon Western detection system (Millipore).
ChIP assay
Chromatin immunoprecipitation (ChIP) assay was carried out as previously described21 using crosslinked chromatin prepared from 6-day cultures of THi or TLo cells that were left untreated or were restimulated with PMA/ionomycin for 2 hours. Antibodies used for immunoprecipitation were anti-NFAT1c (clone 7A6; Santa Cruz Biotechnology) or isotype-matched IgG control (Abcam). Briefly, 5 μg of irrelevant or anti-NFAT1c antibody were incubated overnight with 20 μg chromatin. The complexes were precipitated with Protein G–sepharose beads for 2 hours, then washed sequentially in low-salt buffer (0.1% SDS, 1% Triton X-100, 2mM EDTA [ethylenediaminetetraacetic acid], 20mM Tris-HCl, pH 8.1, 150mM NaCl]), high-salt buffer (0.1% SDS, 1% Triton X-100, 2mM EDTA, 20mM Tris-HCl, pH 8.1, 500mM NaCl), and lithium chloride buffer (0.25 M LiCl, 1% Nonidet P-40, 1% deoxycholate, 1mM EDTA, 10mM Tris-HCl, pH 8.1). Beads were then washed twice with Tris-EDTA (TE) buffer and eluted with 500 mL of elution buffer (1% SDS, 0.1 M NaHCO3). The cross-links were reversed, and DNA was obtained by phenol-chloroform extraction and ethanol purification. Polymerase chain reaction (PCR) amplification of the human IL17A proximal NFAT1c binding site was carried out using specific oligonucleotide primers 5′-gcagctctgctcagcttctaa-3′ and 5′-ttcaggggtgacaccatttt-3′. All reactions were normalized to the isotype control and relative level of transcriptional difference calculated using the following equation: [1/(2A)] × 100.
Statistical analysis
Statistics were performed using Prism 4.0 (GraphPad Software).
Results
Low-strength T-cell stimulation favors Th17 responses
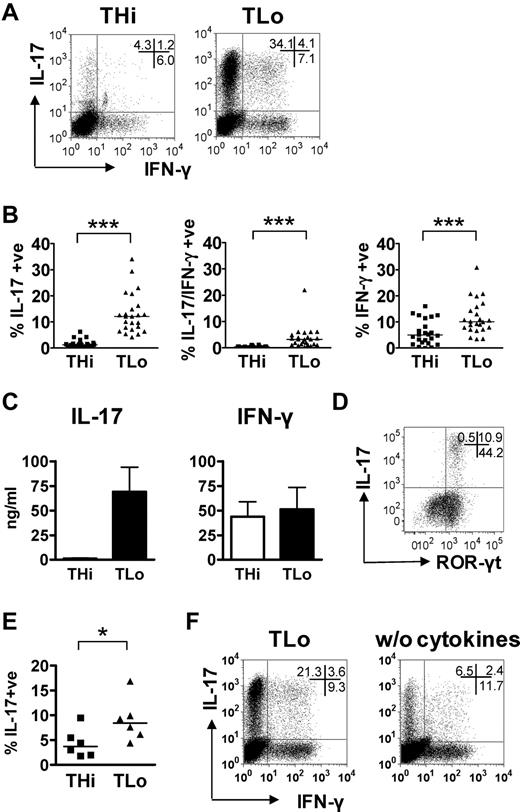
To assess whether the strength of stimulation affects T-helper cell differentiation toward a Th17 phenotype, human CD4+ T cells were stimulated with anti-CD3/anti-CD28– coated beads at either a 1:1 (THi—recommended ratio by the manufacturer) or 1:50 (TLo) bead/T cell ratio in the presence of Th17-inducing cytokines IL-1β, TGF-β, and IL-23. After 6 days of culture, the Th17 and Th1 signature cytokines, IL-17 and IFN-γ, respectively, were measured upon restimulation with PMA/ionomycin. Compared with THi stimulation, TLo stimulation resulted in a substantial increase in both the percentage of IL-17–producing T cells and the amount of IL-17 secreted (Figure 1A-C). A significant, yet less marked, increase in the percentage of IFN-γ–producing T cells, and IL-17/IFN-γ dual-secreting cells, was also observed; however, only a small difference in secreted IFN-γ was detected (Figure 1A-C). Intermediate bead:T cell ratios (1:10 and 1:25) resulted in intermediate Th17 generation (ie, higher than THi, but lower than achieved with TLo stimulation; data not shown). Furthermore, PMA/ionomycin stimulation of cells that had not received a prior CD3/CD28 signal in vitro only yielded approximately 0.5% IL-17 producers (data not shown). The majority of the IL-17–producing TLo cells coexpressed the Th17-associated transcription factor, ROR-γt (Figure 1D), indicating these T cells are Th17 cells. TLo stimulation also led to a higher proportion of IL-17 producers in murine CD4+ T cells than THi stimulation (Figure 1E). Furthermore, optimal generation of human Th17 cells required the presence of pro-Th17 cytokines (Figure 1F).
Low-strength T-cell stimulation favors Th17 responses. (A-D) Human CD4+ T cells were cultured under THi or TLo conditions for 6 days. (A-B) THi and TLo cells were restimulated with PMA/ionomycin for 5 hours, and expression of IL-17 and IFN-γ were determined by intracellular flow cytometry. Plots of 1 example of 24 independent experiments are shown (A). (B) Percentages of IL-17 and IFN-γ single and double producers; data of 24 independent experiments are shown. (C) THi and TLo cells were restimulated with PMA/ionomycin for 24 hours, and cytokines were measured by ELISA (n = 3). (D) Expression of IL-17 and ROR-γt in TLo cells was determined by intracellular flow cytometry. One representative of 3 independent experiments is shown. (E) Mouse CD4+ T cells were cultured under THi or TLo conditions, and expression of IL-17 and IFN-γ were determined by intracellular flow cytometry (n = 6). (F) Human CD4+ T-cells were cultured under TLo conditions in the presence (“TLo”) or absence (“w/o cytokines”) of IL-1β, IL-23, and TGF-β for 6 days. Expression of IL-17 and IFN-γ were determined by intracellular flow cytometry. Results are representative of 3 independent experiments. Horizontal bars represent the median value; error bars represent SEM. P values were calculated using the Wilcoxon test: *P < .05; ***P < .0001.
Low-strength T-cell stimulation favors Th17 responses. (A-D) Human CD4+ T cells were cultured under THi or TLo conditions for 6 days. (A-B) THi and TLo cells were restimulated with PMA/ionomycin for 5 hours, and expression of IL-17 and IFN-γ were determined by intracellular flow cytometry. Plots of 1 example of 24 independent experiments are shown (A). (B) Percentages of IL-17 and IFN-γ single and double producers; data of 24 independent experiments are shown. (C) THi and TLo cells were restimulated with PMA/ionomycin for 24 hours, and cytokines were measured by ELISA (n = 3). (D) Expression of IL-17 and ROR-γt in TLo cells was determined by intracellular flow cytometry. One representative of 3 independent experiments is shown. (E) Mouse CD4+ T cells were cultured under THi or TLo conditions, and expression of IL-17 and IFN-γ were determined by intracellular flow cytometry (n = 6). (F) Human CD4+ T-cells were cultured under TLo conditions in the presence (“TLo”) or absence (“w/o cytokines”) of IL-1β, IL-23, and TGF-β for 6 days. Expression of IL-17 and IFN-γ were determined by intracellular flow cytometry. Results are representative of 3 independent experiments. Horizontal bars represent the median value; error bars represent SEM. P values were calculated using the Wilcoxon test: *P < .05; ***P < .0001.
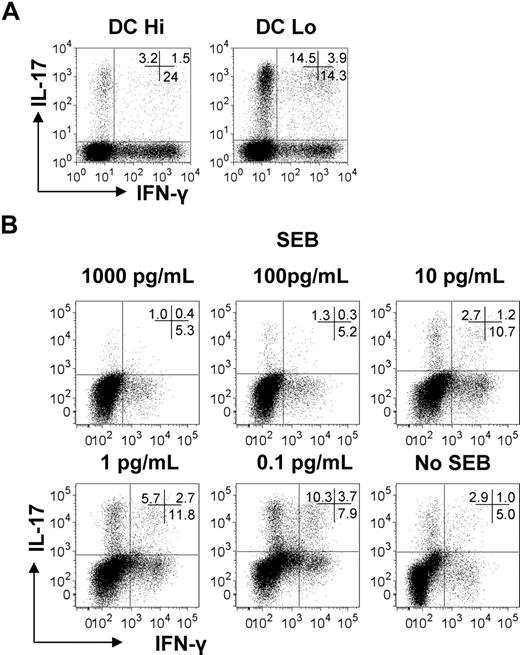
To test whether the strength of T-cell activation also influences Th17 generation, if stimulatory signals are delivered by APC instead of beads, CD4+ T cells were cocultured with either allogeneic DCs at different DC:T cell ratios (Figure 2A) or with autologous DCs that had been pulsed with various concentrations of the superantigen, SEB (Figure 2B). Because pro-Th17 cytokines are required for optimal Th17 responses, DCs were activated with the TLR2 agonist, peptidoglycan, to induce the release of pro-Th17-inducing cytokines22,23 (Figure 2A), or the pro-Th17 cytokine cocktail was added to the DC–T-cell cocultures (Figure 2B). Similarly to TLo stimulation with beads, low-strength stimulation by lowering DC numbers or by lowering SEB concentrations also promoted a substantial increase in the percentage of IL-17–producing T cells (Figure 2A-B).
Low-strength T-cell stimulation by DCs favors Th17 responses. (A) Human CD4+ T cells were cultured for 6 days with allogeneic peptidoglycan-activated DCs at either a 1 DC:1 CD4+ T cell ratio (DC Hi) or 1 DC:50 CD4+ T cell ratio (DC Lo). Expression of IL-17 and IFN-γ was determined by intracellular flow cytometry. Plots are representative of 3 independent experiments. (B) Human CD4+ T cells were cultured with autologous DCs at a 1:10 DC:CD4+ T cell ratio with decreasing concentrations of SEB (1000-0 pg/mL) for 6 days. Expression of IL-17 and IFN-γ was determined by intracellular flow cytometry. Results are representative of 3 independent experiments.
Low-strength T-cell stimulation by DCs favors Th17 responses. (A) Human CD4+ T cells were cultured for 6 days with allogeneic peptidoglycan-activated DCs at either a 1 DC:1 CD4+ T cell ratio (DC Hi) or 1 DC:50 CD4+ T cell ratio (DC Lo). Expression of IL-17 and IFN-γ was determined by intracellular flow cytometry. Plots are representative of 3 independent experiments. (B) Human CD4+ T cells were cultured with autologous DCs at a 1:10 DC:CD4+ T cell ratio with decreasing concentrations of SEB (1000-0 pg/mL) for 6 days. Expression of IL-17 and IFN-γ was determined by intracellular flow cytometry. Results are representative of 3 independent experiments.
Together, these data demonstrate that, compared with high-potency activation, low-strength T-cell stimulation leads to selective enrichment of IL-17–producing CD4+ T cells in a pro-Th17 milieu.
TLo stimulation induces higher numbers of proliferating Th17 cells than THi stimulation
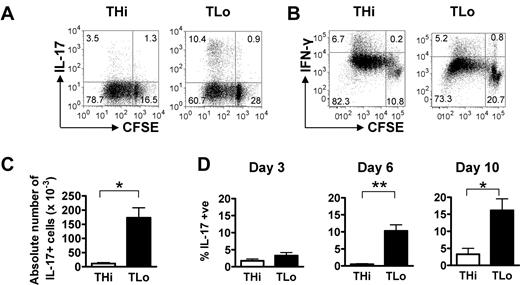
Because it was anticipated that THi cells proliferated more vigorously than TLo cells, we examined the possibility that the TLo-induced Th17-cell enrichment was proportional, rather than absolute. First, we determined the proliferation of CFSE-labeled THi and TLo cells by measuring the dilution of CFSE after 6 days of culture and assessed whether Th17 cells resided in the proliferating or nonproliferating fraction. The majority of both THi and TLo cells were CFSEdim, indicative of proliferation (Figure 3A), although a higher percentage of TLo than THi cells had not undergone division (33% ± 3.7% for TLo vs 14% ± 3.4% for THi; n = 3). Furthermore, IL-17–producing cells in both the THi and TLo cultures were mostly observed in the CFSEdim fraction, indicating that T-cell division does not counteract IL-17 production (Figure 3A). IFN-γ–producing T cells also resided mostly in the proliferating fraction (Figure 3B). Second, we counted the total number of living cells in 6-day cultures to calculate the absolute number of Th17 cells. As shown in Figure 3C, TLo cultures contained a significantly higher total number of Th17 cells than THi cultures. Thus, low-strength stimulation of primary CD4+ T cells leads to an enrichment of Th17 cells by enhancing both the relative proportion and total number of Th17 cells.
TLo stimulation induces higher numbers of proliferating Th17 cells than THi stimulation. (A-B) Human CD4+ T cells were labeled with CFSE and cultured under THi or TLo conditions for 6 days. Proliferating IL-17+ (A) and IFN-γ+ (B) cells were assessed by intracellular flow cytometry. Plots are representative of 3 independent experiments. (C) The number of alive cells on day 6 was determined by cell counting using trypan blue, and the absolute number of IL-17+ cells was then calculated using the proportion of IL-17+ cells after intracellular flow cytometry (n = 3). Error bars represent SEM. P values were calculated using the Student t test: *P < .05. (D) Human CD4+ T cells were cultured under THi or TLo conditions for 3, 6, or 10 days. To generate day 10 cells, beads were removed at day 6 and cells were washed, replated, and cultured for 4 more days. Expression of IL-17 was determined by intracellular flow cytometry. Day 3 and 10 graphs: n = 3; day 6 graphs: n = 4. Error bars represent SEM. P values were calculated using the Mann-Whitney U test: *P < .05
TLo stimulation induces higher numbers of proliferating Th17 cells than THi stimulation. (A-B) Human CD4+ T cells were labeled with CFSE and cultured under THi or TLo conditions for 6 days. Proliferating IL-17+ (A) and IFN-γ+ (B) cells were assessed by intracellular flow cytometry. Plots are representative of 3 independent experiments. (C) The number of alive cells on day 6 was determined by cell counting using trypan blue, and the absolute number of IL-17+ cells was then calculated using the proportion of IL-17+ cells after intracellular flow cytometry (n = 3). Error bars represent SEM. P values were calculated using the Student t test: *P < .05. (D) Human CD4+ T cells were cultured under THi or TLo conditions for 3, 6, or 10 days. To generate day 10 cells, beads were removed at day 6 and cells were washed, replated, and cultured for 4 more days. Expression of IL-17 was determined by intracellular flow cytometry. Day 3 and 10 graphs: n = 3; day 6 graphs: n = 4. Error bars represent SEM. P values were calculated using the Mann-Whitney U test: *P < .05
Because THi cells proliferated more vigorously than TLo cells, we considered the possibility that THi cells were “exhausted” at the day 6 time point, when cytokine production was measured. We therefore performed kinetics experiments, measuring cytokine production by THi and TLo cells on days 3, 6, and 10 after activation with CD3/CD28 beads (Figure 3D). However, the proportion of Th17 cells in THi cultures remained low at all time points tested, even after the removal of beads and resting of T cells (day 10). TLo cultures contained significantly higher proportions of Th17 cells than THi cultures at days 6 and 10 (Figure 3D).
Together, these data indicate that low-strength stimulation of primary CD4+ T cells leads to an enrichment of Th17 cells by enhancing both the relative proportion and total number of Th17 cells, and that low IL-17 production by THi cells is not caused by exhaustion.
Low signal strength through the TCR/CD3 complex favors Th17 cells only in the presence of anti-CD28
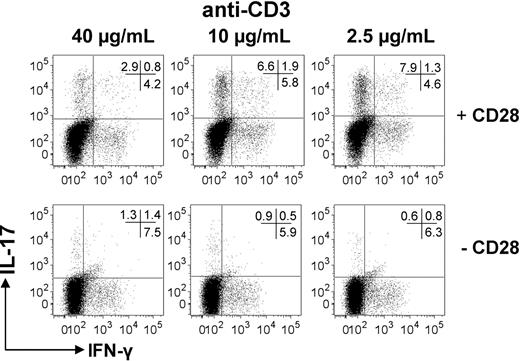
We next addressed the question of whether lowering the signal strength through the TCR/CD3 complex alone was sufficient for optimal Th17-cell generation. We therefore coated beads with different concentrations of anti-CD3 mAb, allowing us to keep the T cell:bead ratio constant in all culture conditions while providing different CD3 signal strengths. In addition, as it was recently shown that anti-CD28 inhibits Th17 responses in murine T cells,20 we performed these experiments in the absence or presence of anti-CD28. As shown in Figure 4, lower concentrations of anti-CD3 mAb selectively promoted Th17 cells, but only in the presence of anti-CD28 signaling.
Low signal strength through the TCR/CD3 complex favors Th17 cells only in the presence of anti-CD28. Human CD4+ T cells were cultured for 6 days at a 1:10 bead:CD4+ T cell ratio. Beads were loaded with decreasing concentrations (40-2.5 μg/mL) of anti-CD3 ± 10 μg/mL anti-CD28. Cells were restimulated with PMA/ionomycin for 5 hours, and expression of IL-17 and IFN-γ was determined by intracellular flow cytometry. Results are representative of 3 independent experiments.
Low signal strength through the TCR/CD3 complex favors Th17 cells only in the presence of anti-CD28. Human CD4+ T cells were cultured for 6 days at a 1:10 bead:CD4+ T cell ratio. Beads were loaded with decreasing concentrations (40-2.5 μg/mL) of anti-CD3 ± 10 μg/mL anti-CD28. Cells were restimulated with PMA/ionomycin for 5 hours, and expression of IL-17 and IFN-γ was determined by intracellular flow cytometry. Results are representative of 3 independent experiments.
TGF-β is required for high IL-17 production by TLo cells and membrane-bound TGF-β expression by THi cells does not inhibit Th17 responses
The reason why high-strength T-cell stimulation did not promote Th17 cells was investigated. The possibility that THi stimulation induced the secretion of factors inhibiting Th17 responses in an autocrine or paracrine fashion was excluded because (1) neutralization of IL-10 or IFN-γ in THi cultures did not restore IL-17 production and (2) addition of rIL-10, rIFN-γ, or conditioned medium from THi cells to TLo cultures did not hinder Th17 responses (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article; and data not shown).
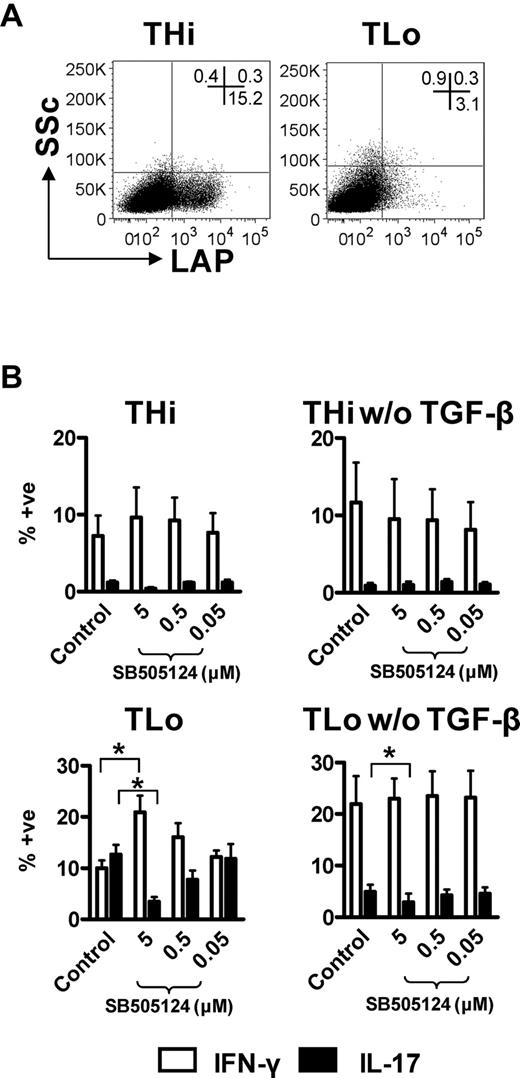
TGF-β plays a central role in Th17 and Treg differentiation. Low TGF-β concentrations favor Th17 differentiation, while high concentrations inhibit Th17 responses and induce Treg differentiation.6 We therefore investigated the possibility that THi cells produced high amounts of membrane-bound TGF-β, inhibiting Th17 differentiation. First, we determined the expression of the TGF-β–binding protein, LAP, by THi and TLo cells, indicative of membrane-bound TGF-β expression. Interestingly, THi cells expressed enhanced levels of LAP, compared with TLo cells (Figure 5A), indicating an increased presence of (latent) TGF-β within THi cultures. Second, to assess the contribution of TGF-β to promoting Th17 responses in our system, TLo cells were cultured in the presence or absence of TGF-β. As shown in Figure 5B (bottom panels), exogenous TGF-β enhanced the percentage of IL-17+ cells by over 2-fold while reducing the proportion of IFN-γ producers, confirming a pro-Th17 effect of this cytokine under TLo conditions. Titration of TGF-β (500-0.01 ng/mL) was also performed to determine the optimal TGF-β concentration in our system (10 ng/mL; data not shown). However, exogenous TGF-β did not have a similar pro-Th17 effect under THi conditions (Figure 5B top panels). To examine whether high endogenous TGF-β in THi cultures inhibited Th17 responses, TGF-β signaling was inhibited using the small-molecule inhibitor, SB505124, blocking ALK5 receptor signaling. The activity of this inhibitor was confirmed in TLo cells; Th17 generation was suppressed by culturing TLo cells in the presence of SB505124 (Figure 5B bottom left panel). Because TGF-β can either inhibit or support Th17 cells, depending on its concentration, SB505124 was titrated, aiming to block a proportion of signaling from endogenously produced TGF-β in THi cultures. However, none of the doses of SB505124 enhanced the percentage of Th17 cells in THi cultures to the percentage observed under TLo conditions (Figure 5B). Thus, high expression of membrane-bound TGF-β by THi cells does not appear to be responsible for low IL-17 production.
TGF-β is required for high IL-17 production by TLo cells, and high latent TGF-β/LAP expression by THi cells does not inhibit Th17 responses. (A) Human CD4+ T cells were cultured under THi or TLo conditions for 6 days, and LAP expression was determined by flow cytometry. Plots are representative of 3 independent experiments. (B) Human CD4+ T cells were cultured under THi or TLo conditions with or without TGF-β in decreasing concentrations of the ALK5 inhibitor, SB505124 (5-0.05μM). Expression of IL-17 and IFN-γ were determined by flow intracellular cytometry on day 6 (n = 3). Error bars represent SEM. P values were calculated using one-way analysis of variance with Tukey correction: *P < .05.
TGF-β is required for high IL-17 production by TLo cells, and high latent TGF-β/LAP expression by THi cells does not inhibit Th17 responses. (A) Human CD4+ T cells were cultured under THi or TLo conditions for 6 days, and LAP expression was determined by flow cytometry. Plots are representative of 3 independent experiments. (B) Human CD4+ T cells were cultured under THi or TLo conditions with or without TGF-β in decreasing concentrations of the ALK5 inhibitor, SB505124 (5-0.05μM). Expression of IL-17 and IFN-γ were determined by flow intracellular cytometry on day 6 (n = 3). Error bars represent SEM. P values were calculated using one-way analysis of variance with Tukey correction: *P < .05.
Inverse relationship between Foxp3 and Th17 in TLo, but not THi, conditions
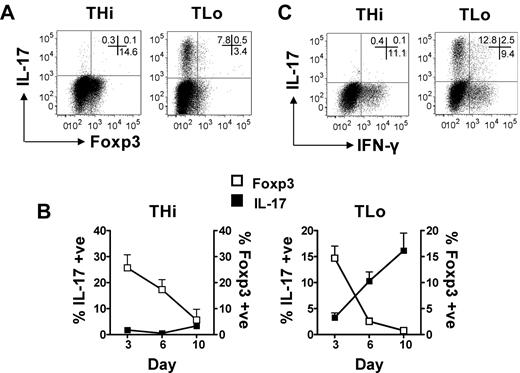
We next hypothesized that THi stimulation induced high levels of Foxp3, potentially resulting in inhibition of ROR-γt transcriptional activities and IL-17 production. We therefore measured Foxp3 expression and IL-17 production in THi and TLo cells after 6 days of culture and found that, indeed, THi cultures contained a higher proportion of Foxp3+ cells (Figure 6A). We also observed that Foxp3 expression and IL-17 production were mutually exclusive.
Inverse relationship between Foxp3 and Th17 in TLo, but not THi, conditions. (A) Human CD4+ T cells were cultured under THi or TLo conditions for 6 days. Expression of IL-17 and Foxp3 was determined by intracellular flow cytometry. Plots are representative of 4 independent experiments. (B) Human CD4+ T cells were cultured under THi or TLo conditions for 3, 6, or 10 days. To generate day 10 cells, beads were removed at day 6 and cells were washed, replated, and cultured for 4 more days. Expression of IL-17 and Foxp3 were determined by intracellular flow cytometry (n = 3). IL-17 data shown in Figure 3D were plotted in this graph. (C) IPEX patient PBMCs were cultured under THi or TLo conditions. Expression of IL-17 and IFN-γ were determined by intracellular flow cytometry.
Inverse relationship between Foxp3 and Th17 in TLo, but not THi, conditions. (A) Human CD4+ T cells were cultured under THi or TLo conditions for 6 days. Expression of IL-17 and Foxp3 was determined by intracellular flow cytometry. Plots are representative of 4 independent experiments. (B) Human CD4+ T cells were cultured under THi or TLo conditions for 3, 6, or 10 days. To generate day 10 cells, beads were removed at day 6 and cells were washed, replated, and cultured for 4 more days. Expression of IL-17 and Foxp3 were determined by intracellular flow cytometry (n = 3). IL-17 data shown in Figure 3D were plotted in this graph. (C) IPEX patient PBMCs were cultured under THi or TLo conditions. Expression of IL-17 and IFN-γ were determined by intracellular flow cytometry.
Activation-induced Foxp3 expression is known to occur in CD4+ T cells, but reduces after resting of T cells.24 We therefore assessed Foxp3 expression and IL-17 production at days 3 and 6 and after an additional 4 days of rest (ie, day 10). For both THi and TLo culture conditions, the highest proportion of Foxp3+ T cells was found at day 3, with levels declining at day 6 and decreasing even further at day 10 (Figure 6B). Interestingly, for TLo-cell populations, an inverse relationship between Foxp3 and IL-17 was observed, with percentages of Th17+ cells increasing over time, while the number of Foxp3+ cells declined. However, for THi cells, no such clear relationship was found; a similar decline in Foxp3+ cells between days 3 and 10 was not accompanied by a rise in IL-17+ cells (Figure 6B).
Nevertheless, the possibility remained that a high proportion of Foxp3+ cells during the first 6 days of THi stimulation inhibited the differentiation of Th17 cells. To address this possibility, we made use of cells from a patient with IPEX syndrome, who expressed a mutated form of Foxp3 causing defective Treg function. However, as depicted in Figure 6C, a similar trend was observed in IPEX cells (ie, low-strength T-cell stimulation favored Th17 differentiation).
NFATc1 binds to the IL-17 promoter in TLo, but not THi, cells
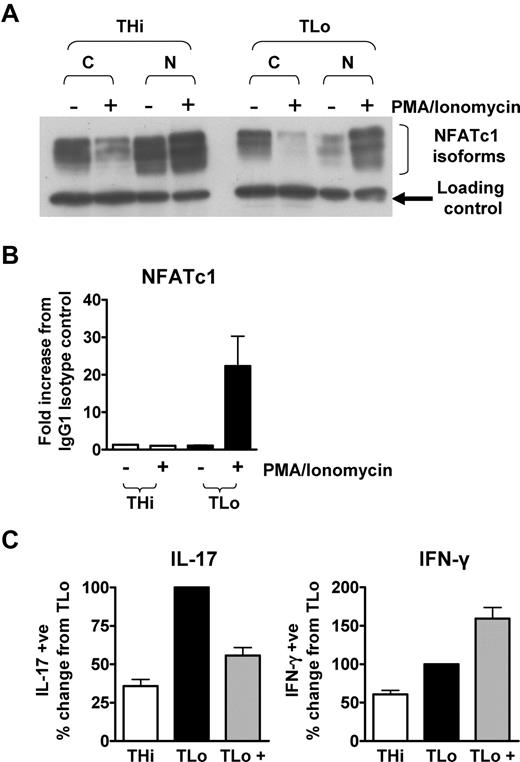
As it was previously shown that low potency of TCR triggering leads to preferential activation of NFATc125 and NFATc1 regulates IL-17 expression,7,8 we addressed the question of whether the low IL-17 production by THi cells was caused by a lack of NFATc1 translocation to the nucleus or a failure of NFATc1 to bind to the proximal region of the IL-17 promoter. Nuclear translocation of NFATc1 was observed in both THi and TLo cells that had been restimulated by PMA/ionomycin (Figure 7A). However, chromatin immunoprecipitation assays revealed that NFATc1 binding to the proximal region of the IL-17 promoter could only be observed in TLo, but not THi, cells, explaining the failure of THi cells to produce high levels of IL-17 (Figure 7B). As NFATc1 activity is regulated by the Ca2+/calcineurin pathway, we next investigated whether high Ca2+ signaling during T-cell activation inhibited Th17 responses. Indeed, addition of the Ca2+ ionophore ionomycin during initial activation under pro-Th17 conditions reduced the ability of TLo cells to produce IL-17, but not IFN-γ, upon restimulation (Figure 7C). Thus, these data suggest that low Ca2+ signaling promotes Th17 responses.
NFATc1 binds to the IL-17 promoter in TLo, but not THi, cells. Human CD4+ T cells were cultured under THi or TLo conditions for 6 days. Day 6 cells were either restimulated for 2 hours with PMA/ionomycin (+) or left untreated (-). (A) Cytoplasmic and nuclear lysates were prepared, and expression of NFATc1 was determined by Western blotting. Blots are representative of 3 independent experiments. (B) NFATc1 binding to the proximal IL-17 promoter was assessed by ChIP (n = 2). Error bars respresent SEM. (C) CD4+ T cells were cultured for 6 days under either THi or TLo conditions or TLo conditions +500nM ionomycin (TLo +) added daily for the first 4 days of culture. Expression of IL-17 was determined by intracellular flow cytometry (n = 3). Error bars represent SEM.
NFATc1 binds to the IL-17 promoter in TLo, but not THi, cells. Human CD4+ T cells were cultured under THi or TLo conditions for 6 days. Day 6 cells were either restimulated for 2 hours with PMA/ionomycin (+) or left untreated (-). (A) Cytoplasmic and nuclear lysates were prepared, and expression of NFATc1 was determined by Western blotting. Blots are representative of 3 independent experiments. (B) NFATc1 binding to the proximal IL-17 promoter was assessed by ChIP (n = 2). Error bars respresent SEM. (C) CD4+ T cells were cultured for 6 days under either THi or TLo conditions or TLo conditions +500nM ionomycin (TLo +) added daily for the first 4 days of culture. Expression of IL-17 was determined by intracellular flow cytometry (n = 3). Error bars represent SEM.
Discussion
Since the discovery of Th17 cells, the delineation of variables influencing their development has become a major topic. Here, we investigated the effect of strength of T-cell stimulation on the capability of human CD4+ T cells to produce IL-17 upon restimulation. Low-strength T-cell activation by CD3/CD28 beads or by APCs (DCs) in a pro-Th17 cytokine milieu profoundly promoted Th17 responses, characterized by the production of IL-17 and expression of RORγt. In contrast, even in the presence of Th17-polarizing cytokines, high-strength stimulation only poorly supported IL-17–producing T cells. Our data suggest that in addition to cytokine milieu, the strength of T-cell activation influences the skewing of CD4+ T-cell responses toward a Th17 phenotype.
The low IL-17 production by THi cells is, most likely, explained by a failure of THi cells to induce the binding of NFATc1 to the IL-17 promoter. NFATc1 has been reported to be a crucial transcription factor for regulating IL-17 promoter activity in response to TCR signaling.7,8 An increase of intracellular Ca2+ levels upon TCR engagement leads to the activation of calcineurin, which induces a translocation of NFAT family members to the nucleus.26 Although the nuclear translocation of NFATc1 occurred in both THi and TLo cells, the binding of this transcription factor to the proximal region of the IL-17 promoter could only be detected in TLo cells. NFAT signaling is complex: depending on the stimulus received, NFAT can interact with distinct transcription factors (eg, activator protein 1) or form NFAT dimers, resulting in the transcriptional activation of distinct sets of genes.26 Thus, it is possible that the inability of NFATc1 to bind to the IL-17 promoter in THi cells may be caused by the interaction of NFATc1 with other transcriptional partners, thereby altering the transcriptional program of NFATc1. Differences in the level of Ca2+ signaling under THi and TLo conditions may be responsible for the observed differences in NFATc1-binding activity to the IL-17 promoter in THi and TLo cells. Indeed, increasing the levels of intracellular Ca2+ under TLo conditions by a Ca2+ ionophore reversed the pro-Th17 effect of TLo stimulation, suggesting that low-level Ca2+ signaling benefits Th17 responses.
The overall strength of T-cell stimulation is determined by various factors that control the rate of TCR triggering (eg, antigen affinity and density) or the degree of signal amplification and prolongation (eg, costimulation through CD28 and duration of DC–T-cell interaction, respectively27 ; and references therein). Stimulation strength plays an important role in determining T-cell fate in terms of cell survival and functional development.27-32 For example, differentiation of CD4+ T cells into Th1 or Th2 effector cell lineages is highly influenced by signal strength. By varying either antigen dose, costimulatory signals, or affinity of antigenic peptide/MHC complexes for the TCR, it has been shown that, in general, low-strength stimulation favors Th2 differentiation, whereas high-strength stimulation promotes Th1 responses.17 In our study, the strength of stimulation was varied by either altering the number of stimulatory beads or DCs or, alternatively, by varying the amount of anti-CD3 or antigen per bead/DC, respectively. Similar results were found in all these different models (ie, low-strength activation favored the generation of Th17 cells). The anti-CD3 and antigen titration experiments suggest that low-potency activation through the TCR/CD3 complex is important for the promotion of Th17 cells, and it would therefore be interesting to investigate, in future studies, whether low-affinity TCR peptides similarly promote Th17 responses.
It has been suggested that T cell–polarizing cytokines can largely over-rule any effects of signal strength on Th1/Th2 differentiation.33 In contrast, we found that a pro-Th17 cytokine milieu did not counteract the negative influence of high stimulation strength on Th17 responses, but was absolutely required for optimal Th17 generation. Thus, low-strength stimulation through CD3/CD28 beads or DCs only promoted Th17 cells, if appropriate cytokines (eg, IL-1β, TGF-β, and IL-23) were added to the cultures (Figures 1,5) or if DCs were preactivated with peptidoglycan (data not shown), a TLR2 ligand that leads to the production of pro-Th17 cytokines IL-23 and IL-1.22,23 It could be argued that the necessity for the appropriate cytokine milieu in promoting IL-17–producing T cells under low-stimulation-strength conditions safeguards against the development of unwanted pathogenic Th17 cells in case autoreactive T cells with low functional avidity or affinity become activated in the absence of polarizing danger signals.
TGF-β plays a critical role in the development of both Treg and Th17 cells; TGF-β alone induces Foxp3 expression and differentiation of Treg, whereas TGF-β in combination with IL-1β or IL-21 drives the development of human Th17 cells.34-37 However, high levels of TGF-β can inhibit Th17 responses even when other pro-Th17 cytokines are present.36 Because endogenous TGF-β levels in bovine sera have been found to suppress Th17 development,36 we set up our experimental model with a TGF-β–free serum-replacement reagent, and found that the addition of 10 ng/mL TGF-β optimally supported Th17 generation. This level of TGF-β is within the range of concentrations reported to be required for the induction of human Th17 cells.37 Furthermore, blocking of TGF-β–mediated signaling under TLo conditions reduced IL-17, but enhanced IFN-γ production, confirming the critical role of TGF-β in the generation of human Th17-cells.
Although TGF-β is required for Th17 responses, high levels of TGF-β can suppress Th17 generation.6 The possibility that high expression of membrane-bound latent TGF-β-LAP on THi cells suppressed their skewing toward a Th17 phenotype was excluded by the partial inhibition of TGF-β–mediated signaling. THi cells were also characterized by high levels of Foxp3. Activation-induced Foxp3 is a well-known phenomenon described for in vitro activated human T cells.38,39 We used the Foxp3 antibody clone, 206D, which does not result in nonspecific binding upon T-cell activation.40 Foxp3 has been shown to repress IL-17 transcription through binding to ROR-γt,6 and we observed an inverse relationship between Foxp3 expression and IL-17 production in TLo cultures. However, high Foxp3 expression in THi cells did not appear to be responsible for impaired IL-17 production, as reducing FoxP3 levels by cell resting did not enhance the proportion of IL-17 producers. Furthermore, THi stimulation of cells from an IPEX patient with defective Foxp3 function also failed to support optimal generation of Th17 cells. Together, these data suggest that activation-induced Foxp3 in human T cells does not inhibit Th17 responses. Our data are in agreement with a recent report showing that activation-induced Foxp3 exerts no suppressive effects on the production of other cytokines by T cells (IFN-γ or IL-2).24
In apparent contrast to our data, 2 studies recently demonstrated that in the mouse, high-strength TCR signaling is required for IL-17 production and Th17 differentiation.8,20 However, conflicting data were presented in these 2 mouse studies with regard to the role of CD28 costimulation: Bouguermouh et al20 reported that CD28 signaling inhibits Th17 development, whereas Gomez-Rodriquez et al8 achieved optimal expression of IL-17 in the presence of CD28 costimulation. Whether variations in the type or concentration of CD3 or CD28 antibodies and/or differences in the pro-Th17 cocktails and/or mouse strain used contribute to the conflicting data in these studies is unclear, at present. Furthermore, in our system, CD28 costimulation was required, as anti-CD3 stimulation alone did not support optimal Th17 generation (Figure 4), a finding supported by another recent human Th17 study.16
Our current data do not distinguish between the possibilities that TLo stimulation primes naive T cells for Th17 development, or leads to the expansion of pre-existing Th17 cells, or enhances further differentiation of memory T-cells toward Th17. Preliminary experiments suggest that TLo stimulation promotes Th17 responses, preferentially, in the memory T-cell pool (data not shown). Whether the pro-Th17 cocktail used in our study does not support Th17 differentiation from human naive T cells, or whether the strength of T-cell stimulation is not an important factor for naive T-cell development toward Th17, requires further investigation. Given that frequencies of Th17 cells in the memory T-cell pool directly measured ex vivo are less than 0.5% (15 and our own unpublished data), we think it is unlikely that the promotion of Th17 responses by TLo stimulation is exclusively due to the expansion of pre-existing Th17 cells. As it has recently emerged that CD4+ T-cell effector lineage commitment is not as stable as previously thought and differentiated memory T cells retain a certain degree of plasticity,41,42 we favor the scenario that TLo stimulation induces further differentiation of memory T cells toward the Th17 phenotype.
In conclusion, this report underlines the importance of stimulation strength for human Th17 responses. The physiologic relevance of low-potency T-cell stimulation in the promotion of Th17 cells is not entirely clear at present, but our data may explain why a variety of autoimmune diseases are characterized by Th17 cells, considering that many autoreactive T cells escaping negative selection in the thymus have low-affinity TCRs. Nevertheless, we propose an effective model for the generation of human Th17 cells, by combining low-strength T-cell stimulation with the pro-Th17 cytokines, IL-1β, IL-23, and TGF-β. This model may provide a useful tool for studying future therapeutics targeting Th17/IL-17.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ian Dimmick and Rebecca Stewart for their help with flow cytometry and Martina Elias for her help with Western blotting.
This work was supported by Arthritis Research UK (grant 18262) and the Medical Research Council (grant G0601211).
Authorship
Contribution: H.A.P. designed experiments, acquired and analyzed data, and wrote the manuscript; J.N.S. acquired and analyzed data and contributed to the writing of the manuscript; J.M. designed experiments and acquired and analyzed data; S.W. and A.E.K. acquired data; S.H. provided IPEX patient samples and contributed to the writing of the manuscript; J.H.R. and J.D.I. contributed to the writing of the manuscript; A.E.A. designed experiments, analyzed data, and contributed to the writing of the manuscript; and C.M.U.H. conceptualized the study, designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Catharien Hilkens, Institute of Cellular Medicine, Musculoskeletal Research Group, Faculty of Medical Sciences, Newcastle University, Framlington Place, Newcastle-upon-Tyne, NE2 4HH, United Kingdom; e-mail: catharien.hilkens@ncl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal