Regulating transition of hematopoietic stem cells (HSCs) between quiescent and cycling states is critical for maintaining homeostasis of blood cell production. The cycling states of HSCs are regulated by the extracellular factors such as cytokines and extracellular matrix; however, the molecular circuitry for such regulation remains elusive. Here we show that tissue inhibitor of metalloproteinase-3 (TIMP-3), an endogenous regulator of metalloproteinases, stimulates HSC proliferation by recruiting quiescent HSCs into the cell cycle. Myelosuppression induced TIMP-3 in the bone marrow before hematopoietic recovery. Interestingly, TIMP-3 enhanced proliferation of HSCs and promoted expansion of multipotent progenitors, which was achieved by stimulating cell-cycle entry of quiescent HSCs without compensating their long-term repopulating activity. Surprisingly, this effect did not require metalloproteinase inhibitory activity of TIMP-3 and was possibly mediated through a direct inhibition of angiopoietin-1 signaling, a critical mediator for HSC quiescence. Furthermore, bone marrow recovery from myelosuppression was accelerated by over-expression of TIMP-3, and in turn, impaired in TIMP-3–deficient animals. These results suggest that TIMP-3 may act as a molecular cue in response to myelosuppression for recruiting dormant HSCs into active cell cycle and may be clinically useful for facilitating hematopoietic recovery after chemotherapy or ex vivo expansion of HSCs.
Introduction
Hematopoietic stem cells (HSCs) are somatic stem cells that can support life-long hematopoiesis.1 They are characterized by the unique capacity to self-renew and differentiate into all blood cell lineages. In a steady state, most HSCs are quiescent in the G0 phase of the cell cycle, being recruited into the cycle intermittently with long intervals.2,3 Once HSCs face hematopoietic demands such as myelosuppression, they are rapidly recruited into the cell cycle to meet the increased need for multipotent progenitors (MPPs), which then expand, differentiate, and replenish the depleted mature blood cell population. Transition of HSCs between quiescent and cycling states has to be tightly regulated, because excess cycling of HSCs eventually leads to their exhaustion through differentiation.4,–6
HSCs reside in the specific microenvironment known as the niche in adult bone marrow (BM).7,8 It was previously reported that the niche is located on the surface of trabecular bones, and osteoblasts lining their surface are a critical niche component.9,10 In addition, later study revealed that BM vasculature also constitutes HSC niche, as many HSCs were found in close contact with sinusoidal endothelium.11 Self-renewal, differentiation, and the cell-cycle status of HSCs are regulated by a variety of signals supplied by the niche, including cytokines, growth factors, adhesion molecules, and extracellular matrix components.12,–14 It has been reported that angiopoietin-1 (Ang-1) produced by osteoblasts plays a critical role in inducing HSC quiescence.15,16 However, the molecular mechanism for recruiting quiescent HSCs into the cell cycle remains poorly understood.
Extracellular signals are often regulated by the extracellular matrix environment, which is modulated by metalloproteinase (MMP) activity.17,–19 Tissue inhibitor of metalloproteinase-3 (TIMP-3) is an endogenous inhibitor of MMPs,20,21 and we have previously proposed that TIMP-3 may play a critical role in HSC physiology.22 TIMP-3 is unique among the TIMP family in its broader inhibition profile, which includes MMPs and a disintegrin and metalloproteinase (ADAM)/a disintegrin and metalloproteinase with a thrombospondin motifs (ADAM-T3) family proteases.23 TIMP-3 is involved in a wide array of biological processes such as extracellular matrix remodeling, cell survival, and modulation of cytokine/growth factor signaling.20,24 These activities are exerted mainly through protease inhibition; however, some TIMP-3 activities do not seem to require MMP inhibition.25
In this study, we revealed an unexpected role for TIMP-3 in HSC regulation. TIMP-3 was induced in the BM before hematopoietic recovery after myelosuppression, and it promoted cell cycle entry of quiescent HSCs, leading to the expansion of the MPP pool. We also provide evidence that inhibition of Ang-1 signaling is a possible mechanism for HSC recruitment by TIMP-3. Furthermore, TIMP-3 facilitated BM recovery from myelosuppression in vivo; in turn, BM recovery from myelosuppression was impaired in TIMP-3–deficient animals. These results demonstrate that TIMP-3 may act as one of the molecular cues in response to myelosuppression for recruiting quiescent HSCs into active cell cycle, which then leads to the propagation of MPPs and eventually to the hematopoietic recovery.
Methods
Mice
C57BL/6 (B6) mice were from Japan CLEA, Inc., and B6-Ly5.1 mice were from Sankyo Lab Service Co. TIMP-3–deficient mice were described previously.26 Mice from 8-12 weeks old were used in all experiments. All animal experiments were reviewed and approved by the Internal Review Board of Keio University School of Medicine and the Institute of Medical Science, University of Tokyo.
Flow cytometry
The following monoclonal antibodies were used for flow cytometric analysis: c-Kit (ACK2), Sca-1 (E13–161.7), CD34 (RAM34), and Flt3 (A2F10.1). All antibodies were purchased from BD Pharmingen. Purification of CD34−KSL cells and the analysis of long-term (LT) HSCs, short-term (ST)-HSCs, and MPPs were done as previously described27 with modifications. Briefly, BM cells were harvested from 8- to 12-week-old mice, and mononuclear cells were separated by density-gradient centrifugation using Lymphoprep (Nycomed). Lineage-positive cells were depleted with Lineage Cell Depletion Kit (Miltenyi Biotec) according to the manufacturer's protocol. For isolation of CD34−KSL cells, lineage-negative cells were stained with anti-CD34-fluorescein isothiocyanate (FITC), anti-Sca-1-phycoerythrin (PE), anti-c-Kit-allophycocyanin (APC), and an anti-lineage antibody cocktail in the Lineage Cell Depletion Kit, followed by staining with streptoavidin-PE-Cy7.28 For the analysis of LT-HSC, ST-HSC, and MPP, cells were stained with anti-CD34-FITC, anti-Flt3-PE, anti-c-Kit-PE-Cy7, anti-Sca-1-APC, and an anti-lineage antibody cocktail in the Lineage Cell Depletion Kit, followed by staining with streptoavidin-peridinin chlorophyll protein Cy5.5. FACS Calibur, FACS Vantage, or FACS Aria was used for analysis and cell sorting.
Cell-cycle analysis
Lineage negative cells were separated from 8-12-week-old mice as described above. Cells were stained with 10 μg/mL of Hoechst 33342 (Sigma-Aldrich) in Hoechst staining buffer (Hank's Balanced Salt Solution + 3% fetal bovine serum [FBS] + 10 mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]) at 37°C. After 45 minutes, Pyronin Y (1 μg/mL, Sigma-Aldrich) was added to the buffer, and the cells were further incubated for 45 minutes. Cells were then subjected to antibody staining with anti-CD34-FITC, anti-Sca-1-PE-Cy5.5, c-Kit-APC, and an anti-lineage antibody cocktail in the Lineage Cell Depletion Kit, followed by staining with streptoavidin-APC-Cy7. Analysis was performed by FACS Vantage.
In vivo BrdU labeling and analysis of BrdU uptake by flow cytometry
In vivo labeling of HSCs was performed by injecting 2 mg of BrdU intraperitoneally into mice. BM cells were harvested at 24 hours after injection and subjected to CD34−KSL staining. Incorporated BrdU was stained using APC BrdU flow kit (BD Pharmingen) according to the manufacturer's protocol. Hoechst 33342 was used for DNA staining.
Culture of hematopoietic stem cells and single-cell colony-forming assays
CD34−KSL cells were sorted into 96-well plates and cultured in S-clone SF-03 (Sanko Junyaku) + 0.5% bovine serum albumin (BSA) + 50 μM 2-mercaptoethanol + 50 ng/mL stem cell factor (SCF) + 50 ng/mL thrombopoietin (TPO) with or without 3 μg/mL recombinant human TIMP-3 (R&D Systems). Single-cell colony-forming assays were performed by adding full-differentiation medium (S-Clone SF-03 supplemented with 10% FBS, 20 ng/mL SCF, 20 ng/mL interleukin-3, 50 ng/mL TPO, and 2 U/mL erythropoietin) to each well. Cells were recovered, cytospun onto glass slides, and then subjected to May-Giemsa staining for morphological examination. GM6001 was purchased from Sigma-Aldrich.
Paired daughter cell assay
Paired daughter cell-colony assay was done as previously described.29 Briefly, CD34−KSL cells were clonally sorted into 96-well plates and allowed to divide once in S-clone + 0.5% bovine serum albumin + 50 ng/mL SCF + 50 ng/mL TPO with or without 3 μg/mL recombinant human TIMP-3. The daughter cells were separated by micromanipulation and deposited into methylcellulose (MethoCult M3434, Stem Cell Technologies). After 7 days of culture, each colony was picked up and recovered onto glass slides. Cells were stained by May-Giemsa solution and subjected to morphological evaluation by microscopy.
Plasmids
Retroviral plasmid expressing TIMP-3 (pMXs-IG/TIMP-3) was described previously.22 Mutant TIMP-3 (Cys1 to Ser) was generated by mutating the first cysteine residue after the signal sequence to serine using a QuikChange site-directed mutagenesis kit (Stratagene). pcDNA3/TIMP-3 were generated by subcloning cDNAs encoding TIMP-3 into pcDNA3 (Invitrogen) vector.
Q-RT-PCR
PolyA+ mRNA was extracted from BM cells using a Micro-FastTrack 2.0 Kit (Invitrogen). cDNAs were reverse-transcribed by SuperScript II reverse transcriptase (Invitrogen). Q-RT-PCR was performed as described previously using ABI StepOne real-time PCR system (Applied Biosystems).30,31 Briefly, from a given test sample, the threshold cycle (CT) values for target gene (TIMP-3) were normalized to the endogenous control gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH; ΔCT = CT target − CT endogenous) and compared with the mean ΔCT from the control sample (ΔΔCT = ΔCT sample − ΔCT control). Calculated fold difference relative to the control is plotted on the graphs. Primer sequences are shown in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Hydrodynamic plasmid delivery
Histologic analysis and immunostaining
Femurs were dissected, fixed in 10% buffered formalin, and decalcified. The bones were then embedded in Optimal Cutting Temperature compound, frozen, and sectioned (5 μm thickness). Sections were subjected to hematoxylin staining or refixed with methanol/acetone for 5 minutes, blocked, and stained with anti-Flag antibody (Sigma-Aldrich) and DAPI (4′,6-diamidino-2-phenylindole; Molecular Probes).
Statistical analysis
All statistical analyses, except for survival curves, were performed using unpaired Student t test. The differences in survival rates were analyzed by Mann-Whitney U test.
Results
TIMP-3 is induced in the bone marrow in response to myelosuppression
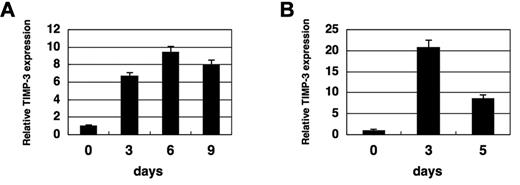
We have previously proposed that TIMP-3 may play a critical role in HSC physiology using HSC/stromal cell coculture system.22 To investigate the involvement of TIMP-3 in HSC regulation in physiological settings, we first examined its expression in BM cells upon myelosuppressive stress, using Q-RT-PCR. Interestingly, after injecting 5-fluorouracil (5-FU) into mice, TIMP-3 was up-regulated on day 3, with the induction persisting through day 9 (Figure 1A and supplemental Table 2). Induction of TIMP-3 was also observed when mice were treated with irradiation in which the induction was even more transient (Figure 1B and supplemental Table 2). These results suggest that TIMP-3 might be involved in the hematopoietic response during a myelosuppressive state.
Myelosuppression induces TIMP-3 in the bone marrow. (A) Induction of TIMP-3 by BM suppression. Mice (C57BL/6) were intraperitoneally injected with 150 mg/kg of 5-FU, and BM mononuclear cells (BMMNCs) were harvested at the indicated time points. RNA was extracted and examined for the expressions of TIMP-3 by Q-RT-PCR. Data were normalized and analyzed by comparative CT method and shown as relative values to day 0. Data are mean ± standard deviation (SD) (n = 3). (B) Induction of TIMP-3 by irradiation. Mice were treated with lethal irradiation (950 R), and BMMNCs were harvested at the indicated time points. The expression of TIMP-3 was examined by Q-RT-PCR. Data were normalized and analyzed by comparative CT method and shown as relative values to day 0. Data are mean ± SD (n = 3).
Myelosuppression induces TIMP-3 in the bone marrow. (A) Induction of TIMP-3 by BM suppression. Mice (C57BL/6) were intraperitoneally injected with 150 mg/kg of 5-FU, and BM mononuclear cells (BMMNCs) were harvested at the indicated time points. RNA was extracted and examined for the expressions of TIMP-3 by Q-RT-PCR. Data were normalized and analyzed by comparative CT method and shown as relative values to day 0. Data are mean ± standard deviation (SD) (n = 3). (B) Induction of TIMP-3 by irradiation. Mice were treated with lethal irradiation (950 R), and BMMNCs were harvested at the indicated time points. The expression of TIMP-3 was examined by Q-RT-PCR. Data were normalized and analyzed by comparative CT method and shown as relative values to day 0. Data are mean ± SD (n = 3).
TIMP-3 stimulates proliferation of HSCs in vitro
To investigate the possible effects of TIMP-3 on the dynamics of HSCs/MPPs, we isolated CD34−c-Kit+Sca-1+Lineage− (CD34−KSL) cells, a well-known and highly enriched fraction of murine HSCs,34 from murine BM and examined their proliferation in vitro in the presence or absence of TIMP-3. Interestingly, TIMP-3 augmented the proliferation of CD34−KSL cells by approximately 2-fold compared with a vehicle control (Figure 2A). Similarly, over-expression of TIMP-3 in CD34−KSL cells, which allows autocrine production of the protein, stimulated their proliferation by approximately 3 times compared with a mock control (supplemental Figure 1A-B). These results clearly show that TIMP-3 facilitates proliferation of HSCs in vitro.
Effects of TIMP-3 on CD34−KSL cells. (A) Enhanced proliferation of CD34−KSL cells by TIMP-3. One hundred CD34−KSL cells/well were sorted into a 96-well plate and cultured as described in “Methods.” (Left) Growth curve (n = 3, mean ± standard deviation [SD]). (Right) Pictures of cells cultured for 5 days (original magnification, ×100). (B) Long-term repopulating activity of CD34−KSL cells cultured with TIMP-3. CD34−KSL cells (Ly5.1) were cultured for 2 weeks as described in “Methods.” All of the cells generated from 10 CD34−KSL cells were transplanted into lethally irradiated congeneic hosts (Ly5.2) with competitors. Percentage of repopulation was not statistically different between control- and TIMP-3–treated groups at any time points. (C) Enhanced production of multipotential progenitor cells from HSCs cultured with TIMP-3. One hundred clonally sorted CD34−KSL cells were cultured in vitro for 1 week with SCF and TPO with or without TIMP-3 as described in “Methods.” Cells were then subjected to colony assays. Data are mean ± SD (n = 3, *P < .05). n, neutrophil; m, macrophage; E, erythroid cell; M, megakaryocyte. (D) TIMP-3 increases multipotential progenitors in vivo. TIMP-3 was over-expressed in vivo by hydrodynamic delivery of TIMP-3 plasmid. After 3 days of injection, mice were killed and examined for LT-HSC (CD34−KSLFlt3−), ST-HSC (CD34+KSLFlt3−), and MPP (CD34+KSLFlt3+) by FACS, and the actual numbers of each progenitor per femur were calculated (n = 3, mean ± SD, *P < .05). Other multipotential (nmEM, nmE) or lineage-restricted (EM, E, nm) progenitors were examined by colony assay. Numbers of colonies per 1 × 104 BM mononuclear cells are shown (n = 3, mean ± SD).
Effects of TIMP-3 on CD34−KSL cells. (A) Enhanced proliferation of CD34−KSL cells by TIMP-3. One hundred CD34−KSL cells/well were sorted into a 96-well plate and cultured as described in “Methods.” (Left) Growth curve (n = 3, mean ± standard deviation [SD]). (Right) Pictures of cells cultured for 5 days (original magnification, ×100). (B) Long-term repopulating activity of CD34−KSL cells cultured with TIMP-3. CD34−KSL cells (Ly5.1) were cultured for 2 weeks as described in “Methods.” All of the cells generated from 10 CD34−KSL cells were transplanted into lethally irradiated congeneic hosts (Ly5.2) with competitors. Percentage of repopulation was not statistically different between control- and TIMP-3–treated groups at any time points. (C) Enhanced production of multipotential progenitor cells from HSCs cultured with TIMP-3. One hundred clonally sorted CD34−KSL cells were cultured in vitro for 1 week with SCF and TPO with or without TIMP-3 as described in “Methods.” Cells were then subjected to colony assays. Data are mean ± SD (n = 3, *P < .05). n, neutrophil; m, macrophage; E, erythroid cell; M, megakaryocyte. (D) TIMP-3 increases multipotential progenitors in vivo. TIMP-3 was over-expressed in vivo by hydrodynamic delivery of TIMP-3 plasmid. After 3 days of injection, mice were killed and examined for LT-HSC (CD34−KSLFlt3−), ST-HSC (CD34+KSLFlt3−), and MPP (CD34+KSLFlt3+) by FACS, and the actual numbers of each progenitor per femur were calculated (n = 3, mean ± SD, *P < .05). Other multipotential (nmEM, nmE) or lineage-restricted (EM, E, nm) progenitors were examined by colony assay. Numbers of colonies per 1 × 104 BM mononuclear cells are shown (n = 3, mean ± SD).
TIMP-3 promotes generation of MPPs from HSCs without affecting their overall long-term repopulating capacity
Enhanced proliferation of CD34−KSL cells in vitro by TIMP-3 could be caused by an expansion of either HSCs, MPPs, or both. To dissect which cell population was expanded by TIMP-3, we first examined the long-term repopulating (LTR) activity, a hallmark of HSCs, of CD34−KSL cells cultured with TIMP-3 by competitive repopulation assay. This revealed that the overall LTR activity of whole cultured output from CD34−KSL cells was not significantly different between TIMP-3- and vehicle-treated groups even after 2 weeks of culture (Figure 2B). Assuming that LTR activity of single CD34−KSL cell was maintained, this result implies that TIMP-3 does not affect the number of HSCs in the culture at least up to 2 weeks. In contrast, CD34−KSL cells cultured with TIMP-3 for 1 week generated approximately 1.5 times more MPPs (shown as nmEM [neutrophil-macrophage-erythroid-megakaryocyte] and nmE [neutrophil-macrophage-erythroid] in Figure 2C) compared with control-treated cells (Figure 2C). Taken together, these results suggest that TIMP-3 promotes asymmetric cell division of HSCs, resulting in the expansion of MPPs without affecting the number of initial HSCs.
Over-expression of TIMP-3 in vivo increases short-term HSCs, MPPs, and downstream progenitors
We next tested whether TIMP-3 expands the MPP pool in vivo. To do this, we over-expressed TIMP-3 in mice by the hydrodynamic plasmid delivery method, achieving transient protein over-expression in vivo for 1 week.32 Because TIMP-3 is a soluble protein, over-expression by this method is expected to increase systemic concentration of the protein including in the BM. In fact, the expression or deposition of TIMP-3 protein was demonstrated at the endosteal surface of the BM by immunostaining, and as was reported previously in hepatocytes,32 a major site of plasmid expression (supplemental Figure 2A-B). As expected, enforced expression of TIMP-3 in vivo resulted in an increase of ST-HSCs (CD34+KSL Flt3−), MPPs (CD34+KSL Flt3+),27 and other multipotent (nmEM and nmE) or lineage-restricted progenitors (EM, E, nm) (Figure 2D). In contrast, the number of LT-HSCs (CD34−KSL Flt3−) did not significantly differ between control- or TIMP-3–treated mice. These results indicate that TIMP-3 promotes expansion of ST-HSCs, MPPs, and their downstream progenitors in vivo, without affecting the size of the LT-HSC pool.
TIMP-3 stimulates the proliferation of long-term HSCs but not short-term HSCs or MPPs
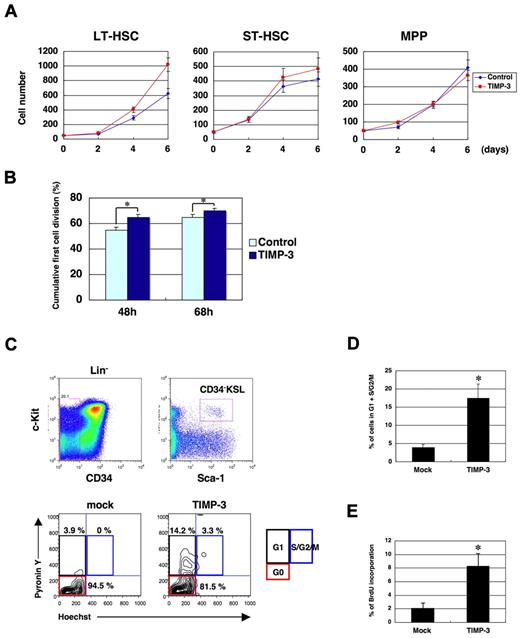
Expansion of MPP pool by TIMP-3 can be caused by an enhanced production of MPPs from HSCs and/or to an increased proliferation of MPPs themselves. To distinguish between these 2 possibilities, we first examined in vitro the effect of TIMP-3 on the proliferation of LT-HSCs, ST-HSCs, and MPPs. As shown in Figure 3A, the growth stimulatory effect of TIMP-3 was most prominently observed in LT-HSCs and was minimal or negligible in ST-HSCs or MPPs, respectively. This suggests that TIMP-3 exclusively affects proliferation of HSCs, but not MPPs. Enhanced HSC proliferation by TIMP-3 was also confirmed by single-cell growth analysis of CD34−KSL cells, which showed that the cumulative percentage of the cells having gone through the first cell division at any defined time points was significantly higher in TIMP-3 (Figure 3B).
TIMP-3 facilitates cell-cycle entry of quiescent HSCs. (A) TIMP-3 stimulates proliferation of LT-HSCs (CD34−KSL Flt3−) but not ST-HSCs (CD34+KSL Flt3−) or MPPs (CD34+KSL Flt3+). Fifty cells/well for each fraction were sorted into a 96-well plate and cultured as described in the Methods. Cell numbers were counted at the indicated time points and plotted onto the graphs (n = 3, mean ± SD). (B) Enhanced rate of the first cell division of CD34−KSL cells in the presence of TIMP-3. CD34−KSL cells were clonally sorted into a 96-well plate; the cell number for each well was counted at 48 and 68 hours after FACS sorting. Percentage of the wells containing 2 or more cells was plotted on the graph (n = 3, mean ± SD). *P < .05. (C-D) TIMP-3 recruits quiescent HSCs into the cell cycle. Mice were injected with TIMP-3 expression plasmids through tail veins by hydrodynamic delivery. (C) After 3 days of injection, BM cells were harvested and examined for the cell-cycle status of CD34−KSL cells, as described in the Methods (D) The percentage of cycling cells (G1 + S/G2/M) was plotted on the graph (n = 3, mean ± SD). *P < .05. (E) TIMP-3 induces DNA synthesis in HSCs. Mice were injected with TIMP-3 expression vector through tail veins by hydrodynamic delivery. After 3 days of injection, mice were injected intraperitoneally with 2 mg of BrdU. BM cells were harvested at day 4 and examined for incorporation of BrdU in CD34−KSL cells. Percentages of cells that have incorporated BrdU are shown (n = 3, mean ± SD). *P < .05.
TIMP-3 facilitates cell-cycle entry of quiescent HSCs. (A) TIMP-3 stimulates proliferation of LT-HSCs (CD34−KSL Flt3−) but not ST-HSCs (CD34+KSL Flt3−) or MPPs (CD34+KSL Flt3+). Fifty cells/well for each fraction were sorted into a 96-well plate and cultured as described in the Methods. Cell numbers were counted at the indicated time points and plotted onto the graphs (n = 3, mean ± SD). (B) Enhanced rate of the first cell division of CD34−KSL cells in the presence of TIMP-3. CD34−KSL cells were clonally sorted into a 96-well plate; the cell number for each well was counted at 48 and 68 hours after FACS sorting. Percentage of the wells containing 2 or more cells was plotted on the graph (n = 3, mean ± SD). *P < .05. (C-D) TIMP-3 recruits quiescent HSCs into the cell cycle. Mice were injected with TIMP-3 expression plasmids through tail veins by hydrodynamic delivery. (C) After 3 days of injection, BM cells were harvested and examined for the cell-cycle status of CD34−KSL cells, as described in the Methods (D) The percentage of cycling cells (G1 + S/G2/M) was plotted on the graph (n = 3, mean ± SD). *P < .05. (E) TIMP-3 induces DNA synthesis in HSCs. Mice were injected with TIMP-3 expression vector through tail veins by hydrodynamic delivery. After 3 days of injection, mice were injected intraperitoneally with 2 mg of BrdU. BM cells were harvested at day 4 and examined for incorporation of BrdU in CD34−KSL cells. Percentages of cells that have incorporated BrdU are shown (n = 3, mean ± SD). *P < .05.
TIMP-3 expands the MPP pool by recruiting quiescent HSCs into active cell cycle and facilitating their cell division
Given that TIMP-3 stimulates proliferation of LT-HSCs, most of which are quiescent in G0 phase, we speculated that TIMP-3 might recruit quiescent HSCs into the cell cycle. To test this possibility, we again over-expressed TIMP-3 in vivo and examined the cell-cycle status of CD34−KSL cells (Figure 3C-D). As expected, approximately 18% of the CD34−KSL cells were recruited into G1 + G2/M phase of the cell cycle in TIMP-3–treated mice, whereas 95% of the cells were resting in G0 phase in the control-treated animals. Importantly, TIMP-3–induced cell-cycle recruitment of quiescent HSCs was accompanied by DNA synthesis as revealed by a BrdU labeling experiment (Figure 3E and supplemental Figure 3). Taken together with the findings from Figure 3A-B, the results indicate that TIMP-3 expands the MPP pool by pushing quiescent HSCs into an actively cycling state, not by stimulating MPP proliferation.
Increased inheritance of multilineage differentiation potential from HSCs to their progenies in the presence of TIMP-3
Facilitated cell division of HSCs could result in an increased loss of multilineage differentiation capacity of their progenies and thus may not necessarily lead to MPP expansion. To confirm whether facilitated cell division of HSCs is actually coupled with increased production of MPPs, we performed paired daughter-cell analysis to evaluate the differentiation potential of the immediate progenies of CD34−KSL cells cultured with TIMP-3 (Table 1). Surprisingly, 73.3 ± 2.6% of CD34−KSL cells cultured with TIMP-3 gave rise to paired progenies, both of which inherited multilineage differentiation potential (see nmEM/nmEM in Table 1), whereas only 45.1 ± 2.2% of the daughter-cell pairs were nmEM/nmEM in the control culture (Table 1). These data clearly show that TIMP-3 increases the chance for HSC progenies to inherit multilineage differentiation potential, further contributing to the TIMP-3–mediated expansion of MPPs from CD34−KSL cells.
Paired-daughter cell colony assay of HSCs cultured with TIMP-3
| . | Control . | TIMP-3 . |
|---|---|---|
| nmEM/nmEM | 45.1 ± 2.2 | 73.3 ± 2.6 |
| nmEM/nmE | 18.4 ± 2.2 | 16.3 ± 2.2 |
| nmEM/nmM | 2.4 ± 0.6 | 1.7 ± 0.6 |
| nmEM/nm | 0.0 ± 0.0 | 1.7 ± 1.6 |
| nmEM/M | 2.4 ± 0.6 | 0.0 ± 0.0 |
| nmE/nmE | 21.9 ± 1.8 | 4.9 ± 1.2 |
| nmM/nmM | 2.4 ± 0.6 | 0.0 ± 0.0 |
| nmE/nm | 1.0 ± 1.0 | 0.0 ± 0.0 |
| nm/nm | 1.7 ± 0.6 | 0.0 ± 0.0 |
| M/M | 4.5 ± 1.2 | 2.1 ± 2.1 |
| . | Control . | TIMP-3 . |
|---|---|---|
| nmEM/nmEM | 45.1 ± 2.2 | 73.3 ± 2.6 |
| nmEM/nmE | 18.4 ± 2.2 | 16.3 ± 2.2 |
| nmEM/nmM | 2.4 ± 0.6 | 1.7 ± 0.6 |
| nmEM/nm | 0.0 ± 0.0 | 1.7 ± 1.6 |
| nmEM/M | 2.4 ± 0.6 | 0.0 ± 0.0 |
| nmE/nmE | 21.9 ± 1.8 | 4.9 ± 1.2 |
| nmM/nmM | 2.4 ± 0.6 | 0.0 ± 0.0 |
| nmE/nm | 1.0 ± 1.0 | 0.0 ± 0.0 |
| nm/nm | 1.7 ± 0.6 | 0.0 ± 0.0 |
| M/M | 4.5 ± 1.2 | 2.1 ± 2.1 |
CD34−KSL cells were clonally sorted into 96-well plates and cultured in the presence of SCF and TPO with or without TIMP-3, as described in “Methods.” Once they divided to generate daughter cell pairs, cells were separated by micromanipulation and subjected to colony assays. Colony types were evaluated by morphological examination as described in “Methods.” The experiments were done in triplicate (96 colony pairs were evaluated in each experiment), and percentages of colony pairs are shown (mean ± SD). Colonies from nmEM progenitors are highlighted in bold.
Metalloproteinase inhibitory activity of TIMP-3 is not required for MPP expansion and enhanced HSC cycling
We next tried to reveal the molecular mechanism for TIMP-3–mediated MPP expansion. As TIMP-3 is an inhibitor of MMPs, we asked whether MMP-inhibitory activity of TIMP-3 is required for its effects on HSCs. It was previously reported that mutating the first cysteine residue of TIMP-3 to serine (TIMP-3C1S) abolishes the MMP-inhibitory activity.25 We therefore over-expressed TIMP-3C1S in vivo and examined its effect on the HSCs or progenitor compartments. Surprisingly, over-expression of TIMP-3C1S resulted in an increase of ST-HSC, MPP, and other multipotent or lineage-restricted progenitors similar to the increase by the wild-type TIMP-3 (Figure 4A). Moreover, enhanced expression of TIMP-3C1S facilitated the cell-cycle entry of HSCs in vivo (Figure 4B). We further tested the requirement for MMP inhibition using a global MMP-inhibitor, GM6001.35 As shown in Figure 4C, inhibition of MMP activity by GM6001 did not affect the proliferation of CD34−KSL cells in vitro, whereas TIMP-3 augmented their proliferation approximately 2-fold in the same setting. Taken together, these data demonstrate that MMP-inhibitory activity of TIMP-3 is not required for the expansion of MPPs and enhanced cell-cycle entry of HSCs.
MMP-inhibitory activity of TIMP-3 is not required for expansion of hematopoietic progenitors and the recruitment of HSCs into the cell cycle. (A) MMP-inhibitory activity of TIMP-3 is not required for expansion of hematopoietic progenitors in vivo. Mutant TIMP-3 lacking MMP-inhibitory activity (TIMP-3C1S) was over-expressed in vivo by hydrodynamic delivery. After 3 days of injection, mice were killed and examined for LT-HSCs (CD34−KSLFlt3−), ST-HSCs (CD34+KSLFlt3−), and MPPs (CD34+KSLFlt3+) by FACS, and the actual numbers of each progenitor per femur were calculated (n = 3, mean ± SD, *P < .05). Other multipotential (nmEM, nmE) or lineage-restricted (EM, E, nm) progenitors were examined by colony assay. Numbers of colonies per 1 × 104 bone marrow mononuclear cells are shown (n = 3, mean ± SD). (B) Metalloproteinase-inhibitory activity is not essential for the recruitment of quiescent HSCs into the cell cycle. TIMP-3C1S was over-expressed in vivo by hydrodynamic delivery. After 3 days of injection, BM cells were harvested and examined for the cell-cycle status of CD34−KSL cells, as described in the Methods. The percentage of cycling cells (G1 + S/G2/M) was plotted on the graph (n = 3, mean ± SD). *P < .05. (C) Effects of metalloproteinase-inhibitor on the proliferation of HSCs. One hundred CD34−KSL cells/well were sorted into a 96-well plate and cultured as described in “Methods.” TIMP-3 (3 μg/mL), GM6001 at various concentrations (1, 5, and 25 μM), or vehicle (0.1% dimethyl sulfoxide [DMSO]) as a control were added to the culture. Cell numbers were counted at the indicated time points (n = 3, mean ± SD).
MMP-inhibitory activity of TIMP-3 is not required for expansion of hematopoietic progenitors and the recruitment of HSCs into the cell cycle. (A) MMP-inhibitory activity of TIMP-3 is not required for expansion of hematopoietic progenitors in vivo. Mutant TIMP-3 lacking MMP-inhibitory activity (TIMP-3C1S) was over-expressed in vivo by hydrodynamic delivery. After 3 days of injection, mice were killed and examined for LT-HSCs (CD34−KSLFlt3−), ST-HSCs (CD34+KSLFlt3−), and MPPs (CD34+KSLFlt3+) by FACS, and the actual numbers of each progenitor per femur were calculated (n = 3, mean ± SD, *P < .05). Other multipotential (nmEM, nmE) or lineage-restricted (EM, E, nm) progenitors were examined by colony assay. Numbers of colonies per 1 × 104 bone marrow mononuclear cells are shown (n = 3, mean ± SD). (B) Metalloproteinase-inhibitory activity is not essential for the recruitment of quiescent HSCs into the cell cycle. TIMP-3C1S was over-expressed in vivo by hydrodynamic delivery. After 3 days of injection, BM cells were harvested and examined for the cell-cycle status of CD34−KSL cells, as described in the Methods. The percentage of cycling cells (G1 + S/G2/M) was plotted on the graph (n = 3, mean ± SD). *P < .05. (C) Effects of metalloproteinase-inhibitor on the proliferation of HSCs. One hundred CD34−KSL cells/well were sorted into a 96-well plate and cultured as described in “Methods.” TIMP-3 (3 μg/mL), GM6001 at various concentrations (1, 5, and 25 μM), or vehicle (0.1% dimethyl sulfoxide [DMSO]) as a control were added to the culture. Cell numbers were counted at the indicated time points (n = 3, mean ± SD).
TIMP-3 inhibits Angiopoietin-1 signaling
To get a further insight into the molecular mechanism of HSC recruitment by TIMP-3, we asked whether TIMP-3 modulates signaling pathways regulating HSC quiescence. Ang-1 is a well-known cytokine that plays a critical role for HSC quiescence.12,15 To test whether TIMP-3 modulates Ang-1 signaling, we examined the effect of TIMP-3 on Ang-1–induced phosphorylation of its receptor, Tie-2 (supplemental Figure 4). Surprisingly, autophosphorylation of Tie-2 induced by Ang-1 was clearly inhibited by pretreating cells with TIMP-3 or by stable expression of TIMP-3 (supplemental Figure 4A-B). This effect was specific to Tie-2/Ang-1 signaling, inasmuch as Janus kinase 2 activation by interleukin-3 was not affected by TIMP-3 (supplemental Figure 4A). The same observation was made in primary human umbilical vein endothelial cells, which express endogenous Tie-2 (supplemental Figure 4C). Of note, Ang-1 stimulation induced rapid decrease of Tie-2 protein levels, and this effect was more profound in TIMP-3–treated cells (supplemental Figure 4A-B), suggesting that inhibition of Tie-2 phosphorylation by TIMP-3 is partly mediated by facilitated degradation of Tie-2 protein. MMP-inhibitory activity of TIMP-3 is not required for Ang-1/Tie-2 inhibition, as TIMP-3C1S equally suppressed Tie-2 phosphorylation (supplemental Figure 4D). To further explore the molecular mechanism of TIMP-3 inhibition of Ang-1 signaling, we next examined the effect of TIMP-3 on constitutively active Tie-2 (CA-Tie-2) mutant.36 TIMP-3 did not affect the autophosphorylation of CA-Tie-2 in the absence of Ang-1 (supplemental Figure 4E). However, up-regulation of CA-Tie-2 phosphorylation by Ang-1 was clearly inhibited by TIMP-3 treatment. These data suggest that TIMP-3 does not affect intracellular signaling pathways of Tie-2 but rather inhibits extracellular steps of Ang-1/Tie-2 signaling. We have actually observed that TIMP-3 directly interfered with the binding of Ang-1 and Tie-2 in Biacore and in vitro binding assays (supplemental Figure 5). Collectively, these data raise a possibility that the enhanced cell-cycle entry of HSCs by TIMP-3 is achieved, at least in part, through inhibition of Ang-1 signaling.
TIMP-3 accelerates recovery from bone marrow suppression and protects mice from lethal irradiation
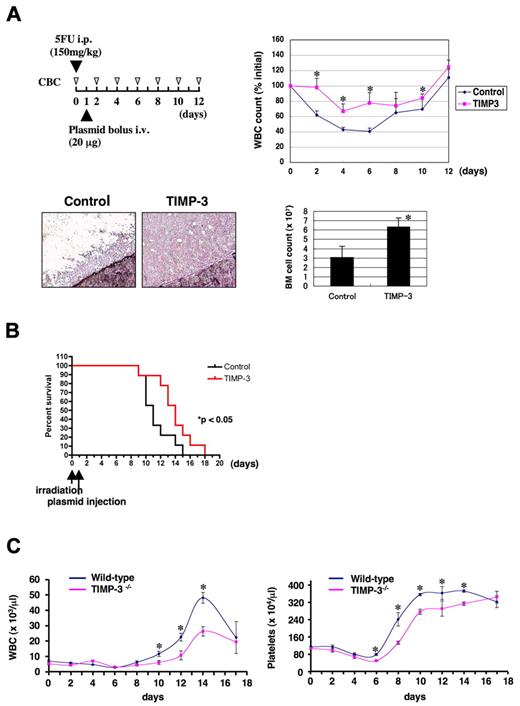
Given that TIMP-3 promotes expansion of ST-HSCs as well as of multipotent or lineage-restricted progenitors in vivo, we thought it might be useful for facilitating hematopoietic recovery from myelosuppression. To test this notion, we over-expressed TIMP-3 in vivo and examined its effect on BM recovery from myelosuppression induced by 5-FU (experimental design depicted in Figure 5A). The lowest average leukocyte count (approximately 40% of the pretreatment level) was observed after 6 days of 5-FU injection in the control-treated animals. As expected, leukocytopenia was less severe in animals treated with TIMP-3 (65%-70% of the pretreatment level) with faster recovery from the nadir (Figure 5A). Surprisingly, after 4 days of plasmid injection, BM cellularity was remarkably higher in mice injected with TIMP-3 plasmid, providing a basis for facilitated hematopoietic recovery (Figure 5A).
TIMP-3 facilitates hematopoietic recovery from BM suppression in vivo. (A) Mice (n = 4 for each group) were injected with 5-FU intraperitoneally on day 0 and then with TIMP-3 expression plasmids through tail veins by hydrodynamic delivery on day 1. (Top left) Experimental schedule. (Top right) Graphs for white blood cell (WBC) counts (initial counts at day 0 are regarded as 100% for each animal). Data are mean ± standard error (SE). *P < .05. (Bottom left) Bone marrow cellularity of animals injected with control or TIMP-3 plasmids (day 4). Hematoxylin staining. Original magnification ×200. (Bottom right) Total number of BM cells (from both femurs) at day 4 was counted and shown (mean ± SE, n = 3, *P < .05). (B) Survival curves of irradiated (900 R) mice treated with TIMP-3. Mice were injected with control or TIMP-3 plasmids 24 hours after irradiation. The curves are statistically different (n = 9 per group, P < .05 by log-rank test). (C) Impaired BM recovery of TIMP-3 deficient mice (TIMP-3−/−). TIMP-3−/− mice and the wild-type littermate controls were treated with 5FU (150 mg/kg, intraperitoneally). White blood cell and platelet counts were monitored at the indicated time points. Data are mean ± SE (n = 5 for wild-type and n = 3 for TIMP-3−/−, *P < .05).
TIMP-3 facilitates hematopoietic recovery from BM suppression in vivo. (A) Mice (n = 4 for each group) were injected with 5-FU intraperitoneally on day 0 and then with TIMP-3 expression plasmids through tail veins by hydrodynamic delivery on day 1. (Top left) Experimental schedule. (Top right) Graphs for white blood cell (WBC) counts (initial counts at day 0 are regarded as 100% for each animal). Data are mean ± standard error (SE). *P < .05. (Bottom left) Bone marrow cellularity of animals injected with control or TIMP-3 plasmids (day 4). Hematoxylin staining. Original magnification ×200. (Bottom right) Total number of BM cells (from both femurs) at day 4 was counted and shown (mean ± SE, n = 3, *P < .05). (B) Survival curves of irradiated (900 R) mice treated with TIMP-3. Mice were injected with control or TIMP-3 plasmids 24 hours after irradiation. The curves are statistically different (n = 9 per group, P < .05 by log-rank test). (C) Impaired BM recovery of TIMP-3 deficient mice (TIMP-3−/−). TIMP-3−/− mice and the wild-type littermate controls were treated with 5FU (150 mg/kg, intraperitoneally). White blood cell and platelet counts were monitored at the indicated time points. Data are mean ± SE (n = 5 for wild-type and n = 3 for TIMP-3−/−, *P < .05).
We also examined the effect of TIMP-3 on protecting mice from myeloablation. Mice were lethally irradiated (900 R) on day 0, followed by an injection of TIMP-3 or mock plasmids on day 1. As shown in Figure 5B, survival of TIMP-3–treated mice was significantly longer than the control (P < .05), indicating that TIMP-3–mediated MPP expansion conferred a survival advantage. These results indicate that increased dosage of TIMP-3 can facilitate hematopoietic recovery from myelosuppression in vivo.
Delayed hematopoietic recovery from myelosuppression in TIMP-3–deficient mice
To get firm evidence for the role of TIMP-3 in BM recovery in physiological settings, we analyzed TIMP-3–deficient mice26 to determine whether they had any defects in the hematopoietic recovery after myelosuppression (Figure 5C). Although the initial suppression phase (day 0-6) was comparable in both wild-type and TIMP-3−/− animals, leukocyte and platelet recovery with the following overshoot after day 6 were clearly impaired in TIMP-3−/− animals compared with wild-type. These data strongly suggest that TIMP-3 plays a critical role in stress hematopoiesis.
Discussion
The precise regulation of HSC cycling is critical for promptly meeting various demands for blood cell production, yet still maintaining stem cell capacities such as multipotentiality and self-renewal.4 Even the slightest dysregulation of these processes would lead to a loss of HSCs through differentiation, leading to a hematopoietic failure. Extracellular signals or cell-cycle regulators such as Ang-112,15 or p2137 have been shown to be critical for HSC quiescence. However, it has been poorly understood how quiescent HSCs wake up and generate progenitors upon hematopoietic needs.
In this study, we demonstrated that TIMP-3 is induced in the BM by myelosuppressive stress and that it recruits quiescent HSCs into the cell cycle leading to the expansion of the MPP pool. As mentioned above, cell-cycle regulators for HSCs must be under stringent control, and in this regard, some of the unique characteristics of TIMP-3 make itself an ideal mediator for HSC regulation. First, TIMP-3 binds with high affinity to the extracellular matrix38,39 and therefore should be concentrated in the niche microenvironment. Second, TIMP-3 has a short half-life in vivo. These characteristics ensure the spatial and temporal specificity of TIMP-3 action, preventing unwanted differentiation of HSCs through excessive cycling.
In the myelosuppressive state, lineage-specific cytokines such as granulocyte colony-stimulating factor or erythropoietin are induced to expand lineage-committed progenitors and facilitate their differentiation.40,41 Consumed progenitors have to be replenished from HSCs to maintain hematopoiesis; however, the factors regulating such replenishment have remained elusive. This study suggests that TIMP-3 could be one such factor promoting progenitor supply from HSCs, indicating that TIMP-3 is an attractive candidate for therapeutic intervention in hematopoietic recovery after chemotherapy or hematopoietic failure. Indeed, we have shown that TIMP-3 facilitates hematopoietic recovery from myelosuppression and prolongs survival for lethally irradiated mice. These results hold great promise for the future application of TIMP-3 in the clinical settings.
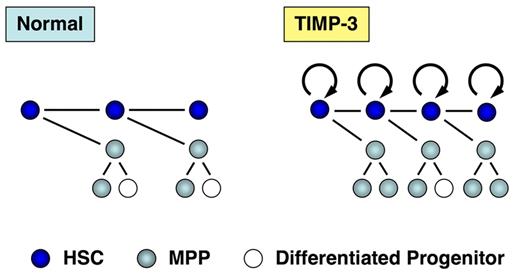
Another possible clinical application of TIMP-3 is ex vivo manipulation of HSCs. As shown in this study, TIMP-3 promotes MPP expansion by facilitating the cell-cycle entry of HSCs without affecting their self-renewal potential. TIMP-3 also acts to maintain multilineage differentiation potential of the direct progenies from HSCs (effects of TIMP-3 on HSCs/MPPs are depicted in Figure 6). These unique properties of TIMP-3 can be applied to the manipulation of cord blood (CB) HSCs, where an expanded MPP pool would help overcome some of the inherent problems seen in the CB transplantation: slow marrow recovery and graft failure.42,43 In this regard, it is intriguing to note that TIMP-3 actually expanded severe combined immunodeficiency (SCID)–repopulating cells in CB in our preliminary experiments (unpublished data).
Effects of TIMP-3 on HSCs and MPPs. TIMP-3 stimulates cell-cycle progression of HSCs and thus promotes production of MPPs. TIMP-3 does not affect proliferation of MPPs. In addition, TIMP-3 acts to preserve multilineage differentiation potential of HSC progenies.
Effects of TIMP-3 on HSCs and MPPs. TIMP-3 stimulates cell-cycle progression of HSCs and thus promotes production of MPPs. TIMP-3 does not affect proliferation of MPPs. In addition, TIMP-3 acts to preserve multilineage differentiation potential of HSC progenies.
The precise molecular mechanism explaining how TIMP-3 wakes up quiescent HSCs into the cell cycle remains obscure. In this study, we have shown that TIMP-3 affects proliferation of only LT-HSCs, but not ST-HSCs or MPPs, suggesting that TIMP-3 modulates regulatory systems specifically working in LT-HSCs. Because Ang-1/Tie-2 signaling is critical for maintaining HSC quiescence and specifically working in LT-HSCs, we speculated that TIMP-3 might be targeting Ang-1/Tie-2 signaling. Indeed, the present study demonstrated that TIMP-3 negatively regulates Ang-1 signaling independently of MMP inhibition. Moreover, we observed that TIMP-3 inhibits the interaction of Ang-1 and Tie-2 through associating with each molecule. These observations raise an intriguing possibility that TIMP-3 inhibits Ang-1/Tie-2 signals by blocking ligand-receptor interactions, thereby releasing HSCs from the quiescent state.
Another possible target of TIMP-3 is the vascular endothelial growth factor (VEGF) pathway. It was previously shown that TIMP-3 inhibits VEGF signaling, which is critical for the maintenance of adult HSCs,44 through associating with VEGF receptor 2.25 However, VEGF actually works “inside” HSCs through an internal autocrine mechanism,44 and therefore TIMP-3 in extracellular environment cannot be accessible for this intracellular regulatory loop. These data suggest that VEGF is not the major target for TIMP-3 in HSC recruitment. Further studies will be required to clarify the precise target of TIMP-3 in this process.
In summary, we have shown that TIMP-3 can drive quiescent HSCs into active cell cycle and expand MPP population without compensation of self-renewal capacity. This result may open up an avenue for clinical application of TIMP-3 for facilitating hematopoietic recovery or manipulating HSCs ex vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank N. Watanabe, T. Shibata, S. Saito (FACS Core Laboratory, Institute of Medical Science, University of Tokyo, IMSUT), and Y. Morita (Laboratory of Stem Cell Therapy, IMSUT) for FACS sorting, Dr K. Tsuji (Division of Cellular Therapy, IMSUT) for valuable advice and critical reading of the manuscript, and Dr Dovie R. Wylie for language assistance.
This work was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Authorship
Contribution: H.N. designed and performed research, analyzed the data, and wrote the paper; M.I., D.S.S., F.S., Y.F., Y.M., Y.I., and F.A. performed research; T.S. and R.K. contributed vital new reagents; and T.K. contributed vital new reagents and supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hideaki Nakajima, MD, PhD, Division of Hematology, Department of Internal Medicine, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan; e-mail: hnakajim@sc.itc.keio.ac.jp.

![Figure 2. Effects of TIMP-3 on CD34−KSL cells. (A) Enhanced proliferation of CD34−KSL cells by TIMP-3. One hundred CD34−KSL cells/well were sorted into a 96-well plate and cultured as described in “Methods.” (Left) Growth curve (n = 3, mean ± standard deviation [SD]). (Right) Pictures of cells cultured for 5 days (original magnification, ×100). (B) Long-term repopulating activity of CD34−KSL cells cultured with TIMP-3. CD34−KSL cells (Ly5.1) were cultured for 2 weeks as described in “Methods.” All of the cells generated from 10 CD34−KSL cells were transplanted into lethally irradiated congeneic hosts (Ly5.2) with competitors. Percentage of repopulation was not statistically different between control- and TIMP-3–treated groups at any time points. (C) Enhanced production of multipotential progenitor cells from HSCs cultured with TIMP-3. One hundred clonally sorted CD34−KSL cells were cultured in vitro for 1 week with SCF and TPO with or without TIMP-3 as described in “Methods.” Cells were then subjected to colony assays. Data are mean ± SD (n = 3, *P < .05). n, neutrophil; m, macrophage; E, erythroid cell; M, megakaryocyte. (D) TIMP-3 increases multipotential progenitors in vivo. TIMP-3 was over-expressed in vivo by hydrodynamic delivery of TIMP-3 plasmid. After 3 days of injection, mice were killed and examined for LT-HSC (CD34−KSLFlt3−), ST-HSC (CD34+KSLFlt3−), and MPP (CD34+KSLFlt3+) by FACS, and the actual numbers of each progenitor per femur were calculated (n = 3, mean ± SD, *P < .05). Other multipotential (nmEM, nmE) or lineage-restricted (EM, E, nm) progenitors were examined by colony assay. Numbers of colonies per 1 × 104 BM mononuclear cells are shown (n = 3, mean ± SD).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/22/10.1182_blood-2010-01-266528/6/m_zh89991061280002.jpeg?Expires=1769096824&Signature=KsdhwTFa6BjsgmQOWbNtdBC7Pk~v~Juyro~zpa3VVApNnSjiEfeKMG8WCKgTvJ6-QX48-Uw7Lm8nvZj6a7obpJ1eUSF~C9AzLYH19Hbe1v08QKbXkBYcPyc~XewmuZ9yDIGJbvDpycNCyaPN5RCLQ~AqkkOUMivMM3SYqRAjBHxXMXbvm5M9-jS1cBryBOY-iZu0p-FKRmXOg0VTquG611hgCxHoluiW8~VrJdSJmLxZYo3vMI7X-sxfeo5oVuttKDjx9vLPox3FvcvkquEGM~~9xjN0WkNkzW3uQnB7MJ8o16p3bd1I4zCMHtgxTj30QfJrJh9wBhGDvVMDQVGlgg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. MMP-inhibitory activity of TIMP-3 is not required for expansion of hematopoietic progenitors and the recruitment of HSCs into the cell cycle. (A) MMP-inhibitory activity of TIMP-3 is not required for expansion of hematopoietic progenitors in vivo. Mutant TIMP-3 lacking MMP-inhibitory activity (TIMP-3C1S) was over-expressed in vivo by hydrodynamic delivery. After 3 days of injection, mice were killed and examined for LT-HSCs (CD34−KSLFlt3−), ST-HSCs (CD34+KSLFlt3−), and MPPs (CD34+KSLFlt3+) by FACS, and the actual numbers of each progenitor per femur were calculated (n = 3, mean ± SD, *P < .05). Other multipotential (nmEM, nmE) or lineage-restricted (EM, E, nm) progenitors were examined by colony assay. Numbers of colonies per 1 × 104 bone marrow mononuclear cells are shown (n = 3, mean ± SD). (B) Metalloproteinase-inhibitory activity is not essential for the recruitment of quiescent HSCs into the cell cycle. TIMP-3C1S was over-expressed in vivo by hydrodynamic delivery. After 3 days of injection, BM cells were harvested and examined for the cell-cycle status of CD34−KSL cells, as described in the Methods. The percentage of cycling cells (G1 + S/G2/M) was plotted on the graph (n = 3, mean ± SD). *P < .05. (C) Effects of metalloproteinase-inhibitor on the proliferation of HSCs. One hundred CD34−KSL cells/well were sorted into a 96-well plate and cultured as described in “Methods.” TIMP-3 (3 μg/mL), GM6001 at various concentrations (1, 5, and 25 μM), or vehicle (0.1% dimethyl sulfoxide [DMSO]) as a control were added to the culture. Cell numbers were counted at the indicated time points (n = 3, mean ± SD).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/22/10.1182_blood-2010-01-266528/6/m_zh89991061280004.jpeg?Expires=1769096824&Signature=B~A4A3LwXemRzXia~dlTK6e3HrZMtp~pfYARKj5I7KdenIf3VLlyyz7mpqPh0ywocWLJuRjUXAQqJswPv~~8Mj66RNx-srHXMY~LOUIxYOtK6lpJDsN5JfISvZQKG-jGi8Bnb7I4jOciWjRKH~Q0kJKWctCxMZGvnEeOfie~fbfC~a0DlpWMcZSL-SLRcHJC4ngoIHBomGk547S4Ui3oiubsJMuGzGrmVQnsG0C5QvAUMdt9ljZ3rnXcqbxliT5WlmEVil3~vjMU0ILY6uYbPR9lZOy6DwMLjtULp2Vst0UKUqF3OzmD0HmjqVKIJ~RDkqCMPtKFeSOipj1tVEXOQg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal