Abstract
von Willebrand factor (VWF) is an important player in hemostasis but has also been suggested to promote inflammatory processes. Gene ablation of VWF causes a simultaneous defect in P-selectin expression making it difficult to identify VWF-specific functions. Therefore, we analyzed whether blocking antibodies against VWF would be able to interfere with neutrophil extravasation. We found that these antibodies inhibited neutrophil recruitment into thioglycollate-inflamed peritoneum and KC-stimulated cremaster by approximately 50%. Whereas platelet-VWF was not involved, the contribution of VWF to granulocyte recruitment was strictly dependent on the presence of platelets and the accessibility of their VWF-receptor glycoprotein Ib. Surprisingly, platelet P-selectin was largely dispensable for leukocyte extravasation, in agreement with our observation that anti-VWF antibodies did not affect leukocyte rolling and adhesion. Searching for possible effects downstream of leukocyte capture, we found that anti-VWF antibodies significantly inhibited thioglycollate-induced vascular permeability. The increase of permeability was independent of circulating granulocytes, showing that it was not a side effect of neutrophil diapedesis. Collectively, our results demonstrate that VWF-associated platelets strongly support neutrophil extravasation at a step downstream of leukocyte docking to the vessel wall. This step could be related to leukocyte diapedesis facilitated by destabilization of the endothelial barrier.
Introduction
Leukocyte interactions with the endothelium leading to extravasation into inflamed tissues have classically been assigned to the action of selectins, chemokine receptors, integrins,1 and various membrane proteins at endothelial cell contacts.2 In addition to these well-established mechanisms, platelets were reported to participate in leukocyte extravasation in various inflammation models.3–6 Whether or to what extent von Willebrand factor (VWF), a major ligand for platelets, is involved in this process is currently unknown.
VWF can be found in high quantities in Weibel-Palade bodies and α-granules of endothelial cells (ECs) and platelets, respectively. Upon activation, these cells release VWF and deposit it on the subendothelium of injured vessels where it serves as an anchoring substance for platelets by binding to platelet glycoprotein Ib (GPIb).7 Thus, VWF initiates the plug formation by platelets leading to resealing of injured vessels. The importance of VWF for hemostasis is impressively illustrated by von Willebrand disease, a strong bleeding disorder caused by functionally defective VWF.8 VWF is most active as ultra-large multimers but is efficiently controlled by a disintegrin-like and metalloproteinase with thrombospondin type I repeats 13 (ADAMTS13), which cleaves ultra-large VWF, thus down-regulating the hemostatic process.9
VWF does not only bind to subendothelial layers but can also be found on the apical surface of activated cultured ECs, where it forms ultra-large VWF multimers that support platelet binding under flow.10,11 As shown in vitro, endothelium-associated platelets can indeed support rolling and adhesion of polymorphonuclear leukocytes (PMNs).12 In vivo, stimuli such as histamine can trigger very rapidly platelet adhesion to endothelium of mesenteric venules, which is mediated by VWF on the endothelial surface.13 However, this interaction progressively decreases already 1 minute after stimulation.13 Most likely this is due to the rapid cleavage of VWF multimers by ADAMTS13.9,14 Indeed, VWF-dependent formation of platelet strings at the vessel wall can only be observed in vivo if ADAMTS13 is either inhibited15 or absent due to genetic ablation.16 Thus, it is unclear whether or to what extent VWF supports leukocyte extravasation when ADAMTS13 is active at physiological levels. Although VWF-deficient mice do display reduced leukocyte rolling and adhesion in vivo,17 these mice lack P-selectin storage and expression due to the loss of intact Weibel–Palade bodies.17 Consequently, it is unclear whether changes of leukocyte-EC interactions in these mice are due to VWF deficiency or the lack of endothelial P-selectin.
Here, we have directly addressed the question whether and to what extent VWF is relevant for the extravasation of leukocytes using anti-VWF antibodies in a murine model of peritonitis. We found that VWF strongly promotes the extravasation of leukocytes from blood vessels in a strictly platelet and GPIb dependent way. Surprisingly, blocking VWF had no effect on the rolling or adhesion of leukocytes. Instead, we found that VWF is required for a step downstream of the leukocyte capturing and adhesion mechanisms. This could be related to the diapedesis process because antibodies against VWF could partially block inflammation-induced vascular permeability, suggesting that VWF-recruited platelets destabilize endothelial barrier integrity.
Methods
Antibodies
The following antibodies were used: Rat anti-mouse monoclonal antibodies (mAbs): anti-Ly-6C/G RB6–8C5 (Gr-1), anti-CD45 (both from BD Biosciences), anti-P-selectin RB40.34,18 anti-L-selectin Mel-14 (no. HB-132; American Type Culture Collection), isotype-matched control mAbs (BD Biosciences), platelet-depleting anti-GPIbα mAb R300, nondepleting blocking anti-GPIbα Fab (p0p/B) and control Fab (Emfret Analytics)19 ; anti-platelet endothelial cell adhesion molecule (PECAM)-1 rat mAb labeled with Alexa 488 dye; anti-CD41/61 rat anti-mouse antibody; secondary antibodies: Alexa donkey anti-rabbit 568, fluorescein isothiocyanate-labeled goat anti-rabbit, Alexa donkey anti-rat 594 (Invitrogen); polyclonal rabbit antibody against human VWF (dialyzed against endotoxin-free phosphate-buffered saline [PBS]; DakoCytomation A0082) strongly cross-reacting with murine VWF (DakoCytomation and own observations); control polyclonal antibodies (pAbs): anti-endothelial cell-selective adhesion molecule (ESAM) pAb VE19, which binds to endothelium but does not affect leukocyte rolling, adhesion, and extravasation20 or rabbit nonimmune serum (immunoglobulin G [IgG] fraction).
Depletion of anti-VWF antibodies with recombinant purified VWF
pAbs against VWF purified from human plasma were depleted of VWF-reactivity by sequentially passing them over a column with 0.6 mg of purified recombinant human VWF (Baxter BioScience) immobilized on CnBr-sepharose beads (GE Healthcare). After 7 rounds of depletion, Western blotting showed that the antibody preparation had lost its reactivity toward recombinant VWF.
Immunofluorescence of mesenteric vessels
All animal experiments were approved by the Landesamt für Umwelt und Verbraucherschutz Nordrhein-Westfalen. Mice were intraperitoneally injected with 1 mL of 3% thioglycollate followed 1.5 hours later by intraveneous injection of 80 μg of labeled anti-PECAM antibody and 100 μg of nonlabeled anti-VWF antibody. After another 30 minutes, mice were anesthetized using intraperitoneal injection of ketamine hydrochloride (125 mg/kg, Sanofi Winthrop Pharmaceuticals), xylazine (12.5 mg/kg, Tranqui Ved, Phonix Scientific), and atropine sulfate (0.025 mg/kg, Fujisawa), and the circulatory system was perfused with PBS to remove unbound antibodies. Mesenteries were dissected and fixed with 4% paraformaldehyde for 2 hours at room temperature (RT). After permeabilization (0.5% Triton X-100 in PBS) for 2 hours at RT, samples were blocked (3% BSA in PBS) for 1 hour at RT and incubated overnight at RT with secondary antibody (donkey anti-rabbit 568). After washing and mounting (Dako mounting medium), samples were analyzed with a ZEISS LSM 510 confocal microscope.
Analysis of neutrophil recruitment and vascular permeability in peritonitis
Peritonitis assays were performed on C57BL/6 mice as described previously21 with intravenous injection of 50 μg complete antibodies or 80 μg F(ab′)2 fragments directly before and peritoneal lavage 2 hours after induction of inflammation. Importantly, injection of polyclonal VWF antibody did not cause significant changes of peripheral platelet and leukocyte counts. In some experiments, 40 μg of the Thromboxan receptor antagonist SQ 29548 were intravenously injected instead of Ab.
Permeability was analyzed as described22 with injection of Evans Blue directly after injection of the antibodies followed by intraperitoneal injection of thioglycollate. Peritoneal lavage (10 mL) was performed after 1 hour. In some experiments, mice received an intraperitoneal injection of 20 μg of Gr-1 mAb (or isotype-matched control from BD Biosciences) for depletion of granulocytes. Depletion was analyzed after 24 hours, just before starting the permeability assay, by hemocytometer analysis of peripheral blood samples stained with Kimura solution and by flow cytometry. Depletion was always ≥ 95%. Platelet depletion was performed and evaluated as described previously.21
Bone marrow transplantation
Mice were irradiated (950 rad) before they were injected intravenously with 3 × 106 bone marrow cells obtained either from strain and sex-matched wild-type (wt) mice, P-selectin−/− mice,23 or VWF−/− mice,17 respectively. The success of transplantation was confirmed by the lack of P-selectin expression on thrombin-stimulated platelets in flow cytometry. Lack of VWF expression in platelets was confirmed by staining of blood smears with polyclonal anti-VWF antibodies.
Intravital microscopy of leukocyte-endothelial interactions in the mesentery
C57BL/6 mice (6-8 weeks old) were anaesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). Peritonitis was induced as described above 2 hours prior to intravital microscopic observation. At the same time, 50 μg of anti-VWF antibodies or control anti-ESAM antibodies (diluted in 200 μL PBS) were injected intravenously. Mice were laparotomized, and a segment of the jejunum was exteriorized for intravital fluorescence microscopy.24 Leukocyte rolling and firm adhesion were analyzed in postcapillary mesenteric venules (inner diameter 17-40 μm) using 0.1 mL of 0.05% rhodamine-6G (Sigma-Aldrich) for in vivo leukocyte staining.25 In each animal, 7-15 single unbranched postcapillary venules were analyzed. Videotaped images were evaluated off-line using CAPIMAGE software (Zeintl). Rolling leukocytes were defined as those moving slower than the associated blood flow and were quantified for 30 seconds. The number of adherent leukocytes was determined for each venule segment (100 μm) and was expressed per 104 μm2 of venule surface area.
Intravital microscopy of leukocyte-endothelial interactions in the cremaster
Mice were anesthetized using intraperitoneal injection of ketamine hydrochloride (125 mg/kg, Sanofi Winthrop Pharmaceuticals), xylazine (12.5 mg/kg; Tranqui Ved, Phonix Scientific), and atropine sulfate (0.025 mg/kg; Fujisawa), and the cremaster muscle was prepared for intravital imaging as previously described.26,27 Intravital microscopy was performed on an upright microscope (Axioskop; Zeiss) with a 40× 0.75 numerical aperture saline immersion objective. In order to induce leukocyte recruitment into inflamed tissue, the cremaster muscle was superfused with keratinocyte-derived chemokine (KC) (chemokine [C-X-C motif] ligand 1; R&D Systems) at 5nM in superfusion buffer at 37°C. Neutrophil recruitment was monitored 1h after the preparation of the cremaster muscle. This approach has been shown to induce leukocyte emigration into inflamed tissue.28 Numbers of rolling and adherent leukocytes were determined by transillumination intravital microscopy, whereas leukocyte extravasation was investigated by reflected light oblique transillumination microscopy as previously described.29 Recorded images were analyzed off-line using ImageJ and AxioVision (Carl Zeiss) software. Emigrated cells were determined in an area stretching 75 μm toward each side of a vessel 100 μm long (representing 1.5 × 104 μm2 tissue area). The microcirculation was recorded using a digital camera (Sensicam QE; Cooke). Blood flow centerline velocity was measured using a dual photodiode sensor system (Circusoft Instrumentation). Centerline velocities were converted to mean blood flow velocities by multiplying with an empirical factor of 0.625.
Statistics
Data sets were analyzed for normality (Shapiro-Wilk) and equal variance. When possible, P values were determined by the Student t test. Otherwise, the Mann-Whitney rank sum test was applied. Analysis was done using SigmaPlot 10.0. Error bars indicate SEM.
Results
VWF mediates PMN recruitment into inflamed peritoneum
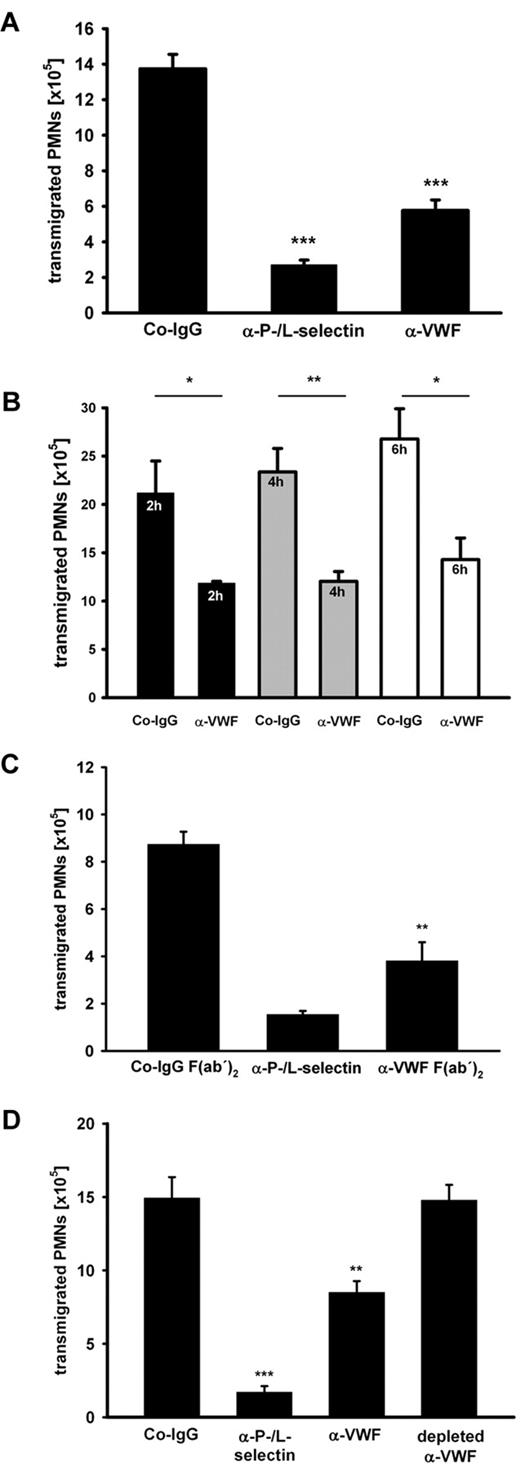
We studied the role of VWF in inflammation using function-blocking pAbs against purified human VWF in the well-established murine model of thioglycollate-induced peritonitis. To this end, the antibodies that are strongly reactive with murine VWF (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), were injected intravenously, inflammation was induced 15 minutes later, and PMNs recruited to the inflamed peritoneum over a period of 2 hours were removed and counted. As a positive inhibitory control, we used a cocktail of anti-P- and L-selectin antibodies that reduced PMN recruitment by 85% ± 4% (mean ± SD). Antibodies against VWF had an unexpectedly strong inhibitory effect on PMN extravasation of 54% ± 13% (average over 10 experiments). A representative experiment is shown in Figure 1A. Anti-VWF–mediated inhibition persisted also at later time points, as shown by comparing PMN recruitment at 2, 4, and 6 hours after thioglycollate stimulation (Figure 1B). Strong inhibition was also seen when F(ab)′2 fragments of the anti-VWF antibody were injected ruling out any Fc-dependent effects (Figure 1C). Because the commercially obtained polyclonal anti-VWF antibodies had been raised against VWF purified from human plasma, we wanted to exclude that any antibodies against possible minor impurities of the VWF preparations would be responsible for the detected in vivo effects. Therefore, we subjected the antibodies to serial rounds of incubation with immobilized recombinant VWF. This treatment successfully depleted all anti-VWF antibodies and as expected removed all inhibitory activity from the antibody preparation as tested in subsequent peritonitis assays (Figure 1D). Taken together, these data show that VWF is of major importance for neutrophil extravasation.
Antibodies against VWF inhibit PMN recruitment to the inflamed peritoneum. (A) Mice were treated with either control rabbit IgG (Co-IgG), anti-P- and L-selectin antibodies, or rabbit anti-VWF antibodies (5 mice per group). Upon antibody injection peritonitis was induced by i.p. application of thioglycollate. After 2 hours, PMNs were collected and counted. (B) Mice were injected with Co-IgG or anti-VWF antibodies (4 mice per group) as indicated. Peritonitis was induced as mentioned in panel A, and transmigrated PMNs were analyzed after 2 hours (black bars), 4 hours (gray bars), and 6 hours (white bars). (C) Mice were either treated with Co-IgG F(ab′)2, anti-P- and L-selectin antibody, or anti-VWF F(ab′)2 (3 mice per group). Peritonitis was induced and analyzed as indicated in panel A. (D) Peritonitis assays were performed as indicated in panel A with an additional group where the anti-VWF antibody was depleted of VWF-reactivity with immobilized recombinant VWF protein (4 mice per group). Error bars show SEM values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. Representative analyses from 10 experiments (A), 2 experiments (B-C), and 1 experiment (D) are shown.
Antibodies against VWF inhibit PMN recruitment to the inflamed peritoneum. (A) Mice were treated with either control rabbit IgG (Co-IgG), anti-P- and L-selectin antibodies, or rabbit anti-VWF antibodies (5 mice per group). Upon antibody injection peritonitis was induced by i.p. application of thioglycollate. After 2 hours, PMNs were collected and counted. (B) Mice were injected with Co-IgG or anti-VWF antibodies (4 mice per group) as indicated. Peritonitis was induced as mentioned in panel A, and transmigrated PMNs were analyzed after 2 hours (black bars), 4 hours (gray bars), and 6 hours (white bars). (C) Mice were either treated with Co-IgG F(ab′)2, anti-P- and L-selectin antibody, or anti-VWF F(ab′)2 (3 mice per group). Peritonitis was induced and analyzed as indicated in panel A. (D) Peritonitis assays were performed as indicated in panel A with an additional group where the anti-VWF antibody was depleted of VWF-reactivity with immobilized recombinant VWF protein (4 mice per group). Error bars show SEM values. *P ≤ .05; **P ≤ .01; ***P ≤ .001. Representative analyses from 10 experiments (A), 2 experiments (B-C), and 1 experiment (D) are shown.
VWF supports PMN extravasation via platelets
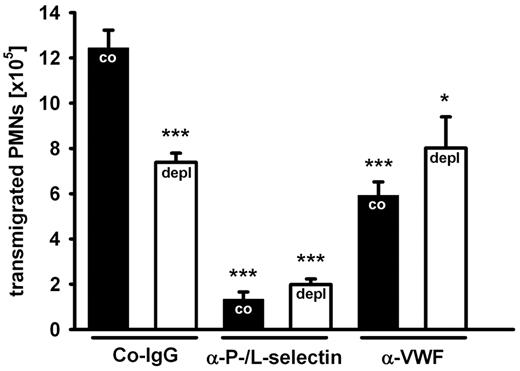
Since VWF is a major ligand for platelets, we assumed that platelet depletion would result in an inhibitory effect comparable to blocking VWF. Indeed, we found that depletion of platelets from the circulation by intravenous injection of a depleting anti-GPIb antibody inhibited PMN recruitment to the inflamed peritoneum by 47% ± 8% (average over 3 experiments). A representative experiment is shown in Figure 2. This inhibitory effect is similar to the one induced by anti-VWF antibodies. Importantly, blocking VWF and depleting platelets had no additive inhibitory effects (Figure 2), demonstrating that the VWF contribution to PMN extravasation requires platelets.
Platelets are required for the VWF effect on PMN recruitment. Mice were treated with an isotype control (black bars) or with a platelet-depleting antibody (white bars). After 24 hours, control rabbit IgG (Co-IgG), anti-P- and L-selectin antibodies, or rabbit anti-VWF antibodies were injected, and peritonitis was induced 15 minutes later with thioglycollate. Transmigrated PMNs were counted after 2 hours. For determination of statistical significance, data were compared with nondepleted Co-IgG-treated mice. Error bars show SEM (groups of 4 or more). Comparable results were achieved in 2 independent experiments. ***P ≤ .001; *P ≤ .05.
Platelets are required for the VWF effect on PMN recruitment. Mice were treated with an isotype control (black bars) or with a platelet-depleting antibody (white bars). After 24 hours, control rabbit IgG (Co-IgG), anti-P- and L-selectin antibodies, or rabbit anti-VWF antibodies were injected, and peritonitis was induced 15 minutes later with thioglycollate. Transmigrated PMNs were counted after 2 hours. For determination of statistical significance, data were compared with nondepleted Co-IgG-treated mice. Error bars show SEM (groups of 4 or more). Comparable results were achieved in 2 independent experiments. ***P ≤ .001; *P ≤ .05.
Platelet-derived VWF does not contribute to PMN recruitment
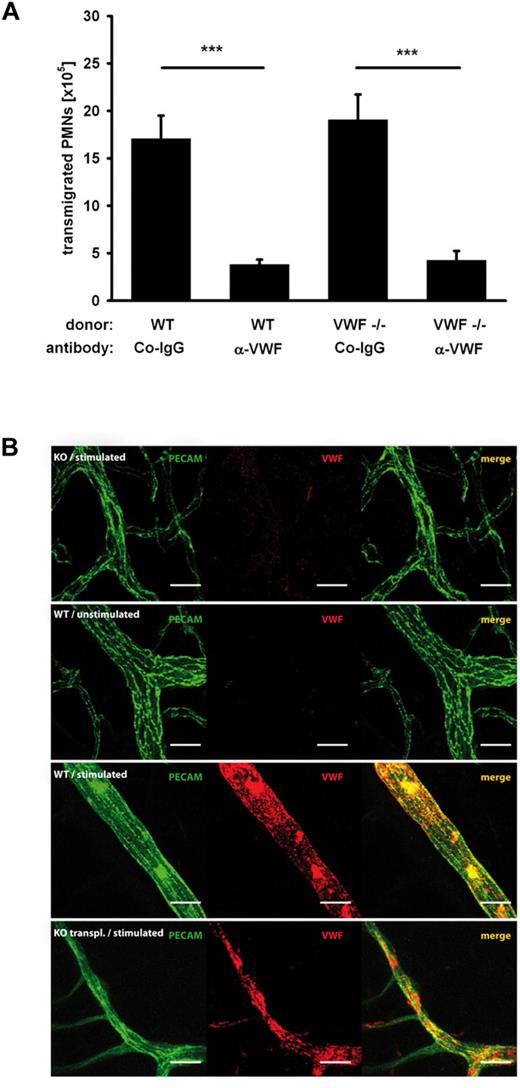
As both, ECs and platelets release VWF, we asked which source would be responsible for the extravasation of neutrophils. Since the absence of VWF in ECs results in Weibel-Palade body defects and thus makes data interpretation difficult, we studied the effect of VWF-deficiency in platelets. To this end, lethally irradiated wild-type mice were reconstituted with bone marrow from VWF-deficient mice. Analyzing blood smears of resulting chimeric animals by staining of platelets for VWF revealed that no platelets were detectable that expressed VWF (data not shown). We found that these chimeric mice showed normal PMN recruitment, whereas blocking VWF with antibodies caused strong inhibition (Figure 3A). We conclude that it is VWF from endothelial cells and not from platelets that contributes to the promotion of PMN recruitment. To visualize the presence of VWF on the vessel wall upon inflammatory stimulation, we stimulated mice intraperitonally with thioglycollate for 1.5 hours followed by intravenous injection of nonlabeled rabbit anti-VWF antibodies and a directly labeled anti PECAM-1 rat mAb (to visualize endothelium). After 30 minutes, the animals were anesthetized, and the vascular system was perfused with PBS to wash away nonspecifically attached antibodies. Then the mesentery was stained as whole mount with a labeled secondary antibody against rabbit IgG to visualize specifically bound anti-VWF antibodies. In this way, we observed strong VWF staining in mesenteric blood vessels only in mice treated with thioglycollate, but not in unstimulated mice (Figure 3B). This staining was specific because it was not seen in VWF−/− mice. Importantly, VWF staining was not due to platelets associated with the vessel wall because it was still strongly detectable in mice transplanted with VWF-/- bone marrow cells (Figure 3B). Thus, thioglycollate stimulates luminal deposition of VWF in blood vessels, which is accessible for antibodies from within the blood vessels and which originates from endothelium.
PMN recruitment is independent of platelet-derived VWF and thioglycollate stimulates luminal deposition of endothelial VWF. (A) Mice were lethally irradiated and reconstituted either with wt or VWF-/- donor bone marrow. Peritonitis was induced with thioglycollate. After 2 hours, PMNs were collected and counted in the different groups (n ≥ 5). Error bars show SEM. ***P ≤ .001. (B) Wild-type (WT), VWF−/− (KO), or wild-type mice transplanted with VWF−/− bone marrow (KO transplanted) were injected intraperitoneally with either thioglycollate (stimulated) or PBS (unstimulated). After 1.5 hours, primary labeled anti-PECAM antibodies and nonlabeled anti-VWF antibodies were intravenously injected. After 30 minutes, the vascular system was perfused with PBS to remove unbound antibodies, mesenteries were dissected and stained as whole mounts with a labeled secondary antibody for VWF. Scale bar = 20 μm.
PMN recruitment is independent of platelet-derived VWF and thioglycollate stimulates luminal deposition of endothelial VWF. (A) Mice were lethally irradiated and reconstituted either with wt or VWF-/- donor bone marrow. Peritonitis was induced with thioglycollate. After 2 hours, PMNs were collected and counted in the different groups (n ≥ 5). Error bars show SEM. ***P ≤ .001. (B) Wild-type (WT), VWF−/− (KO), or wild-type mice transplanted with VWF−/− bone marrow (KO transplanted) were injected intraperitoneally with either thioglycollate (stimulated) or PBS (unstimulated). After 1.5 hours, primary labeled anti-PECAM antibodies and nonlabeled anti-VWF antibodies were intravenously injected. After 30 minutes, the vascular system was perfused with PBS to remove unbound antibodies, mesenteries were dissected and stained as whole mounts with a labeled secondary antibody for VWF. Scale bar = 20 μm.
VWF promotes PMN extravasation via platelet GPIb
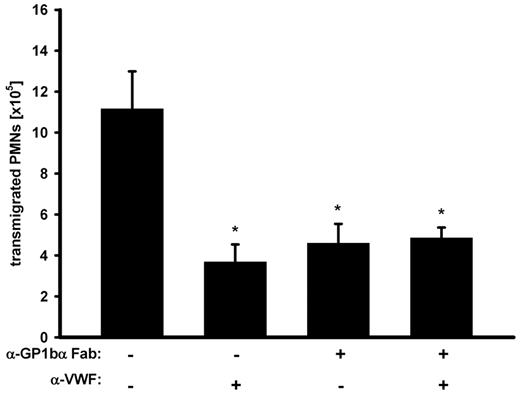
The data presented above suggest that EC-derived VWF acts on PMN extravasation by recruiting platelets to the vessel wall. Because GPIb is the prime platelet receptor for VWF, we asked whether VWF exerts its effect on PMN extravasation via GPIb. As shown in Figure 4, function-blocking Fab antibody-fragments against GPIb (p0p/B)30 that did not deplete platelets (data not shown) reduced PMN recruitment to a degree similar to that of anti-VWF antibodies. Importantly, when GPIb was blocked, VWF-antibodies could not further decrease PMN migration (Figure 4). This clearly indicates that VWF supports neutrophil extravasation by recruiting platelets via GPIb.
GPIbα is required for the VWF effect on PMN recruitment. Mice were treated with adhesion-blocking anti-GPIbα Fab-fragments, anti-VWF antibodies, or a combination of both before peritonitis was induced with thioglycollate. After 2 hours, PMNs were collected and counted. The experiment was repeated 3 times with n ≥ 3 mice per group, giving similar results. Error bars show SEM. *P ≤ .05.
GPIbα is required for the VWF effect on PMN recruitment. Mice were treated with adhesion-blocking anti-GPIbα Fab-fragments, anti-VWF antibodies, or a combination of both before peritonitis was induced with thioglycollate. After 2 hours, PMNs were collected and counted. The experiment was repeated 3 times with n ≥ 3 mice per group, giving similar results. Error bars show SEM. *P ≤ .05.
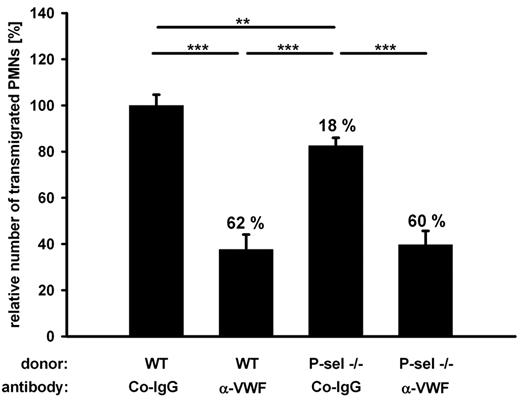
Minor role for platelet P-selectin in PMN recruitment
Leukocytes have been described to roll on and adhere to activated platelets bound to endothelial cells in vitro,12 and platelet P-selectin is believed to be an important mediator of leukocyte extravasation in acute lung injury.31 To study the function of platelet P-selectin in PMN recruitment to the inflamed peritoneum, we reconstituted lethally irradiated mice with bone marrow of P-selectin deficient mice. To our surprise, we observed only a very small reduction (18%) of PMN extravasation by platelet P-selectin deficiency, which was not statistically significant in single experiments (with n = 5) but appeared significant when evaluated over 3 experiments (Figure 5). In addition, the absence of P-selectin caused no additive inhibitory effect when combined with the block of VWF with antibodies (Figure 5). This shows that only a very minor part of VWF-mediated PMN recruitment can be attributed to PMN interactions with platelet P-selectin and that the very significant contribution of VWF to the extravasation of PMNs is largely independent of P-selectin-mediated interactions between platelets with PMNs.
Platelet P-selectin plays a minor part in VWF-mediated PMN recruitment. Mice were lethally irradiated and reconstituted either with wt or P-selectin−/− donor bone marrow. Animals were treated with control antibodies (Co-IgG) or anti-VWF antibodies before peritonitis was induced with thioglycollate. Normalized results of 3 independent assays are shown (number of mice per group ≥ 11). Numbers indicate the percentage of reduction in comparison to wt transplanted, Co-IgG treated mice. Error bars show SEM. ***P ≤ .001; **P ≤ .01.
Platelet P-selectin plays a minor part in VWF-mediated PMN recruitment. Mice were lethally irradiated and reconstituted either with wt or P-selectin−/− donor bone marrow. Animals were treated with control antibodies (Co-IgG) or anti-VWF antibodies before peritonitis was induced with thioglycollate. Normalized results of 3 independent assays are shown (number of mice per group ≥ 11). Numbers indicate the percentage of reduction in comparison to wt transplanted, Co-IgG treated mice. Error bars show SEM. ***P ≤ .001; **P ≤ .01.
VWF promotes leukocyte extravasation in peritoneum and cremaster but does not promote leukocyte rolling and adhesion
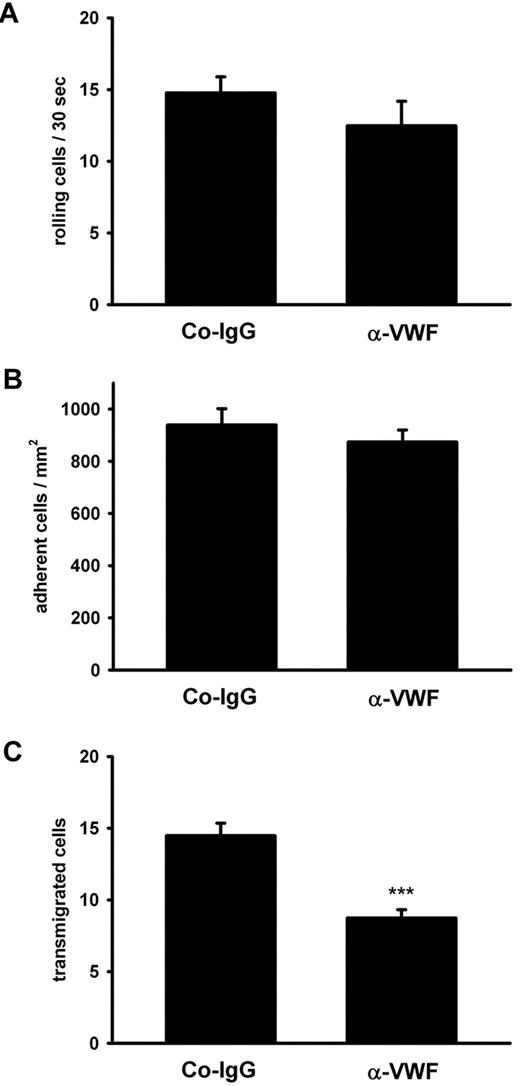
The minute contribution of platelet P-selectin to the strongly VWF-dependent extravasation of PMNs in thioglycollate-induced peritonitis prompted us to test whether VWF would at all influence leukocyte rolling and adhesion on the blood vessel wall. We used intravital microscopy of mesenteric venules and observed that these vessels were clearly activated by thioglycollate injection as judged from a strong increase of leukocyte rolling and adhesion over the PBS control (Table 1). However, we found that neither of these types of interactions was affected by blocking VWF with antibodies (Table 1). Similar results were found for another inflammation model. Upon superfusion of the exteriorized cremaster with the chemokine KC for 1 hour of intravital microscopy revealed that KC enhanced neutrophil rolling and adhesion in postcapillary venules as well as leukocyte extravasation. Anti-VWF antibodies clearly inhibited extravasation without affecting rolling or adhesion (Figure 6).
Leukocyte rolling and adhesion in inflamed mesenteric venules
| Parameter* . | PBS . | Thioglycollate + control Ab . | Thioglycollate + anti-VWF Ab . |
|---|---|---|---|
| Leukocyte rolling (n/30 s) | 6.1 × 6.0 | 30.9 × 5.0 | 29.2 × 3.0 |
| Leukocyte adhesion (n/104 μm2) | 0.2 × 0.1 | 3.3 × 0.4 | 3.1 × 0.2 |
| Vessel diameter (μm) | 23.6 × 1.8 | 29.5 × 0.4 | 28.1 × 0.4 |
| Parameter* . | PBS . | Thioglycollate + control Ab . | Thioglycollate + anti-VWF Ab . |
|---|---|---|---|
| Leukocyte rolling (n/30 s) | 6.1 × 6.0 | 30.9 × 5.0 | 29.2 × 3.0 |
| Leukocyte adhesion (n/104 μm2) | 0.2 × 0.1 | 3.3 × 0.4 | 3.1 × 0.2 |
| Vessel diameter (μm) | 23.6 × 1.8 | 29.5 × 0.4 | 28.1 × 0.4 |
Intravital microscopic assessment of leukocyte-endothelial interactions. Two hours after thioglycollate-mediated induction of peritonitis and injection of VWF- or anti-ESAM negative control antibodies intravital microscopy was performed on 7-15 single unbranched postcapillary mesenteric venules per animal (5 mice per group). Control animals received PBS instead of thioglycollate. Rolling leukocytes were quantified for 30 seconds. The number of adherent leukocytes was determined for each venule segment (100 μm) and was expressed per 104 μm2 of venule surface area. Data from 1 representative analysis of 3 are shown.
Antibodies against VWF inhibit PMN extravasation in the inflamed cremaster. The cremaster muscle of mice (4 mice per group) was prepared for intravital microscopy. Inflammation was induced by superfusion with keratinocyte-derived chemokine (KC), and the vessels (n ≥ 4 per mouse) were analyzed for rolling (A), adherent (B), and transmigrated (C) leukocytes. Error bars show SEM. ***P ≤ .001.
Antibodies against VWF inhibit PMN extravasation in the inflamed cremaster. The cremaster muscle of mice (4 mice per group) was prepared for intravital microscopy. Inflammation was induced by superfusion with keratinocyte-derived chemokine (KC), and the vessels (n ≥ 4 per mouse) were analyzed for rolling (A), adherent (B), and transmigrated (C) leukocytes. Error bars show SEM. ***P ≤ .001.
VWF promotes vascular permeability
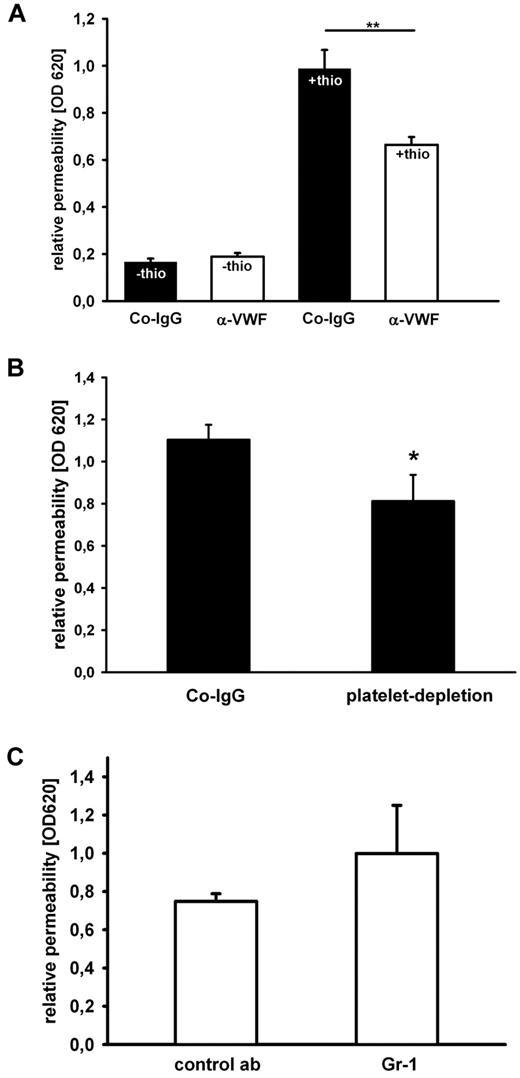
These data from 2 different inflammation models strongly suggest that VWF promotes PMN extravasation via a mechanism that acts downstream of leukocyte rolling and adhesion to the luminal surface of the endothelium. We hypothesized that such a mechanism could be related to the diapedesis process, the actual transmigration of PMNs through the endothelial cell barrier. Therefore, we tested whether VWF could contribute to PMN extravasation via modulating the integrity of the endothelial barrier. It is well known that inflammatory mediators enhance vascular permeability mainly via paracellular mechanisms,32 and, in agreement with this, we found that thioglycollate stimulation of the peritoneum caused a strong increase in the efflux of the dye Evans blue from blood vessels into the peritoneal cavity (Figure 7A black bars). This effect on permeability was not simply a consequence of the leukocyte diapedesis process, because it was also observed under conditions when peripheral myeloid cells were depleted by ≥ 95% by injecting anti-Gr1 antibodies 24 hours before the inflammatory stimulus (Figure 7C). Intriguingly, injection of blocking VWF-antibodies significantly reduced the efflux of Evans blue by 33% (Figure 7A), and depletion of platelets had a similar inhibitory effect (Figure 7B). Thus, VWF and also platelets contribute significantly to the thioglycollate-induced destabilization of the endothelial barrier function in mesenteric venules. This suggests that platelets binding to VWF presented on the endothelium of inflamed mesenteric venules promote the opening of endothelial junctions and thereby facilitate the diapedesis process.
Antibodies against VWF and platelet-depeletion reduce blood vessel permeability in the inflamed peritoneum. (A) Mice were intravenously injected with Evans blue dye and either control IgG (black bars) or anti-VWF antibody (white bars) before they were treated with PBS or thioglycollate (thio). Evans Blue was removed from the peritoneum by lavage 1 hour later. One representative experiment out of 3 is shown (n = 3-5). (B) Mice received an intravenous injection of either Co-IgG or a platelet-depleting antibody. After 24 hours, mice were injected intravenously with Evans blue dye then intraperitonally with thioglycollate. After 1 hour, Evans blue dye was quantified from peritoneal lavage (n ≥ 9). (C) Mice were intravenously injected with Evans blue dye and either isotype control Ab or Gr-1 Ab for PMN depletion before inflammation was induced with thioglycollate. Permeability was determined by optical density 620 measurement of the peritoneal lavage. Error bars show SEM. *P ≤ .05; **P ≤ .01.
Antibodies against VWF and platelet-depeletion reduce blood vessel permeability in the inflamed peritoneum. (A) Mice were intravenously injected with Evans blue dye and either control IgG (black bars) or anti-VWF antibody (white bars) before they were treated with PBS or thioglycollate (thio). Evans Blue was removed from the peritoneum by lavage 1 hour later. One representative experiment out of 3 is shown (n = 3-5). (B) Mice received an intravenous injection of either Co-IgG or a platelet-depleting antibody. After 24 hours, mice were injected intravenously with Evans blue dye then intraperitonally with thioglycollate. After 1 hour, Evans blue dye was quantified from peritoneal lavage (n ≥ 9). (C) Mice were intravenously injected with Evans blue dye and either isotype control Ab or Gr-1 Ab for PMN depletion before inflammation was induced with thioglycollate. Permeability was determined by optical density 620 measurement of the peritoneal lavage. Error bars show SEM. *P ≤ .05; **P ≤ .01.
Discussion
We show here that blocking antibodies against VWF or against its prime platelet receptor GPIb as well as depletion of platelets all have similarly strong inhibitory effects on neutrophil recruitment into the thioglycollate-inflamed peritoneum. These effects were not additive, strongly suggesting that platelets promote leukocyte recruitment via binding through GPIb to VWF deposited on the vessel wall. This VWF originated from endothelial cells but not from platelets. Importantly, VWF also contributed significantly to the extravasation of neutrophils in another inflammation model, the KC stimulated cremaster. In both models, VWF acted downstream of leukocyte rolling and adhesion to the blood vessel wall.
It is well documented that inflammatory stimuli can trigger secretion of VWF, leading to the deposition of VWF strings on the vessel wall that capture strings of platelets. However, in mesenteric venules, this deposition is only seen if the protease ADAMTS13 is either inhibited or absent due to gene ablation.15,16 Because we did not interfere in our experiments with the physiological ADAMTS13 levels, we do not expect that VWF was present in our experiments in its ultra-large form. Thus, our results suggest that ADAMTS13-cleaved products of ultra-large VWF are still able to capture platelets in sufficient numbers or for sufficient time to affect leukocyte extravasation. In agreement with the reports on rapid and efficient cleavage of ultra-large VWF by ADAMTS13 in vivo,16 we did not see any contribution of VWF, and thereby VWF-captured platelets, to capturing, rolling, and adhesion of leukocytes to the vessel wall. Thus, platelets captured by the cleaved forms of ultra-large VWF are not able to capture leukocytes but are able to facilitate leukocyte extravasation via steps downstream of leukocyte tethering and adhesion.
Besides GPIb, the integrin αIIbβ3 is a second potent platelet receptor for VWF.33 Our finding that anti-VWF antibodies do not block leukocyte extravasation when the adhesive function of GPIb is blocked argues against a significant role of αIIbβ3 in supporting platelet-VWF interactions that promote leukocyte recruitment. PMNs were recently described to be able to interact directly via P-selectin glycoprotein ligand-1 and β2-integrins with VWF.34 We can exclude that this interaction is important for the VWF support of PMN extravasation in the peritonitis model, because VWF exerted its effect via platelets, and anti-VWF factor antibodies did not interfere with rolling and adhesion of PMNs to the vessel wall.
The mechanisms whereby platelets contribute to the extravasation of leukocytes in various inflammatory models seem to vary considerably. In contrast to our peritonitis results, in acid-induced acute lung injury, PMN recruitment and the complete inflammatory process was critically dependent on platelet P-selectin.5 This study also demonstrated that the interaction of platelets with neutrophils and endothelia was associated with thromboxaneA2 formation, which enhanced vascular permeability and PMN accumulation in the alveolar space. Employing the same protocol for the analysis of thromboxane function by administering the thromboxane receptor antagonist SQ 29548, we found no effect on leukocyte recruitment in peritonitis (data not shown) corroborating different functions of platelets in acute lung injury and peritonitis.
In contrast to other inflammation models,3,5,6 platelet P-selectin played only a very minor role in leukocyte extravasation in the peritionitis model described here. Because anti-VWF antibodies had no inhibitory effect on leukocyte rolling, we can exclude that platelets captured by VWF to the endothelial cell surface would support leukocyte capturing or rolling via platelet P-selectin. A potential mechanism for the minor contribution of platelet P-selectin to leukocyte extravasation could be a stimulation of PMNs via P-selectin glycoprotein ligand-1, which can lead to activation of RAS, mitogen-activated protein kinases, and eventually the release of interleukin-8, signaling steps that have been described.35 A role of platelet P-selectin only as signaling ligand and not as support for leukocyte capturing would be in agreement with the fact that VWF is probably not available in its ultra-large multimeric form in our experimental setting, because ADAMTS13 is active at physiological levels. Thus, platelets are probably not captured in large numbers or possibly bind only transiently to the endothelium and therefore cannot contribute to leukocyte capturing.
Instead, platelets must contribute to leukocyte extravasation in another way. Concomitant with the platelet support of neutrophil recruitment, we observed a significant contribution of VWF to the thioglycollate stimulated increase of vascular permeability in the peritoneum. This effect was independent of the presence of neutrophils in the circulation. Thus, thioglycollate-stimulated vascular permeability is not a side effect of neutrophil diapedesis and is to a significant degree dependent on VWF and, by inference, dependent on vessel-associated platelets. Because we saw no effect of the anti-VWF antibodies on neutrophil rolling and adhesion but a clear cut effect on neutrophil extravasation, we conclude that VWF and interacting platelets promote neutrophil extravasation at a step downstream of leukocyte tethering, rolling, and adhesion. A likely explanation would be that the platelet-mediated stimulation of vascular permeability would contribute to the destabilization and opening of endothelial junctions, thereby facilitating neutrophil diapedesis.
Platelets can induce vascular permeability in various ways. They release soluble factors such as vascular endothelial growth factor, platelet activating factor, and thrombin, which stimulate endothelial cells.33,36 Each of these factors has been shown to cause opening of interendothelial junctions, with platelet activating factor inducing relocation of the junctional molecules ZO-1 and VE-cadherin,37 and thrombin activating myosin light chain kinase, cortical actin contraction, and β-catenin dissociation from VE-cadherin resulting in the disassembly of interendothelial tight junctions.32
Collectively, our data support a model where peritoneal inflammation triggers the release and cell surface deposition of endothelial VWF. This allows platelets to adhere to the endothelium via GPIb, presumably in a transient manner. Platelets tethered in this way to the vessel wall are not sufficient to directly support leukocyte capturing, rolling, or adhesion, but they are obviously able to affect the endothelial barrier integrity and efficiently enhance neutrophil extravasation into the inflamed peritoneum. Our results establish VWF as an important proinflammatory mediator of leukocyte extravasation in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Friedemann Kiefer (Max Planck Institute for Molecular Biomedicine, Münster, Germany) for help with transplantations, and Cecile Denis and Denisa Wagner for kindly providing the VWF−/− mice.
This work was supported by Deutsche Forschungsgemeinschaft (DFG) grant GO1360/41 to T.G.; by DFG grant SFB/TR23, subproject A9 to S.W.S.; and by the Max Planck Society.
Authorship
Contribution: B.P., A.B., H.L., A.G.K., A.Z., T.G., C.J., and M.K.W. designed, performed, and analyzed experiments; S.W.S. and B.N. provided valuable reagents; F.K. designed experiments and interpreted results; D.V. initiated the study; and M.K.W. and D.V. analyzed and interpreted data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation of B.P. is Immunology Research Group, Department of Physiology and Biophysics, Calvin, Phoebe and Joan Snyder Institute for Infection, Immunity and Inflammation, University of Calgary, Calgary, AB. The current affiliation of S.W.S. is Department of Dermatology, Venereology and Allergology, Experimental Dermatology, Medical Faculty Mannheim, Heidelberg Ruprecht-Karls-University, Mannheim, Germany.
Correspondence: Dietmar Vestweber, Max Planck Institute for Molecular Biomedicine, Röntgenstr 20, 48149 Münster, Germany; e-mail: vestweb@mpi-muenster.mpg.de; or Martin K. Wild, Max Planck Institute for Molecular Biomedicine, Röntgenstr 20, 48149 Münster, Germany; e-mail: mwild@mpi-muenster.mpg.de.
References
Author notes
B.P. and A.B. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal