Monoclonal antibodies and T cells modified to express chimeric antigen receptors specific for B-cell lineage surface molecules such as CD20 exert antitumor activity in B-cell malignancies, but deplete normal B cells. The receptor tyrosine kinase-like orphan receptor 1 (ROR1) was identified as a highly expressed gene in B-cell chronic lymphocytic leukemia (B-CLL), but not normal B cells, suggesting it may serve as a tumor-specific target for therapy. We analyzed ROR1-expression in normal nonhematopoietic and hematopoietic cells including B-cell precursors, and in hematopoietic malignancies. ROR1 has characteristics of an oncofetal gene and is expressed in undifferentiated embryonic stem cells, B-CLL and mantle cell lymphoma, but not in major adult tissues apart from low levels in adipose tissue and at an early stage of B-cell development. We constructed a ROR1-specific chimeric antigen receptor that when expressed in T cells from healthy donors or CLL patients conferred specific recognition of primary B-CLL and mantle cell lymphoma, including rare drug effluxing chemotherapy resistant tumor cells that have been implicated in maintaining the malignancy, but not mature normal B cells. T-cell therapies targeting ROR1 may be effective in B-CLL and other ROR1-positive tumors. However, the expression of ROR1 on some normal tissues suggests the potential for toxi-city to subsets of normal cells.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) and mantle cell lymphoma (MCL) are common B-cell malignancies that respond to chemotherapy but are rarely cured. Allogeneic hematopoietic stem cell transplantation (HCT) enables a T cell–mediated graft-versus-leukemia (GVL) effect and induces durable remissions in a subset of patients with chemotherapy-refractory B-CLL and MCL, demonstrating that these malignancies are susceptible to recognition and elimination by T cells.1,2 In a previous study, we identified tumor-reactive CD8+ T cells directed against minor histocompatibility (H) and tumor-associated antigens (TAA) expressed by B-CLL in patients with sustained tumor regression after allogeneic HCT.3 These results have encouraged the development of T cell–adoptive immunotherapy to augment the GVL effect after HCT. However, major challenges for therapy with αβ Τ-cell receptor (TCR)–bearing T cells include the need to identify antigens with restricted expression on malignant cells to avoid graft-versus-host disease, and the population distribution and requirement for human leukocyte antigen (HLA)–restriction for both minor H antigens and TAA.4
An approach that could overcome these challenges and also enable T-cell therapy for B-CLL and MCL in the nontransplant setting is to genetically modify T cells to express a chimeric antigen receptor (CAR) that is specific for a cell surface protein expressed by malignant cells. CARs consists of a single-chain antibody fragment (scFv) that is derived from the variable heavy (VH) and variable light (VL) chains of a monoclonal antibody (mAb) linked to the TCR CD3ζ chain that mediates T-cell activation and cytotoxicity.5 Costimulatory signals can also be provided through the CAR by fusing the costimulatory domain of CD28 or 4-1BB to the CD3ζ chain.5,6 CARs are specific for cell surface molecules independent from HLA, thus overcoming the limitations of TCR-recognition including HLA-restriction and low levels of HLA-expression on tumor cells. B-cell lineage differentiation molecules such as CD19 and CD20 are retained on most B-cell tumors, and T cells modified with CD19- and CD20-specific CARs are currently being evaluated in clinical trials.7,8 However, targeting B-cell lineage-specific antigens with immunotherapy has the disadvantage of eliminating normal mature B cells, which can increase the risk of infection.9,10
Here, we evaluate a strategy to selectively eliminate malignant B cells without damaging the mature normal B-cell compartment by targeting the receptor tyrosine kinase-like orphan receptor 1 (ROR1). ROR1 was identified as a highly expressed gene in B-CLL by expression profiling and it has been shown that ROR1-protein is uniformly expressed on the cell surface of B-CLL.11,,–14 The ROR1-gene encodes a 105-kDa protein with a 45-kDa extracellular domain that contains immunoglobulin (Ig)–like, Frizzled, and Kringle domains.13,15 Functional data suggest that ROR1 may act in Wnt-signaling and promote the survival of malignant cells.16,17 Here, we characterize the expression of ROR1 in B-cell malignancies and human tissues, and show that in addition to B-CLL, ROR1 is expressed uniformly at high levels in MCL and transiently at a specific stage of normal B-cell development but not in major adult tissues. CD8+ T cells engineered to express a ROR1-specific CAR selectively lyse primary B-CLL and MCL, but not normal mature B cells in vitro, suggesting that ROR1-specific T-cell therapy may be an effective treatment for patients with ROR1-positive B-cell tumors.
Methods
Human subjects
Blood samples were obtained from patients and healthy donors who provided written informed consent in accordance with the Declaration of Helsinki to participate in research protocols approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center. Peripheral blood mononuclear cells (PBMCs) and bone marrow mononuclear cells (BMMCs) were isolated by centrifugation over Ficoll-Hypaque (Sigma-Aldrich) and cryopreserved in RPMI containing 20% human serum and 10% dimethyl sulfoxide.
Cell lines
Epstein-Barr virus transformed B cells (EBV-LCL) were generated as described.18 The tumor lines Jeko-1, Rec-1, BALL-1, RPMI-8226, RCH-ACV, SU-DHL-4, FL-18, and SUP-B15 were provided by Drs Oliver Press and Jerald Radich (Fred Hutchinson Cancer Research Center). All cell lines were maintained in RPMI, 10% fetal calf serum, 0.8mM l-glutamine, and 1% penicillin-streptomycin (LCL medium). K562, Jurkat, and 293T cells were obtained from the American Type Culture Collection and cultured as directed.
Adipocytes were derived by in vitro differentiation of human white preadipocytes obtained from and differentiated in media provided by Promo Cell. Preadipocytes and adipocytes were stained with 0.1 μg/mL Nile red (Invitrogen) for 10 minutes, washed with phosphate-buffered saline (PBS), and analyzed by fluorescent microscopy.
The human embryonic stem (ES) cell lines HUES2 and H1 were a kind gift of Drs Carol Ware and Tony Blau (University of Washington). Undifferentiated ES cells were cultured in mouse embryonic fibroblast conditioned medium.19 Undirected differentiation was performed by cul-ture in Dulbecco modified Eagle medium, 10% fetal calf serum, and 1% penicillin-streptomycin for 6 and 12 days. Expression of stage-specific embryonic antigen-4 (SSEA-4) and SSEA-1 was analyzed with phycoerythrin (PE)–conjugated specific monoclonal antibodies (mAbs) and corresponding isotype controls (Millipore).20
Transfection of K562 cells with ROR1
For polymerase chain reaction (PCR)–amplification of the ROR1-gene, total RNA was obtained from B-CLL cells (RNeasyPlusKit; QIAGEN) and reverse transcribed into cDNA with M-MLV Reverse Transcriptase (Invitrogen). PCR was performed with specific primers (ROR1-F: 5′-XhoI-AGAGGAGGAATGCACCGGCC-3′ and ROR1-R: 5′-XhoI-CACAGAAGGTACTTGTTGCGATGT-3′) using Herculase-II DNA Polymerase (Stratagene). The PCR product was cloned into the MIGR-1 retroviral vector21 and sequence verified. Effectene transfection reagent (QIAGEN) was used to transfect Platinum-A cells (Cell Biolabs) with MIGR-1/ROR1 and produce ROR1-encoding retrovirus. K562 cells were retrovirally transduced by centrifugation at 2500 rpm for 60 minutes at 32°C, expanded, and the ROR1-positive subset was sort-purified.
Real-time quantitative PCR
First-strand cDNA of B-CLL, normal resting and activated B cells, and EBV-LCL was prepared as described in the previous paragraph. First-strand cDNA from normal tissues (Human Tissue panels I/II, Blood Fractions) was obtained from Clontech. Expression of ROR1 mRNA was analyzed in duplicate and normalized to GAPDH. Amplifications were performed on an ABI Prism 7900 (Applied Biosystems) in a 50 μL reaction consisting of 25 μL Power SYBR Green PCR Master Mix (Applied Biosystems), 2.5 ng of cDNA, and 300nM gene-specific forward and reverse primers: ROR1-F 5′-AGCGTGCGATTCAAAGGATT-3′, ROR1-R 5′-GACTGGTGCC GACGATGACT-3′, GAPDH-F 5′-GAAGGTGAAGGTCGGAGTC-3′ and GAPDH-R 5′-GAAGATGGTGATGGGATTTC-3′. The cycle threshold (Ct) was determined using SDS software v2.2.2 (Applied Biosystems) and the level of gene expression calculated using the comparative Ct method (2−(ΔΔCt)).
Immunophenotyping
Cell surface expression of ROR1 was analyzed using specific polyclonal goat-anti–human-ROR1 antibody and goat IgG as isotype control (R&D Systems). In brief, 1 × 106 cells were washed, resuspended in 100 μL PBS/0.5% bovine serum albumin and stained with 10 μL of 25 μg/mL anti-ROR1 antibody or isotype for 30 minutes at 4°C. After washing, secondary staining was performed with 0.5 μL of APC-conjugated donkey-anti–goat-IgG antibody (R&D Systems) for 30 minutes at 4°C.
Primary B-CLL, normal B cells, PBMCs, and BMMCs were stained with 1 or more of the following conjugated mAbs: CD3, CD5, CD8, CD10, CD19, CD34, CD38, CD45, CD45RO, CD62L, CD86 and matched isotype controls (BD Pharmingen). Flow analyses were done on a FACSCanto and LSRII, sort-purifications on a FACSAriaII (Becton Dickinson). Data were analyzed using FlowJo software v9.0.2 (TreeStar).
Activation of normal B cells
Primary B cells were isolated from PBMCs of healthy donors using CD19-specific magnetic beads (Miltenyi). B cells were cultured at 1-2 × 106 cells/well in 24-well plates in Iscove Modified Dulbecco Medium (Invitrogen), 10% human serum, 5 μg/mL transferrin (Roche), and 5 μg/mL insulin (Sigma-Aldrich). B-cell receptor (BCR)–crosslinking was performed by incubation with 10 μg/mL anti–human IgM immunobeads (Irvine Scientific) for 3 hours. Thereafter, B-cells were washed, resuspended in fresh medium, and incubated for 24 hours. Activation with phorbol-12-myristate-13-acetate (PMA) and ionomycin (both Sigma-Aldrich) was performed by culture for 24 hours in medium containing 25 ng/mL PMA and 1 μg/mL ionomycin. For CD40-activation, we used CD40L-transfected NIH-3T3 cells as described.3
Vector construction and generation of lentivirus
CD20-CAR (CD20R-epHIV7) and green fluorescent protein (GFP)–encoding lentiviral vectors (GFP-epHIV7) were described previously.22 The ROR1-CAR was encoded in the same vector. A mouse mAb (clone 2A2) that demonstrated specific binding to human ROR1 expressed on primary B-CLL and MCL tumor lines was generated, cloned, and characterized in a previous study (S.B. and C.R., manuscript in preparation). A codon-optimized nucleotide sequence encoding a scFv containing the VL and VH chain of mAb 2A2 was synthesized (GENEART) and cloned into CD20R-epHIV7 using NheI and RsrII restriction sites to replace the CD20-specific scFv. Lentivirus was produced in 293T cells cotransfected with the lentiviral vector and the packaging vectors pCHGP-2, pCMV-Rev2, and pCMV-G using Effectene (Qiagen). Medium was changed 16 hours after transfection and lentivirus collected after 48 hours.
Lentiviral transduction and isolation of CAR-transduced T-cell clones
PBMC from healthy donors and B-CLL patients, and sort-purified CD8+CD45RO+CD62L+ central memory T cells (TCM) were activated with anti-CD3 mAb (30 ng/mL),18 and transduced in lentiviral supernatant supplemented with 1 μg/mL polybrene (Sigma-Aldrich) and 50 IU/mL recombinant human interleukin-2 (IL-2) on day 2 and 3 after activation by centrifugation at 2500 rpm for 60 minutes at 32°C. T cells were expanded in RPMI containing 10% human serum, 2mM l-glutamine, and 1% penicillin-streptomycin (CTL medium).18
After expansion, an aliquot of each transduced T-cell line was stained with biotin-conjugated anti-EGFR (epithelial growth factor receptor) mAb, streptavidin-PE, and anti-CD8 mAb. EGFR+CD8+ T cells were sort-purified and cloned by limiting dilution (0.5 cells/well).18 ROR1-CAR transduced T cells were identified by staining with biotinylated recombinant Fc-ROR1 extracellular domain fusion protein and streptavidin-PE. Recombinant ROR1-protein was produced in transiently transfected 293F cells (Invitrogen), purified as described13 and biotinylated using the BiotinTag kit (Sigma). GFP-transduced CD8+ T cells were identified by flow cytometry, sort-purified, and cloned in similar fashion.
Chromium release and cytokine secretion assays
Target cells were labeled with 51Cr (PerkinElmer) overnight, washed and incubated in triplicate at 1-2 × 103 cells/well with effector T cells at various effector to target (E:T) ratios. Supernatants were harvested for γ-counting after a 4-hour incubation, and specific lysis was calculated using the standard formula.18
For analyses of cytokine secretion, target and effector cells were plated in triplicate wells at an E/T ratio of 2:1, and interferon-γ (INF-γ), tumor necrosis factor-α (TNF-α), and IL-2 were measured by multiplex cytokine immunoassay (Luminex) in supernatant removed after a 24-hour incubation.
CFSE proliferation assay
T cells were labeled with 0.2μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen), washed, and plated with stimulator cells at a ratio of 2:1 in CTL medium containing 10 U/mL recombinant human IL-2. After a 72-hour incubation, cells were labeled with anti-CD8 mAb and propidium iodide (PI) to exclude dead cells from analysis. Samples were analyzed by flow cytometry, and cell division of live CD8+ T cells assessed by CFSE dilution.
Evaluation of the subset of CLL cells that effluxes Hoechst 33342
Side population experiments were performed as described.23 In brief, primary B-CLL cells were diluted to 2 × 106 cells/mL in LCL medium and labeled with 5 μg/mL Hoechst 33342 (Sigma-Aldrich) for 120 minutes at 37°C. In selected cases, verapamil (Sigma-Aldrich) was added at a final concentration of 50μM. After staining, cells were washed; resuspended in ice-cold PBS/0.5% bovine serum albumin; and stained for expression of CD5, CD19, and ROR1 and with PI.
Results
ROR1 is highly expressed in B-CLL and not in mature normal B cells and adult organs
Microarray studies have demonstrated that ROR1 is highly expressed in B-CLL relative to diffuse large B-cell lymphoma (DLBCL), normal resting and activated B cells and T cells.11,12 The ROR1-protein is also expressed at the surface of B-CLL, suggesting it may serve as a target for immunotherapy using mAbs or CAR-modified T cells.13 However, the extent to which ROR1 is expressed in normal cells has not been rigorously examined. We used real-time quantitative PCR (qPCR) to measure the expression of ROR1 mRNA in normal cells from a variety of hematopoietic and nonhematopoietic tissues, and in a panel of primary B-CLL. There are 2 isoforms of ROR1, a long splice variant 1 that encodes a 937-amino acid (AA) type I transmembrane protein, and splice variant 2 that encodes a 393-AA truncated protein that lacks both the transmembrane and tyrosine kinase domain and is not present on the cell surface.15 We examined the expression of ROR1 splice variant 1 using PCR primers that amplify the sequence of ROR1 cDNA that corresponds to the transmembrane domain of the protein. ROR1 mRNA was present at high levels in 8/8 primary B-CLL samples, and expression was 50- to 100-fold lower in normal peripheral blood and EBV-transformed B cells, CD8+ and CD4+ T cells, and total PBMC (Figure 1A). Analysis of normal adult nonhematopoietic tissues revealed that ROR1-expression was markedly lower (> 50-fold) compared with CLL in all tissues except pancreas and adipose tissue where the relative expression of ROR1 mRNA was 35-fold and 10-fold lower, respectively (Figure 1A).
ROR1 is uniformly expressed in B-CLL and shows expression in human ES cells. (A) ROR1 mRNA expression in B-CLL cells, normal primary and EBV-transformed B cells and a panel of normal hematopoietic and nonhematopoietic tissues was analyzed by qPCR. One B-CLL sample (B-CLL #1) was used as a reference (expression = 1), and the relative expression of ROR1 was compared with GAPDH as housekeeping gene. (B) Analysis of ROR1-protein expression on the cell surface of PBMCs obtained from B-CLL patients and normal donors using specific polyclonal antibodies (solid black line) compared with isotype controls (gray histogram). Data are representative for 10 B-CLL patients and 4 normal donors. (C) ROR1 expression on mature adipocytes that were generated in vitro by differentiation from human white preadipocytes, compared with B-CLL cells analyzed in the same experiment. (D) Expression of ROR1, SSEA-4, and SSEA-1 on the human ES cell line H1 before and after undirected in vitro differentiation for 6 and 12 days. Histograms show staining with specific monoclonal (SSEA-4/1) and polyclonal (ROR1) antibodies (solid black line) versus matched isotype control antibodies (gray histogram).
ROR1 is uniformly expressed in B-CLL and shows expression in human ES cells. (A) ROR1 mRNA expression in B-CLL cells, normal primary and EBV-transformed B cells and a panel of normal hematopoietic and nonhematopoietic tissues was analyzed by qPCR. One B-CLL sample (B-CLL #1) was used as a reference (expression = 1), and the relative expression of ROR1 was compared with GAPDH as housekeeping gene. (B) Analysis of ROR1-protein expression on the cell surface of PBMCs obtained from B-CLL patients and normal donors using specific polyclonal antibodies (solid black line) compared with isotype controls (gray histogram). Data are representative for 10 B-CLL patients and 4 normal donors. (C) ROR1 expression on mature adipocytes that were generated in vitro by differentiation from human white preadipocytes, compared with B-CLL cells analyzed in the same experiment. (D) Expression of ROR1, SSEA-4, and SSEA-1 on the human ES cell line H1 before and after undirected in vitro differentiation for 6 and 12 days. Histograms show staining with specific monoclonal (SSEA-4/1) and polyclonal (ROR1) antibodies (solid black line) versus matched isotype control antibodies (gray histogram).
To determine whether ROR1 mRNA expression correlated with ROR1-protein levels, we used multiparameter flow cytometry to analyze surface expression of ROR1 on mononuclear cell subsets in blood obtained from B-CLL patients with newly diagnosed or advanced disease (n = 10), and from healthy individuals (n = 4). Consistent with previous reports, we found selective and uniform expression of ROR1-protein on the surface of CD19+CD5+ B-CLL cells in all 10 cases, but not on normal CD19+CD5− B cells, CD19−CD5+ T cells or CD19−CD5− PBMC, which contains natural killer cells and monocytes (Figure 1B).13,14,24 Adipose tissue was the normal tissue with the highest level of ROR1 mRNA, but it was difficult to isolate intact primary human adipocytes for analysis by flow cytometry. As a surrogate, we generated adipocytes from human white preadipocytes by in vitro differentiation25 and identified mature adipocytes by accumulation of packed intracellular fat droplets that stained positive with Nile red by fluorescent microscopy26 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Mature adipocytes derived by this method expressed detectable cell surface ROR1, but the level of expression was significantly lower than on B-CLL (Figure 1C).
The absence or very low levels of ROR1 mRNA in all major adult organs is consistent with studies in rats, which demonstrated that ROR1 is expressed during early embryonic development and then down-regulated in late embryogenesis and in mature adult cells.27 We used flow cytometry to analyze ROR1-expression on the surface of 2 human ES cell lines H1 and HUES2 and on the progeny of these cells after undirected differentiation. Undiffer-entiated H1 and HUES2 cells expressed the characteristic SSEA-4 antigen20 and expressed cell surface ROR1 (Figure 1D and supplemental Figure 2). Undirected differentiation of the ES cells was induced by culture in conditioned medium, which resulted in loss of SSEA-4 and up-regulation of the differentiation marker SSEA-1.20 Differentiation of ES cells resulted in dramatic down-regulation of ROR1 surface expression (Figure 1D and supplemental Figure 2). Collectively, our findings demonstrate that human ROR1 has characteristics of an oncofetal antigen that is not expressed in major adult organs or mature blood cells but is highly expressed in B-CLL.
ROR1 is uniformly expressed in mantle cell lymphoma
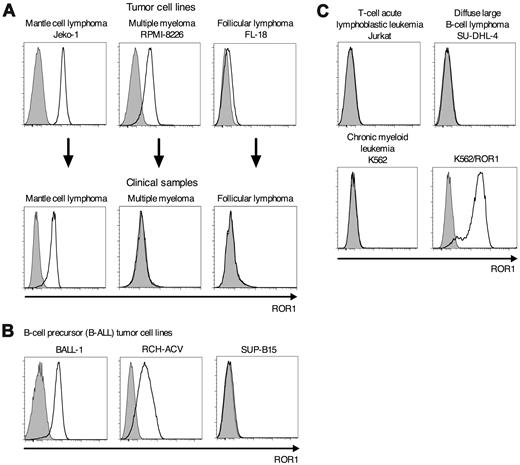
To investigate whether ROR1 might be overexpressed by B-cell malignancies other than CLL, we analyzed B-lymphoid tumor cell lines and clinical samples by flow cytometry. We detected uniformly high levels of ROR1 on Jeko-1 and Rec-1 (MCL), RPMI-8226 (multiple myeloma [MM]), but not on FL-18 (follicular lymphoma [FL]; Figure 2A). Uniform high-level expression of ROR1 on MCL was confirmed in 6/6 primary clinical samples. However, we did not detect expression of ROR1 on primary clinical samples of MM (n = 6) and FL (n = 5). We screened a panel of B-ALL, DLBCL, T-lymphoid, and chronic myeloid leukemia tumor cell lines and detected ROR1-expression only on the B-ALL cell lines BALL-1 and RCH-ACV but not on the B-ALL cell line SUP-B15 (Figure 2B-C), which is consistent with an earlier report of ROR1-expression on a subset of primary B-ALL.24 These data demonstrate that, in addition to B-CLL, specific therapies targeting ROR1 could be applied in MCL and in a subset of B-ALL.
ROR1 is uniformly expressed on MCL. (A) Screening for expression of ROR1 on tumor cell lines and clinical samples of MCL (Jeko-1), multiple myeloma (RPMI-8226), and follicular lymphoma (FL-18). Data of clinical samples are representative for 6 MCL, 6 multiple myeloma, and 5 follicular lymphoma patients, respectively. (B) The B-cell precursor (B-ALL) tumor cell lines BALL-1 and RCH-ACV but not SUP-B15 are positive for ROR1-protein expression by flow cytometry. (C) The tumor cells lines Jurkat (T-cell acute lymphoblastic leu-kemia), SU-DHL-4 (diffuse large B-cell lymphoma), and K562 (chronic myeloid leukemia) are negative for ROR1 surface expression. Expression of ROR1 on K562 cells after stable transfection with the ROR1-gene (K562/ROR1).
ROR1 is uniformly expressed on MCL. (A) Screening for expression of ROR1 on tumor cell lines and clinical samples of MCL (Jeko-1), multiple myeloma (RPMI-8226), and follicular lymphoma (FL-18). Data of clinical samples are representative for 6 MCL, 6 multiple myeloma, and 5 follicular lymphoma patients, respectively. (B) The B-cell precursor (B-ALL) tumor cell lines BALL-1 and RCH-ACV but not SUP-B15 are positive for ROR1-protein expression by flow cytometry. (C) The tumor cells lines Jurkat (T-cell acute lymphoblastic leu-kemia), SU-DHL-4 (diffuse large B-cell lymphoma), and K562 (chronic myeloid leukemia) are negative for ROR1 surface expression. Expression of ROR1 on K562 cells after stable transfection with the ROR1-gene (K562/ROR1).
ROR1 is expressed on a subset of normal B-cell precursors in adult bone marrow
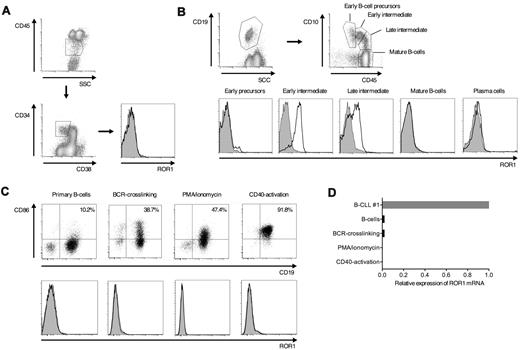
The expression of ROR1 in a subset of B-cell malignancies suggested ROR1 might be expressed at some stage of normal B-cell development. We obtained BMMC from healthy individuals (n = 3) and examined ROR1-expression on CD34+CD38−lin− hematopoietic progenitor cells and CD34+ and CD34− B-cell precursors at distinct stages of maturation.28 ROR1 was not expressed on CD34+CD38−lin− cells or on the earliest B-cell precursors (CD34+CD38+CD19+CD10bright; Figure 3A-B). However, we detected ROR1 on B-cell precursors at an intermediate stage of maturation (CD34−CD38+CD19+CD10high/intermediate; Figure 3B). Late B-cell precursors (CD34−CD38+CD19+CD10low), mature B cells (CD34−CD38+CD19+CD10−), and plasma cells (CD34−CD38brightCD19+CD10−) were negative for ROR1 (Figure 3B).
ROR1 is expressed on a subset of normal B-cell precursors in adult bone marrow. (A-B) Multicolor flow cytometric analysis of BMMCs from healthy donors. Data are representative for results from 3 healthy donors. (A) Analysis of ROR1-expression on CD34+CD38−lin− hematopoietic progenitor cells. (B) Identification of B-cell precursors at distinct stages of B-cell development, mature B cells and plasma cells based on coexpression of CD19, CD10 and CD45, and analysis of ROR1-expression on the cell surface. Staining with specific polyclonal anti–human ROR1 antibodies (solid black line) versus isotype controls (gray histogram). (C) Activation of primary peripheral B cells of healthy donors by BCR-crosslinking, with PMA/ionomycin and stimulation through CD40. Expression of CD86 (top panel) and ROR1 (bottom panel) before and after activation. (D) ROR1 mRNA expression in normal resting, activated and EBV-transformed B cells analyzed by qPCR. The same B-CLL sample as in Figure 1A (B-CLL #1) was used as a reference and the relative expression of ROR1 compared with GAPDH as housekeeping gene.
ROR1 is expressed on a subset of normal B-cell precursors in adult bone marrow. (A-B) Multicolor flow cytometric analysis of BMMCs from healthy donors. Data are representative for results from 3 healthy donors. (A) Analysis of ROR1-expression on CD34+CD38−lin− hematopoietic progenitor cells. (B) Identification of B-cell precursors at distinct stages of B-cell development, mature B cells and plasma cells based on coexpression of CD19, CD10 and CD45, and analysis of ROR1-expression on the cell surface. Staining with specific polyclonal anti–human ROR1 antibodies (solid black line) versus isotype controls (gray histogram). (C) Activation of primary peripheral B cells of healthy donors by BCR-crosslinking, with PMA/ionomycin and stimulation through CD40. Expression of CD86 (top panel) and ROR1 (bottom panel) before and after activation. (D) ROR1 mRNA expression in normal resting, activated and EBV-transformed B cells analyzed by qPCR. The same B-CLL sample as in Figure 1A (B-CLL #1) was used as a reference and the relative expression of ROR1 compared with GAPDH as housekeeping gene.
Both B-CLL and MCL are thought to arise from transformation of mature B cells, suggesting that the expression of ROR1 might be reinduced in normal B cells by activation and/or engagement of the B-cell receptor (BCR). We stimulated purified CD19+ B cells from the peripheral blood of healthy donors with anti–human IgM beads to crosslink the BCR, with PMA/ionomycin, and CD40L. We confirmed that each of these stimuli induced B-cell activation as measured by up-regulation of CD86 (Figure 3C). However, we did not detect ROR1 on activated B cells by cell surface staining, nor did we observe up-regulation of ROR1 mRNA by qPCR (Figure 3C-D). Overall, these findings show that ROR1 is transiently expressed only at an early stage of normal B-cell development in the bone marrow and suggest that therapies targeting ROR1 would spare the mature resting and activated naive and memory B-cell pool.
Expression of a ROR1-specific CAR in human T cells confers recognition of ROR1-positive tumors
Several approaches might be used to target ROR1-positive tumors in vivo, including engineering autologous T cells with a ROR1-specific CAR. We had previously generated, cloned, and characterized mouse mAb 2A2 that binds to an epitope located in the extracellular domain of human ROR1 (S.B. and C.R., manuscript in preparation). Using the AA sequences of the variable domains of mAb 2A2, we constructed a synthetic codon-optimized gene that encoded the original AA sequence in scFv format, with the VH located upstream of the VL chain. The scFv was then linked to an IgG4-Fc domain, a CD28 costimulatory domain and the TCR CD3ζ chain in a CAR cassette that included a truncated extracellular EGFR encoded downstream of a T2A sequence as transduction marker. The construct was packaged in a lentiviral vector (Figure 4A), and PBMCs from healthy donors were transduced with ROR1-CAR-encoding lentivirus. Transduced CD8+ T cells were sort-purified using a biotinylated anti-EGFR mAb and streptavidin-conjugated dyes. Similarly, ROR1-CAR expression on the surface of T cells was evaluated by staining the cells with a biotinylated recombinant Fc-ROR1 extracellular domain fusion protein that directly binds to the scFv of the ROR1-CAR and costaining with streptavidin-conjugates. Fc-ROR1-protein specifically stained CD8+ T cells transduced with the ROR1-CAR lentiviral vector but not CD8+ T cells transduced with a control lentiviral vector encoding GFP (Figure 4B).
A ROR1-specific CAR expressed in human CD8+ T cells confers specific recognition of ROR1-positive tumor cells. (A) Design of the transgene encoding the ROR1-CAR. The CAR-cassette contains a truncated extracellular EGFR transduction marker, separated by a cleavable T2A element to identify transduced T cells. (B) Phenotype and transgene expression of a ROR1-CAR transduced and a GFP-transduced CD8+ T-cell clone obtained from a healthy donor. To identify CAR-transduced T cells, primary staining was either performed with biotinylated anti-EGFR mAb or biotinylated recombinant Fc-ROR1 fusion protein that contained the extracellular domain of ROR1 and binds directly to the scFv of the CAR, and compared with staining with Fc-protein alone. Secondary staining was performed with streptavidin-conjugated PE. (C) Specific cytotoxicity of ROR1-CAR transduced CD8+ T cells against primary B-CLL and ROR1-transfected K562 cells by chromium release assay. Cytotoxicity data are presented as mean values of triplicate wells. The standard deviation of each triplicate was ≤ 3% in all cases (C-F). (D) ROR1-CAR modified CD8+ T cells but not T cells transduced with a GFP-encoding control vector specifically recognize primary B-CLL and MCL samples from multiple patients and a panel of ROR1-positive tumor cell lines. Cytotoxicity was analyzed by chromium release assay at an E/T ratio of 20:1. (E) Chromium release assay comparing the cytotoxicity of ROR1-CAR and CD20-CAR transduced CD8+ T cells obtained from the same donor against primary B-CLL cells and autologous resting and EBV-transformed B cells. (F) Recognition of autologous B cells that had been activated by BCR-crosslinking, with PMA/ionomycin, and stimulation through CD40 by ROR1-CAR, CD20-CAR, and control GFP-transduced CD8+ T cells by chromium release assay at an E/T ratio of 20:1.
A ROR1-specific CAR expressed in human CD8+ T cells confers specific recognition of ROR1-positive tumor cells. (A) Design of the transgene encoding the ROR1-CAR. The CAR-cassette contains a truncated extracellular EGFR transduction marker, separated by a cleavable T2A element to identify transduced T cells. (B) Phenotype and transgene expression of a ROR1-CAR transduced and a GFP-transduced CD8+ T-cell clone obtained from a healthy donor. To identify CAR-transduced T cells, primary staining was either performed with biotinylated anti-EGFR mAb or biotinylated recombinant Fc-ROR1 fusion protein that contained the extracellular domain of ROR1 and binds directly to the scFv of the CAR, and compared with staining with Fc-protein alone. Secondary staining was performed with streptavidin-conjugated PE. (C) Specific cytotoxicity of ROR1-CAR transduced CD8+ T cells against primary B-CLL and ROR1-transfected K562 cells by chromium release assay. Cytotoxicity data are presented as mean values of triplicate wells. The standard deviation of each triplicate was ≤ 3% in all cases (C-F). (D) ROR1-CAR modified CD8+ T cells but not T cells transduced with a GFP-encoding control vector specifically recognize primary B-CLL and MCL samples from multiple patients and a panel of ROR1-positive tumor cell lines. Cytotoxicity was analyzed by chromium release assay at an E/T ratio of 20:1. (E) Chromium release assay comparing the cytotoxicity of ROR1-CAR and CD20-CAR transduced CD8+ T cells obtained from the same donor against primary B-CLL cells and autologous resting and EBV-transformed B cells. (F) Recognition of autologous B cells that had been activated by BCR-crosslinking, with PMA/ionomycin, and stimulation through CD40 by ROR1-CAR, CD20-CAR, and control GFP-transduced CD8+ T cells by chromium release assay at an E/T ratio of 20:1.
We established ROR1-CAR transduced (n > 10) and control GFP-transduced CD8+ T-cell clones (n = 4) by limiting dilution, and confirmed the stable surface expression of the CAR after multiple rounds of in vitro expansion. There was no apparent difference in the growth of ROR1-CAR transduced compared with untransduced or GFP-transduced T-cell clones (data not shown). The ROR1-CAR transduced T-cell clones efficiently lysed primary B-CLL and K562 cells that were stably transfected with the ROR1-gene, but not native, ROR1-negative K562 cells, demonstrating specific recognition of ROR1 (Figure 4C). ROR1-specific and GFP-transduced T cells were then tested against an extended panel of primary CLL and MCL cells and ROR1-positive and ROR1-negative tumor cell lines. Primary CLL (n = 6) and MCL (n = 1); the ROR1-positive tumor cell lines Jeko-1 and Rec-1 (MCL), BALL-1, and RCH-ACV (B-ALL); and RPMI-8226 (MM) were lysed by ROR1-CAR–transduced but not GFP-transduced T cells (Figure 4D). As expected, ROR1-CAR and GFP-transduced T-cell clones did not lyse any of the ROR1-negative tumor lines (SUP-B15, FL-18, SU-DHL-4, and Jurkat).
A potential advantage of targeting ROR1 rather than B-cell lineage-specific molecules such as CD20 or CD19 is that ROR1 is not expressed on mature normal B cells. We compared the ability of CD8+ T-cell clones that expressed either the ROR1-CAR or a similarly designed CD20-CAR to recognize autologous primary and EBV-transformed B-cells, and primary B-cells that had been activated by BCR-crosslinking, CD40L, and incubation with PMA/ionomycin. CD20-CAR expressing T-cells efficiently lysed primary B-CLL, autologous resting and activated normal B-cells and EBV-transformed B-cells. In contrast, T-cells modified with the ROR1-CAR selectively recognized B-CLL, and did not lyse normal resting, activated, and EBV-transformed B-cells (Figure 4E-F).
ROR1-CAR transduced CD8+ T cells can be generated from B-CLL patients and recognize autologous tumor cells
The function of T cells from B-CLL patients is compromised due to tumor cell induced suppression of actin polymerization in T cells leading to the inability to form functional immune synapses and poor recognition through the endogenous TCR.29,30 However, it would be desirable to use T cells from CLL patients for adoptive therapy in an autologous setting without the need for allogeneic HCT. Therefore, we analyzed whether CD8+ T cells from B-CLL patients could be efficiently engineered to recognize autologous CLL cells by introduction of the ROR1-CAR. We obtained PBMC from 3 B-CLL patients with advanced disease and lentivirally transduced bulk PBMC and sort-purified CD8+CD45RO+CD62L+ TCM, which prior studies have suggested may be the preferred cell population for adoptive therapy.31 The transduction efficiency of patient T cells was equivalent to CD8+ T cells from normal donors, and ROR1-CAR expressing CD8+ T-cell lines and clones were readily derived from 3 of 3 patients (Figure 5A). Patient derived ROR1-CAR modified T-cell lines and clones generated from both unselected and TCM-enriched CD8+ T-cell subsets efficiently lysed autologous primary B-CLL and K562/ROR1 at equivalent levels compared with ROR1-CAR T cells derived from healthy individuals, but not ROR1-negative K562 cells (Figures 4C, 5B, and supplemental Figure 3).
ROR1-CAR transduced CD8+ T cells obtained from B-CLL patients specifically recognize autologous tumor cells. (A) Phenotype and transgene expression of a ROR1-CAR and a GFP-transduced CD8+ T-cell clone obtained from a B-CLL patient. Data are representative for results obtained in 3 B-CLL patients. (B) ROR-CAR CD8+ T cells from 3 B-CLL patients specifically recognize autologous primary B-CLL and ROR1-positive tumor cells by chromium release assay. Cytotoxicity data are presented as mean values of triplicate wells. The standard deviation of each triplicate was ≤ 3% in all cases (B,F). (C) Secretion of INF-γ (left axis), TNF-α and IL-2 (right axis) of ROR1-CAR transduced CD8+ T cells obtained from a healthy donor and a B-CLL patient in response to primary (autologous) B-CLL and ROR1-positive tumor cell lines after incubation for 24 hours at an E:T ratio of 2:1 quantified by Luminex Assay. (D) Proliferation of ROR1-CAR expressing CD8+ T cells obtained from a healthy donor and a B-CLL patient after stimulation with primary (autologous) B-CLL and ROR1-positive tumor cell lines assessed by CFSE dilution after incubation for 72 hours at an E/T ratio of 2:1. Specific proliferation (black solid line) versus background proliferation (gray histogram). (E) Detection of a side population of Hoechst 33342 effluxing B-CLL cells by flow cytometry after staining of primary B-CLL cells with Hoechst 33342 (left dot plot). Expression of ROR1 on SP and non-SP B-CLL cells (center histograms). Inhibition of Hoechst 33342 efflux by addition of 50μM verapamil (right dot plot). Data are representative for results obtained in 3 B-CLL patients. (F) Specific and equivalent elimination of sort-purified SP and non-SP primary B-CLL cells by ROR1-CAR transduced CD8+ T cells by chromium release assay at an E/T ratio of 20:1.
ROR1-CAR transduced CD8+ T cells obtained from B-CLL patients specifically recognize autologous tumor cells. (A) Phenotype and transgene expression of a ROR1-CAR and a GFP-transduced CD8+ T-cell clone obtained from a B-CLL patient. Data are representative for results obtained in 3 B-CLL patients. (B) ROR-CAR CD8+ T cells from 3 B-CLL patients specifically recognize autologous primary B-CLL and ROR1-positive tumor cells by chromium release assay. Cytotoxicity data are presented as mean values of triplicate wells. The standard deviation of each triplicate was ≤ 3% in all cases (B,F). (C) Secretion of INF-γ (left axis), TNF-α and IL-2 (right axis) of ROR1-CAR transduced CD8+ T cells obtained from a healthy donor and a B-CLL patient in response to primary (autologous) B-CLL and ROR1-positive tumor cell lines after incubation for 24 hours at an E:T ratio of 2:1 quantified by Luminex Assay. (D) Proliferation of ROR1-CAR expressing CD8+ T cells obtained from a healthy donor and a B-CLL patient after stimulation with primary (autologous) B-CLL and ROR1-positive tumor cell lines assessed by CFSE dilution after incubation for 72 hours at an E/T ratio of 2:1. Specific proliferation (black solid line) versus background proliferation (gray histogram). (E) Detection of a side population of Hoechst 33342 effluxing B-CLL cells by flow cytometry after staining of primary B-CLL cells with Hoechst 33342 (left dot plot). Expression of ROR1 on SP and non-SP B-CLL cells (center histograms). Inhibition of Hoechst 33342 efflux by addition of 50μM verapamil (right dot plot). Data are representative for results obtained in 3 B-CLL patients. (F) Specific and equivalent elimination of sort-purified SP and non-SP primary B-CLL cells by ROR1-CAR transduced CD8+ T cells by chromium release assay at an E/T ratio of 20:1.
To further characterize the effector function of patient derived ROR1-CAR modified T cells, we compared their ability to produce effector cytokines and to proliferate after engagement of antigen with ROR1-CAR T cells from normal donors. After stimulation with autologous B-CLL and other ROR1-positive tumor cells, patient derived ROR1-CAR CD8+ T cells produced similar levels of IFN-γ, TNF-α, and IL-2 as their counterparts obtained from healthy individuals (Figure 5C). Furthermore, patient and healthy donor derived CD8+ ROR1-CAR T cells proliferated equivalently in response to stimulation with autologous B-CLL and ROR1-positive tumor cells (Figure 5D).
ROR1-CAR transduced T cells recognize the subset of drug effluxing CLL cells
A subset of primary B-CLL cells that have the ability to efflux cell permeable fluorescent dyes can be distinguished as a side population (SP) using flow cytometry.23 SP cells in B-CLL and other hematologic malignancies have been shown to be chemotherapy resistant, and in AML this subset has stem cell characteristics and can be serially transplanted in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice.23,32,33 We loaded PBMCs from 3 B-CLL patients with Hoechst 33342 to segregate SP and non-SP B-CLL cells, and determine whether these cells exhibited differential expression of ROR1 and were recognized equivalently by ROR1-CAR transduced T cells. We detected a subpopulation of Hoechst 33342 effluxing CD19+CD5+ SP cells in all 3 patients (range 0.02-0.11%), and coculture of the cells with the ABCG2-transporter inhibitor verapamil blocked dye efflux (Figure 5E). Both the SP and noneffluxing subsets of B-CLL cells uniformly expressed the same high levels of cell surface ROR1 (Figure 5E). In 1 patient, we had sufficient primary tumor cells to sort-purify SP B-CLL cells and evaluate them as targets in a cytotoxicity assay. Sort-purified SP, non-SP, and unsorted primary B-CLL cells were equivalently lysed by ROR1-CAR CD8+ T cells (Figure 5F). Thus, T cells obtained from B-CLL patients can be genetically modified with a CAR specific for the shared B-CLL tumor antigen ROR1 and specifically eliminate autologous tumor cells in vitro, including the subset of primary B-CLL cells that has enhanced chemotherapy resistance as a consequence of drug efflux.
Discussion
Adoptive immunotherapies that employ CAR-modified T cells are being investigated in clinical trials for B-cell malignancies. The surface molecules that are being targeted are B-cell lineage–specific and include CD19, which is expressed on normal B-lineage cells from the pro-B-cell stage to plasma cells, and CD20, which is expressed on normal B cells from the pre-B-cell stage to memory B cells. Thus, an anticipated outcome of effective therapy targeting these molecules is depletion of normal B cells and B-cell precursors. Gene expression profiling studies have identified genes that are preferentially or exclusively expressed by malignant but not by normal B cells and ROR1 emerged as a CLL signature gene in 2 independent analyses.11,12 Specific antibodies to ROR1 developed in CLL patients after vaccination with autologous tumor cells that had been modified to express CD154 and treatment with lenalidomide without apparent toxicity to normal tissues, suggesting this tumor antigen may be a suitable target for immunotherapy.16,34
The studies here provide a detailed analysis of the distribution of ROR1-expression in normal adult nonhematopoietic and hematopoietic cells, including normal precursor and mature B cells. We tailored our qPCR assay to specifically amplify the dominant splice variant 1, which encodes ROR1-protein that has a transmembrane domain and is expressed on the cell surface.15,27 The ROR1-gene was not expressed at significant levels in normal adult nonhematopoietic tissues apart from low levels in pancreas and adipose tissue. Of nonhematopoietic tissues, adipose tissue had the highest level of ROR1 mRNA, and we confirmed ROR1-expression on adipocytes that were differentiated in vitro from preadipocytes, although the surface staining for ROR1 on adipocytes was much lower than that observed in CLL or MCL. Our results are in agreement with previous studies showing that ROR1 is absent from normal mature B cells, T cells, monocytes, and natural killer cells in the peripheral blood.13,14,24 In addition, we show that ROR1-expression is not induced on mature B cells after activation through mitogens, engagement of the BCR, or CD40 ligation. However, we found that, although it was absent on CD34+CD38− hematopoietic progenitors and the earliest B-cell precursors in the bone marrow, ROR1 was transiently expressed at an intermediate stage of normal B-cell development at a level that is similar to that in B-CLL and MCL. Our analysis of ROR1-expression in thymus-derived mature CD8+ and CD4+ T cells by qPCR and the ability to readily transduce and expand T cells expressing a ROR1-specific CAR suggest that ROR1 is not expressed at significant levels in developing and mature, resting, and activated T cells. Studies in rodents have shown that ROR1 is expressed during embryonic development but not in mature adult tissues, leading to its designation as an oncofetal antigen.16,27 We found ROR1 to be expressed in undifferentiated human ES cells, and expression was down-regulated upon differentiation in conditioned medium. Thus, ROR1 has characteristics of an oncofetal antigen, although its expression in adipocytes and in a subset of immature B cells suggests this protein also has a role in a very restricted set of adult tissues.
Our understanding of the function and regulation of ROR1 expression is incomplete, particularly as it relates to a potential role in the pathogenesis and propagation of B-cell malignancies. In our analysis of primary tumors and tumor cell lines, we show that ROR1 is expressed uniformly at high levels on primary MCL and MCL lines, and confirm prior work showing that primary B-CLL and a subset of B-ALL uniformly expresses high levels of ROR1.13,14,24 Both B-CLL and MCL are presumed to derive from mature, antigen-experienced B cells35 that are negative for ROR1-expression, and the mechanism, timing, and role of ROR1 re-expression in malignant transformation is unknown. ROR1 was identified as a “survival” kinase in an in vitro screen for molecules that, when knocked down with shRNA, sensitized HeLa cells to chemotherapy, suggesting that ROR1 may function in maintaining the survival of a variety of malignant cells, including solid tumors.17 Preliminary analyses in our laboratory have demonstrated that ROR1 is expressed on a subset of solid tumor cell lines (M.H. and S.R.R., unpublished data), suggesting careful annota-tion of ROR1-expression in primary and metastatic solid tumors is warranted.
Our studies illustrate the potential to target ROR1-positive malignant cells with engineered T cells expressing a ROR1-CAR. CD8+ ROR1-CAR T cells could be derived from both normal donors and CLL patients after lentiviral transduction of either bulk PBMCs or sort-purified TCM, that in animal models persist for extended periods after adoptive transfer.31 ROR1-CAR transduced T cells efficiently lysed primary B-CLL, primary MCL, and ROR1-positive tumor cell lines including the rare subset of SP CLL cells that can efflux fluorescent dyes and chemotherapy,23 but not normal resting or activated B-cells. These T cells produced effector cytokines including TNF-α, IFN-γ, and IL-2, and were capable of proliferating in response to ROR1-expressing tumor cells.
The potential for other normal cells to be damaged by ROR1-CAR modified T cells will require in vivo studies. It is noteworthy that several case reports of antibody responses to ROR1 in B-CLL patients were associated with reductions in tumor cell burden without adverse events or toxicities to normal tissues.16,34 Our data suggest that immature B-cell precursors in the bone marrow would be subject to elimination by ROR1-CAR modified T cells similar to that expected if CD20 or CD19 were targeted. However, targeting ROR1 has the advantage of sparing the mature B-cell pool that would be eliminated with T cells specific for CD20 or CD19. Because the half-life of mature B cells is estimated to be several weeks to months,36 targeting ROR1 would be expected to preserve greater B-cell function than targeting CD20 or CD19, although there may be consequences of targeting immature B-cell precursors for the long-term durability of B-cell function. Preclinical evaluation of ROR1-specific T-cell therapy in a suitable animal model would be ideal to determine whether targeting ROR1 on developing B cells will indeed preserve the mature B-cell compartment or lead over time to B-cell depletion. Our group has developed a nonhuman primate model to address issues of safety and efficacy in adoptive T-cell therapy.31 The ROR1-gene is highly conserved in macaques including the sequence that encodes the epitope recognized by our ROR1-CAR, and we have shown in preliminary experiments that our ROR1-CAR is functional in macaque T cells (M.H., C. Berger, S.R.R., unpublished data). ROR1-specific therapies might also be associated with toxicities to adipose tissue although it is not clear how effectively T cells would migrate to adipose tissue in the absence of inflammation, or whether the level of ROR1 expression on adipocytes in vivo will be sufficient to trigger T-cell recognition. ROR1-protein may also be expressed on other (rare) subsets of cells that we cannot easily interrogate by qPCR or flow cytometry. Thus, future studies of adoptively transferring autologous ROR1-specific T cells in the nonhuman primate model will be important for determining the safety and potential toxicities of targeting ROR1 with adoptive immunotherapy and how significant these might be.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Carol Ware and Tony Blau for providing the H1 and HUES2 ES cell lines, and Drs Oliver Press and Jerald Radich for providing the Jeko-1, Rec-1, BALL-1, RPMI-8226, RCH-ACV, SU-DHL-4, FL-18, and SUP-B15 tumor cell lines.
This work was supported by grants from the Leukemia & Lymphoma Society (S.R.R. and D.G.M.), National Institutes of Health CA18029 and CA136551 (S.R.R.), and a gift from Rae and Mark Lembersky. M.H. was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft).
National Institutes of Health
Authorship
Contribution: M.H. designed and performed research, analyzed data, and wrote the manuscript; T.M.S., M.T.L.S., T.N., and T.N.Y. designed and performed research; C.R., S.B., W.C.C., M.C.J., C.J.T., M.B., and D.G.M. provided expert advice and reagents; H.A.G. and B.W. performed research and analyzed data; and S.R.R. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael Hudecek, Fred Hutchinson Cancer Research Center, Clinical Research Division, Program in Immunology, D3-100, 1100 Fairview Ave N, PO Box 19024, Seattle, WA 98109; e-mail: mhudecek@fhcrc.org.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal